Abstract
Background:
The accessibility of prescription drugs produced outside of the United States, most notably sildenafil citrate (innovator product, Viagra®), has been made much easier by the Internet. Of greatest concern to clinicians and policymakers is product quality and patient safety. The US Food and Drug Administration (FDA) has issued warnings to potential buyers that the safety of drugs purchased from the Internet cannot be guaranteed, and may present a health risk to consumers from substandard products.
Objective:
The objective of this study was to determine whether generic sildenafil citrate tablets from international markets obtained via the Internet are equivalent to the US innovator product regarding major aspects of pharmaceutical quality: potency, accuracy of labeling, and presence and level of impurities. This will help identify aspects of drug quality that may impact public health risks.
Methods:
A total of 15 sildenafil citrate tablets were obtained for pharmaceutical analysis: 14 generic samples from international Internet pharmacy websites and the US innovator product. According to US Pharmacopeial guidelines, tablet samples were tested using high-performance liquid chromatography for potency of active pharmaceutical ingredient (API) and levels of impurities (impurities A, B, C, and D). Impurity levels were compared with International Conference on Harmonisation (ICH) limits.
Results:
Among the 15 samples, 4 samples possessed higher impurity B levels than the ICH qualification threshold, 8 samples possessed higher impurity C levels than the ICH qualification threshold, and 4 samples possessed more than 1% impurity quantity of maximum daily dose (MDD). For API, 6 of the samples failed to fall within the 5% assay limit.
Conclusions:
Quality assurance tests are often used to detect formulation defects of drug products during the manufacturing and/or storage process. Results suggest that manufacturing standards for sildenafil citrate generic drug products compared with the US innovator product are not equivalent with regards to potency and levels of impurities. These findings have implications for safety and effectiveness that should be addressed by clinicians to safeguard consumers who choose to purchase sildenafil citrate and foreign-manufactured drugs, in general, via the Internet.
Keywords: drug importation, drug quality, drug safety, Internet pharmacy, quality assurance testing, sildenafil citrate, Viagra®
Introduction
According to the National Association of Boards of Pharmacy (NABP), 97% of the over 10,000 Internet drug ‘outlets’ that NABP has assessed maybe illegitimate or illegal, and out of compliance with pharmacy laws and practice standards [National Association of Boards of Pharmacy, 2014]. Sildenafil (Viagra®) is a top-selling, perhaps the best-selling, illicit drug sold on the Internet [BBC News, 2002]. In fact, the pharmaceutical company Pfizer, who first brought Viagra to market in 1998, is setting up its own site to sell Viagra online, in what it says is an effort to curb the sale of counterfeit versions of the drug [Isidore, 2013].
Viagra was the first treatment for erectile dysfunction in an oral dosage form. Initially, it was studied for treatment of hypertension and angina pectoris, but early clinical trials suggested that although it had little effect on angina, it was observed to induce penile erections. As a result, Pfizer made the decision to market Viagra for erectile dysfunction [Ghofrani et al. 2006]. What followed was an immediate healthcare, if not absolute, business success. According to reports at the time: ‘Within two weeks, newspapers reported physicians were writing 15,000 to 20,000 prescriptions a day for the medication’ [Rodriguez, 1998]. In the week of 8 May 1998, and 1 month after Viagra’s release, more than 300,000 total prescriptions were written for Viagra in the US, and Pfizer reported that more than US$400 million worth of Viagra was sold in the first quarter [Keith, 2000].
More recently, according to the healthcare consulting firm IMS Health, approximately 8 million prescriptions for Viagra were written in 2012, which translates into total sales of about US$2 billion. And of the ‘market share’ for pharmaceuticals for erectile dysfunction, which also includes Cialis, Levitra, and Staxyn [Drugs.com, 2014], Viagra has approximately 45% of the market, just ahead of Eli Lilly’s Cialis [Wilson, 2013].
As an illustration of the popularity of online sildenafil, an Internet search using the search terms ‘online sale Viagra’ resulted in over 1.5 million hits, directing the user to locations of Internet pharmacies selling generic versions of this product [Lowe and Costabile, 2011]. Email spam for Viagra is so pervasive that Microsoft and Pfizer jointly filed several lawsuits against pharmacy spam rings running sites that allegedly sell generic Viagra. Millions of disruptive email messages potentially lure consumers to those URLs selling illegal Viagra. According to some estimates, Viagra and other erectile dysfunction medicines or cures may account for as much as a quarter of all spam [Information Week, 2005].
An important key issue of Internet drug importation, and of considerable concern to clinicians and healthcare policymakers, is patient safety due to poor product quality. According to the US Food and Drug Administration, ‘FDA cannot ensure the safety and effectiveness of products that are not FDA-approved and come from unknown sources and foreign locations, or that may not have been manufactured under proper conditions. These unknowns put patient’s health at risk if they cannot be sure of the products identity, purity, and source. For these reasons, FDA recommends only obtaining medicines from legal sources in the U.S.’ [US Food and Drug Administration, 2010]. Several reports validate the assertion that drugs produced in many foreign countries do not meet equivalent standards of quality as in the US [US Food and Drug Administration, 2004; Qureshi and McGilveray, 1998; Westenberger et al. 2005; Veronin and Youan, 2004].
In a previous study, a high-performance liquid chromatography (HPLC) assay method was developed and validated for sildenafil citrate and its related substances that might coexist in tablets’ formulation [Daraghmeh et al. 2001]. These compounds related to sildenafil may be impurities or may appear as degradation products and were prepared and identified as impurity A, impurity B, impurity C, and impurity D.
The requirements for assessing safety of impurities in drug products can be found in the guidelines from the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). Founded in 1990, the ICH is ‘unique in bringing together the regulatory authorities and pharmaceutical industry of Europe, Japan and the US to discuss scientific and technical aspects of drug registration.’ [ICH, 2014].
ICH has developed guidelines for threshold of impurity quantity in new drug products [ICH, 2006], which are presented in Table 1. Per ICH guidelines, identification threshold is the limit above which a degradation product should be identified. Reporting threshold is the limit above which the degradation product should be reported to regulatory bodies. Qualification is the process of acquiring and evaluating data which establishes the biological safety of an individual impurity or a given degradation profile at a specified level. TDI is the total daily intake of the drug. If the impurity is unusually toxic, lower threshold values are appropriate. Maximum daily dose (MDD) is the maximum amount of drug administered per day.
Table 1.
Thresholds for impurities in new drug products [ICH, 2006].
| Reporting thresholds | ||
| Maximum daily dose | Threshold | |
| ≤ 1 g | 0.10% | |
| > 1 g | 0.05% | |
| Identification thresholds | ||
| Maximum daily dose | Threshold | |
| < 1 mg | 1.0 % or 5 µg TDI, whichever is lower | |
| 1–10 mg | 0.5% or 20 µg TDI, whichever is lower | |
| > 10 mg–2 g | 0.2% or 2 mg TDI, whichever is lower | |
| 2g | 0.10% | |
| Qualification thresholds | ||
| Maximum daily dose | Threshold | |
| < 10 mg | 1.0 % or 50 µg TDI, whichever is lower | |
| 10–100 mg | 0.5% or 200 µg TDI, whichever is lower | |
| > 100 mg–2 g | 0.2% or 3 mg TDI, whichever is lower | |
| 2g | 0.15% |
TDI, total daily intake
This study reports on the drug product variability of sildenafil. The objective of this study is to determine whether generic sildenafil tablets from international markets obtained via the Internet are comparable with the US innovator product with regard to major aspects of pharmaceutical quality: potency and accuracy of labeled amount, and level of impurities.
Currently, the innovator product for sildenafil (Viagra®, Pfizer) is protected by a US patent [Milford, 2011], yet is readily available to consumers in generic form from the Internet. The maximum recommended daily dose of sildenafil citrate is 100 mg [Drugs.com, 2014], and for this reason, this was the dosage strength chosen for analysis in this study.
Materials and methods
Sample acquisition
Searches of the World Wide Web were conducted with the browser Internet Explorer 7.0. The websites for the Internet pharmacies were located by using the advanced search options of Google (http://www.google.com.) The keywords selected for entry into the query box of the browser included the search terms ‘generic Viagra’, ‘generic sildenafil’, ‘online pharmacy’, and ‘Internet pharmacy’. All proprietary forms of sildenafil 100 mg tablets were identified on the Websites for prospective procurement. Our perspective was that of a consumer seeking to purchase these prescription medications online or comparison price shopping. If necessary, drug products were purchased a second time from the same website to validate test findings of the initial sample.
During January 2010 through July 2010, 15 sample lots were purchased from the US and international markets. The US innovator product was obtained by prescription from Harrel’s Kingsville Pharmacy (Kingsville, TX, USA), and other samples from Internet pharmacy websites. A checklist of attributes was created for each specific drug product (Table 2).
Table 2.
Characteristics of sildenafil tablet samples.
| Sample No. | Product Name (Dosage form) | Product Source (Laboratory) | Lot No. | Expiration date | Shipping Source | Product Source (website URL) |
|---|---|---|---|---|---|---|
| 1 | Viagra Innovator product - Pfizer (tablet) | Pfizer | A023501 | 29 Feb 16 | Not applicable | Harrell’s Pharmacy (Kingsville, TX) |
| 2 | Aurogra-100 | Aurochem Labs | SD 239 | May 13 | Mumbai, India | PrescriptionRxPharma.com |
| http://www.prescriptionrxpharma.com | ||||||
| 3 | Mygra-100 | Jpee Drugs | SK9001 | Oct 12 | Mumbai, India | My-xlpharmacy.com |
| http://www.my-xlpharmacy.com | ||||||
| 4 | Kamagra | Ajanta Pharma | BN10039K | Oct 13 | Mumbai, India | Generic4u |
| http://www.generic4u.com | ||||||
| 5 | Perfopil-100 | Aurochem Laboratories | SD224M2 | Jan 13 | Mumbai, India | ShopPillRx |
| http://ww2.shoppillrx.com. | ||||||
| 6 | P-Force-100 (capsule) | Sunrise Remedies | SC- 492 | Dec 12 | Mumbai, India | Half Price Pharmacy |
| http://www.half-price-pharmacy.com. | ||||||
| 7 | Malegra-100 | Sunrise Remedies | E- 134 | May 13 | Mumbai, India | Online Pharmacy VG |
| http://onlinepharmacy.vg | ||||||
| 8 | Sildigra-100 (Tablet) | DRJLPL | S-06 | Apr 13 | Nagpur, India | Quality-Online-Generics |
| http://review-pharmacy.net/Quality-Online-Generics.com.htm. | ||||||
| 9 | Viprogra-100 | Vipro Life Science | 10SGDT026 | Mar 12 | Ahmedabad, India | Official Canadian Pharmacy |
| http://www.ednf.org/index.php?option=com_content&task=view&id=1204&Itemid=88888988 | ||||||
| 10 | Vigora-100 | Cadila HealthCare | VG020001 | Jan 12 | Ahmedabad, India | ED Pharmacy |
| http://www.buysildenafil.eu | ||||||
| 11 | Integra (tablet) | G.S. Pharmaceuticals, Pvt., Ltd. | 1003003 | Feb 12 | Ahmedabad, India | 1drugstore-online |
| http://www.1drugstore-online.com. | ||||||
| 12 | G.S. Pharmaceuticals, Pvt., Ltd. | 1004056 | Mar 12 | Ahmedabad, India | Mexican Online Pharmacy | |
| http://www.mexicanonlinepharmacy.net. | ||||||
| 13 | Sildenafil tablets 100 mg (no brand name specified) | Not labeled | SD 234 | Mar 13 | Mumbai, India | XL Pharmacy |
| http://www.xlpharmacy.com | ||||||
| 14 | Not labeled | SD 237M5 | Mar 13 | Mumbai, India | Mexican Export Pharmacy | |
| http://www.mexicanexportpharmacy.com. | ||||||
| 15 | Not labeled | SD 184M3 | Feb 12 | Victoria, Seychelles | International drug mart.com | |
| http://www.internationaldrugmart.com. |
For analyses involving HPLC, the pure pharmaceutical grade chemical for sildenafil was obtained from Toronto Research Chemicals Inc. (Toronto, Ontario, Canada; Lot #5-KPA-93-1, Catalog #A617415). Impurity A was obtained from ChemPacific (Baltimore, MD; Lot #CHP-20-10232-207-6, Catalog #33854), impurity B was obtained from Equinox Chemicals (Albany, GA; Lot EQX-2009-D-030, Catalog #E-03-0316), impurity C was obtained from Specs (The Netherlands; Product ID #AP-312/40633649), impurity D was obtained from Sigma-Aldrich (St. Louis, MO; Lot #04710EH, Product #535524).
Preparation of solutions
To prepare standard solutions of active pharmaceutical ingredient (API), 25 mg of sildenafil citrate was accurately weighed and dissolved in 50 ml of mobile phase (500 µg/ml concentration), sonicated for 15 minutes and filtered through 0.45 µm filter. Further dilutions were carried out to obtain sildenafil citrate within a target concentration range of 5–500 µg/ml.
To prepare standard solution of impurities, 1 mg each of impurities A, B, C, and D were weighed and dissolved in 100 ml of mobile phase (10 µg/ml concentration), sonicated for 15 minutes, and filtered through a standard 0.45 µm filter. Further dilutions were carried out to obtain impurities with in target concentration range of 5–100 µg/ml.
HPLC analysis
Prior to testing, HPLC analytical equipment was calibrated according to manufacturer’s specifications by certified technicians. Where applicable, the appropriate USP guidelines were applied for testing procedures [United States Pharmacopeial Convention, 2003].
An HPLC methodology for sildenafil analysis has been reported previously by Daraghmeh and colleagues [Daraghmeh et al. 2001], and this analytical method was modified and employed in this study.
HPLC analysis was performed on a Waters 1525 Binary HPLC pump equipped with an inline degasser AF, a Waters 2487 dual λ absorbance detector, and a Waters 717 plus autosampler. Data was analyzed using Waters Breeze Software. Centrifugation was performed by the Eppendorf Centrifuge 5430, and sonification was performed with the Fisher Scientific Ultrasonic Cleaner FS20.
Measurements were made with a 20 µl injection volume at 30°C; the detector wavelength was set at 240 nm and the retention time was 6.4 minutes. Routine analyses were carried out isocratically on a Waters X Bridge C18, 5 µm column (250 mm × 4.6 mm), eluted with a mobile phase consisting of acetonitrile/ammonium acetate buffer (50:50 v/v), pH 7.0, at a flow rate of 1.0 ml/min. Pure sildenafil (Sigma-Aldrich, St. Louis, MO) acetonitrile (Aldrich Laboratories, Milwaukee, WI), ammonium acetate buffer (Fisher Scientific, Rochester, NY), water (Barnstead Nanopure ultrapure water purification system, Fisher Scientific, Rochester, NY), were used to prepare standard curves for each assay. Quantitation was accomplished through comparison of peak area to a standard curve. All analyses were performed in triplicate and the error expressed as standard deviation.
To prepare a solution of tablet samples of 1 mg/ml concentration, 10 tablets were pulverized with mortar and pestle and portions equivalent to 100 mg sildenafil citrate were weighed and transferred into 100 ml standard volumetric flasks. Upon addition of mobile phase (100 ml), sonication, centrifugation (15 minutes at 3000 rpm), 20 µl of the supernatant was injected into the chromatograph. This analysis was used to calculate the average impurity and average API quantity present in a dosage form one individual sample set. This method can remove the error caused by tablet weight variation in determination of average impurity and API quantities.
To quantify the impurity and API levels, the calibration curve method using standard plots was used. For sildenafil, and impurities A, B, C, and D, linear curves were plotted using respective standard compounds. As per ICH Q2R1 guidelines for analytical procedure validation, the standard plot range should be at least 80–120% of the sample. For sildenafil citrate the standard plot range was 0.8–1.2 mg and for impurities the range was 2–20 µg, which meets the ICH guidelines.
Results
A total of 15 sildenafil citrate tablets were obtained for pharmaceutical analysis: 14 generic samples from international Internet pharmacy websites and one sample of the US innovator product (Table 2).
HPLC analysis
For sildenafil, an official assay method for content uniformity using HPLC has not been reported in the United States Pharmacopeia (USP), the British Pharmacopoeia (BP), or European Pharmacopoeia (EP). For the 100 mg tablet dosage form of sildenafil, general acceptable assay limits have been published in scientific journals. Daraghmeh and colleagues report an assay limit for sildenafil citrate of 98–102% and any individual impurity should not exceed 0.5% of the MDD with total impurity quantity not exceed 1% of the total daily dose [Daraghmeh et al. 2001]. However, most USP product monographs (as compared with substance monographs) have the standard range for both manufactured products and compounded preparations as 90.0–110.0% [United States Pharmacopeial Convention, 2003; Allen, 2012].
HPLC analysis indicated that 14 of the 15 drug samples were within the general USP range of 90–110% of the labeled claim for each tablet dosage unit. However, for the range of 98–102% of the labeled claim, only six of the drug samples were within this range (Table 3).
Table 3.
Analysis of API quantity in collected samples.
| No. | Sample | Lot No. | label claim | Recovery |
|---|---|---|---|---|
| 1 | Viagra Pfizer | A023501 | 100 mg | 99.7% |
| 2 | Aurogra | SD 239 | 100 mg | 99.3% |
| 3 | Mygra | SK9001 | 100 mg | 101.9% |
| 4 | Kamagra | BN10039K | 100 mg | 96.2% |
| 5 | Perfopil | SD224 | 100 mg | 94.6% |
| 6 | P-Force | SC- 492 | 100 mg | 93.0% |
| 7 | Malegra | E- 134 | 100 mg | 95.0% |
| 8 | Sildigra | S-06 | 100 mg | 96.7% |
| 9 | Viprogra | 10SGDT026 | 100 mg | 98.1% |
| 10 | Vigora | VG020001 | 100 mg | 87.2% |
| 11 | Integra | 1003003 | 100 mg | 99.8% |
| 12 | 1004056 | 100 mg | 105.5% | |
| 13 | Sildenafil Citrate 100 (no brand name specified) | SD 234 | 100 mg | 93.6% |
| 14 | SD 237 | 100 mg | 97.0% | |
| 15 | SD 184 | 100 mg | 100.1% |
Note: Bold values are outside the +/- 2% assay range limit.
Using the linear regression method, linear curves were plotted using respective standard compounds for impurities A–D. HPLC analysis of the 15 samples provides variable data about the quantities of impurities (Table 4).
Table 4.
Impurity quantities (in micrograms) in sildenafil samples.
| Name | Impurity A | Impurity B | Impurity C | Impurity D | Total |
|---|---|---|---|---|---|
| Viagra Pfizer | 21.3 | 15.2 | 0.0 | 0.0 | 36.5 |
| Aurogra | 64.4 | 153.0 | 704.5 | 0.0 | 921.9 |
| Mygra | 98.9 | 177.2 | 32.6 | 22.6 | 331.3 |
| Kamagra | 106.5 | 187.3 | 66.1 | 1.1 | 361.0 |
| Perfopil | 58.4 | 109.4 | 704.9 | 0.0 | 872.7 |
| P-Force | 74.2 | 123.3 | 242.9 | 0.0 | 440.4 |
| Malegra | 155.7 | 1205.2 | 186.9 | 0.0 | 1547.7 |
| Sildigra | 139.9 | 141.0 | 3.4 | 0.0 | 284.3 |
| Viprogra | 135.8 | 119.9 | 284.7 | 0.0 | 540.4 |
| Vigora | 154.1 | 104.9 | 2.7 | 0.0 | 261.6 |
| Integra (1003003) | 132.3 | 1743.5 | 179.4 | 0.0 | 2055.2 |
| Integra (1004056) | 120.8 | 250.0 | 205.4 | 0.0 | 576.2 |
| Sildenafil (SD 234) | 66.1 | 122.9 | 648.3 | 0.0 | 837.4 |
| Sildenafil (SD 237) | 174.3 | 157.6 | 716.1 | 0.0 | 1048.0 |
| Sildenafil (SD 184) | 143.5 | 200.7 | 705.1 | 0.0 | 1049.3 |
Note: Bold values indicate values that are above the ICH qualification threshold. For total impurity levels, bold values indicate total impurity quantity more than 1% of MDD.
According to ICH guidelines, the qualification threshold for a dosage form with a MDD of 100 mg, such as sildenafil, is 200 µg. For impurity A, all of the samples were observed to be under the qualification threshold. The maximum impurity observed was 174.3 µg for sample lot number SD237. For impurity B, 4 of the 15 samples showed more than 200 µg, with 2 samples more than 6 times the qualification threshold. For impurity C, eight samples showed higher impurity limits than the qualification threshold. Impurity D was observed to be present in only in two samples in minute quantities. Among all samples, nine samples showed more than 0.5% of the MDD. In four samples, the total impurity quantity was more than the 1% of MDD. In sample #7 (Malegra, lot number E-134), the total impurity quantity was more than the 1.5% of MDD, and for sample #11 (Integra lot number 1003003), the total impurity quantity was more than 2% of MDD.
The innovator product contained relatively low impurity quantities. Impurities C and D were below the limit of detection, and impurities A and B constituted less than 37 µg, which is far below the qualification threshold.
Discussion
A ‘cultural icon’ can be described as ‘an object that represents some aspect of the values, norms or ideals perceived to be inherent in a culture, or section of a culture’ [Wikipedia, 2014]. In ‘The Viagra Phenomenon’ Tiefer asserts ‘certain medicines, such as aspirin, penicillin, birth control pills, mood regulators (Valium and Prozac), and now Viagra, have become cultural icons, reaching far beyond just their impact on human physiology to shape social norms and practices’ [Tiefer, 2006]. In fact, the American English word ‘viaggravated’ is being adopted in the American lexicon, and is an adjective that means ‘exhibiting a degree of sexual interest that is incongruent to one’s age’ [Merriam-Webster, 2014].
Although still a multibillion dollar industry, the market for Viagra and other ‘virility’ drugs has slowed in recent years. In 2010, the total number of prescriptions for drugs used for erectile dysfunction fell by 5% after growth of only 1% annually the previous 4 years. Specifically, prescriptions for Viagra and Levitra declined 7% and 18%, respectively [James, 2011].
This can be contrasted with massive growth of Internet pharmacies, which have made prescription-only medicine, such as Viagra, readily available online, and the growth and popularity does not appear to be declining [Maxwell and Webb, 2008]. For instance, a search for ‘Internet drug store’ revealed 6.4 million hits and of 7000 Internet pharmacies identified by the Verified Internet Pharmacy Practice Sites Program (VIPPS) [Young, 2013]. Of these sites that were identified, only 4% were in proper compliance with regulations. What would be the impact on domestic sales of Viagra? According to Lowe and Costabile, ‘The role Internet pharmacies play in counterfeit PDE5 or abuse is not well documented; however based on easy access, direct patient marketing, and low advertised cost it is likely this role is underreported’ [Lowe and Costabile, 2011]. It is not unreasonable to assume that illegal access to Viagra via the Internet has had a negative impact on sales of Viagra through conventional healthcare channels.
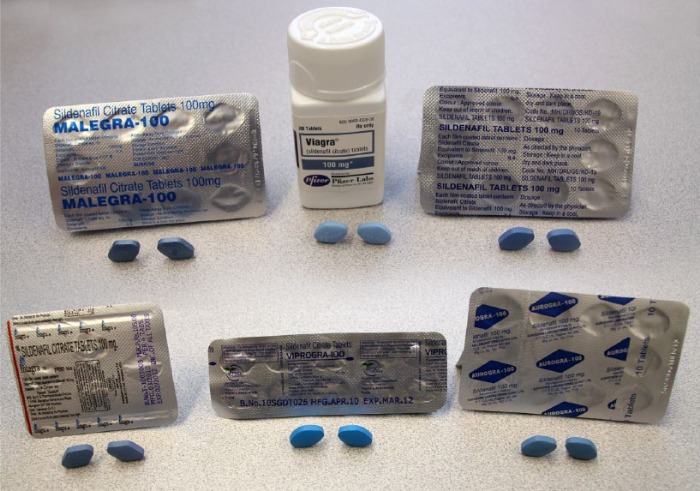
The documented risks of taking Viagra include: counterfeit drugs, potentially harmful drug interactions, and accidental overdose, not to mention improper packaging and labeling (Figure 1) [Young, 2013; Webb et al. 1999; Hung and Yang, 2001; Veronin, 2011]. If one considers the potential harmful effects of drug impurities, perhaps we have added to this list of health risks if a consumer chooses to purchase sildenafil via the Internet.
Figure 1.
Generic sildenafil tablets obtained via the Internet compared to the innovator product.
The picture shows the innovator product and typical generic sildenafil products from the Internet with impurity levels higher than acceptable limits in the US. The generic products appear to be from legitimate drug manufacturers and similar in appearance to the innovator product.
Photo courtesy of Andrew Ramirez, Digital Media Specialist, Office of Student Affairs, Rangel College of Pharmacy, Kingsville, Texas.
According to the US Pharmacopeia, when it comes to determining the quality of a drug product, ‘knowing its impurity profile is as important as knowing about the quality of its active pharmaceutical ingredient (API) and excipients’ [The Standard, 2012]. What is the source of an impurity in a drug product? An impurity can be produced as a result of the drug’s manufacturing process, or it can be a degradation byproduct formed when improper environmental conditions exist, perhaps during storage or transit. It may even be a substance intentionally added during the manufacturing process, such as a catalyst during drug synthesis. In short, impurities are substances that can coexist in pharmaceuticals that serve no purpose for which the drug was designed [Rahman et al. 2006].
In a previous study, four major impurities were isolated in the oral dosage form of sildenafil citrate, and an HPLC method was developed and validated that could differentiate between the active moiety and these impurities. The question then arises, if impurities have no functional role in a drug product, is there a chance that their existence may be detrimental to a person’s health or well-being?
At the different stages during the drug manufacturing process, a variety of analytical tests are conducted to ensure that the final drug product meets standards for identity, strength, quality, purity, and stability as specified in the United States Pharmacopeia and National Formulary (USP/NF). The USP/NF provides guidelines for testing procedures and acceptance criteria for these tests [Chow et al. 2002].
One of the most common methods for measuring API assay in oral dosage forms is HPLC [Rudnic and Schwartz, 2000]. HPLC analysis can assess the potency of tablets, and hence safety if tablets are found to be outside of the acceptable quality assurance range.
For oral dosage forms, when considering deviations from the label claim for potency, and levels of impurities, a fair question would be, what are the short- and long-term health risks?
Since there is no USP monograph for sildenafil tablets, assay limits were derived from standard assay guidelines for oral dosage forms and research studies. For most commercial drugs manufactured in the US, the acceptable potency range is 95–105% or 90–110% of the labeled quantity [Allen, 2012]. Of 14 samples analyzed from international markets, 6 of the products were at or beyond the ±5% range, and 1 of the products was beyond the ±10% range and hence would not be acceptable for patients in the US (Table 3).
Regarding levels of impurities, all samples registered detectable levels of impurities. Levels of impurity A were detectable in all samples, but none were above ICH qualification threshold. For impurity D, only 2 samples registered detectable amounts, and 13 of the 15 samples registered no detectable amounts. However, for impurities B and C, for several samples, the levels detected were above the ICH threshold, and the tablets would not be acceptable for patients in the US. For impurity B, four samples were above both the ICH qualification threshold. The highest levels of impurities were evident for impurity C. Eight samples were above both the ICH qualification threshold limit. For samples above ICH limits for impurities B and C, they would not only be unacceptable therapeutic agents for patients in the US, but also would be a cause for alarm with regards to health risks. These findings suggest dissimilar manufacturing and/or storage processes compared with the US, which in turn may affect quality, therapeutic effectiveness, and safety. Based on this information, tests of safety for short- and long-term use are warranted.
Limitations of the study
The relatively small sample size in this study may not be an accurate representation of other sildenafil tablets sold on the Internet and to generalize should be done with a large degree of caution. However, the findings in this report support results with previously published work [Westenberger et al. 2005; Veronin and Youan, 2004], and further studies are warranted to identify trends in accessibility and health risks for these types of drug products sold on the Internet.
When using analytical equipment, instrument and user error must be allowed for when considering precision and accuracy of test results [Watson, 2005]. In this report, although every precaution was taken into account the possibility exists that the values outside of acceptable ranges may or may not be as extreme as observed, if taking into account the margin of error of laboratory measurement.
A goal of this study was to assess quality attributes of potency and impurity levels that may indirectly convey information on safety and effectiveness through the dosage form’s production and/or storage properties. A direct association between drug manufacturing attributes and clinical outcomes has not been established, so the findings in this report must be interpreted with some reservation.
Conclusions
As this study has shown, consumers can obtain prescription drugs via the Internet with little difficulty and without professional oversight. Although generic drug substitution is acceptable practice in the US, consumers need to be aware that irrespective of advertising claims on Internet pharmacy websites, consumers may receive a drug product nonequivalent to the US counterpart and unlike what would be allowed for consumers in the US. In addition, it is quite likely that the average individual seeking to import sildenafil from the Internet would obtain a product that does not meet quality specifications equivalent to the US-made product. Further, it is virtually impossible to evaluate the quality of drugs purchased from the Internet without specialized analytical testing. The results of this study indicate variability of sildenafil sold on the Internet based on potency and impurity levels, which may give pause to healthcare professionals and consumers of these products.
At a minimum, findings of this study suggest that sildenafil tablets purchased from the Internet should not be considered interchangeable with the US innovator product. These findings have implications for safety and effectiveness that should be considered by clinicians to potentially safeguard patients who choose to purchase foreign-manufactured drugs via the Internet. Beyond this, further testing of the pharmacological effects and drug interaction profiles of the impurities A, B, C, and D are warranted, to assess the potential short- and long-term health risks.
Footnotes
Funding: This research project was funded in part by the American Heart Association Texas Affiliate #0565153Y.
Conflict of interest statement: The authors declare no conflict of interest in preparing this article.
Contributor Information
Michael A. Veronin, Texas A&M University Health Science Center Rangel College of Pharmacy - Pharmaceutical Sciences, MSC 131 1010 West Avenue B, Kingsville, TX 78363-8202, USA
Mohammad T. Nutan, Texas A&M University Health Science Center Rangel College of Pharmacy - Pharmaceutical Sciences, Kingsville, TX, USA
Uday Krishna Reddy Dodla, TechData Service Company, LLC - Statistical Programming, Morrisville, NC, USA.
References
- Allen L. (2012) Science and technology for the hospital pharmacist. Compounding with Commercial Drug Products Can Cause Errors! Available at: http://compoundingtoday.com/Newsletter/Science_and_Tech_1205.cfm (accessed 10 June 2014).
- BBC News (2002) Online Pharmacy Warning. Available at: http://news.bbc.co.uk/2/hi/health/1938890.stm (accessed 10 June 2014).
- Chow S., Shao J., Wang H. (2002) Probability lower bounds for USP/NF tests. J Biopharm Stat 12: 79-92 [DOI] [PubMed] [Google Scholar]
- Daraghmeh N., Al-Omari M., Badwan A., Jaber A. (2001) Determination of sildenafil citrate and related substances in the commercial products and tablet dosage form using HPLC. J Pharm Biomed Anal 25: 483–492 [DOI] [PubMed] [Google Scholar]
- Drugs.com (2014) Erectile Dysfunction Medications. Available at: http://www.drugs.com/condition/erectile-dysfunction.html (accessed 10 June 2014).
- Ghofrani H., Osterloh I., Grimminger F. (2006) Sildenafil: from angina to erectile dysfunction to pulmonary hypertension and beyond. Nat Rev Drug Discov 5: 689-702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung D., Yang D. (2001) Sildenafil overdose in a female patient. J Toxicol Clin Toxicol 39: 423-424 [DOI] [PubMed] [Google Scholar]
- ICH (2006) International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use 2006. ICH Harmonised Tripartite Guideline. Impurities in New Drug Products Q3B(R2), Current Step 4 Version, 2 June 2006. Available at: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q3B_R2/Step4/Q3B_R2__Guideline.pdf (accessed 10 June 2014).
- ICH (2014) Harmonisation for Better Health 2014. Available at: http://www.ich.org (accessed 10 June 2014).
- Information Week (2005) Microsoft, Pfizer Sue Viagra spammers. Available at: http://www.informationweek.com/microsoft-pfizer-sue-viagra-spammers/60300237 (accessed 10 June 2014).
- Isidore C. (2013) Pfizer to Start Selling Viagra Online, CNNMoney. Available at: http://money.cnn.com/2013/05/06/news/companies/pfizer-viagra-online/index.html (accessed 10 June 2014).
- James S. (2011) Honeymoon With Viagra Could Be Over, Say Doctors, ABC News. Available at: http://abcnews.go.com/Health/viagra-prescription-sales-sexual-expectations/story?id=13794726 (accessed 10 June 2014).
- Keith A. (2000) The economics of Viagra. Health Affairs 19: 147-157 [DOI] [PubMed] [Google Scholar]
- Lowe G., Costabile R. (2011) Phosphodiesterase type 5 inhibitor abuse: a critical review. Curr Drug Abuse Rev 4: 87-94 [DOI] [PubMed] [Google Scholar]
- Maxwell S., Webb D. (2008) Internet pharmacy: a web of mistrust? Br J Clin Pharmacol 66: 196-198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriam-Webster (2014) New Words & Slang 2014: Viaggravated. Available at: http://nws.merriam-webster.com/opendictionary/newword_display_alpha.php?letter=vi (accessed 10 June 2014).
- Milford P. (2011) Pfizer Wins Viagra Patent Infringement Case Against Teva Pharmaceuticals, Bloomberg. Available at: http://www.bloomberg.com/news/2011-08-15/pfizer-wins-viagra-patent-infringement-case-against-teva-pharmaceuticals.html (accessed 10 June 2014).
- National Association of Boards of Pharmacy (2014) Buying Medicine Online. Available at: http://www.nabp.net/programs/consumer-protection/buying-medicine-online/ (accessed 10 June 2014).
- Qureshi S., McGilveray I. (1998) Assessment of pharmaceutical quality of furosemide tablets from multinational markets. Drug Dev Industr Pharm 24: 995-1005 [DOI] [PubMed] [Google Scholar]
- Rahman N., Azmi S., Wu H. (2006) The importance of impurity analysis in pharmaceutical products: an integrated approach. Accred Qual Assur 11: 69–74 [Google Scholar]
- Rodriguez A. (1998) Men clamoring to try out new impotence pill. Chicago Sun-Times, 22 April, Section A:1 column 2. [Google Scholar]
- Rudnic E., Schwartz J. (2000) Oral solid dosage forms. In Gennaro A. (ed.), Remington: the Science and Practice of Pharmacy, 20th edn. Philadelphia College of Pharmacy and Science. Philadelphia, PA: Lippincott Williams & Wilkins [Google Scholar]
- Teasdale A. (2011) Risk assessment of drug impurities: how quality of medicines assures safety. In American Association of Pharmaceutical Scientists (AAPS) Annual Meeting and Exposition, Washington DC. October 2011. Macclesfield: AstraZeneca R&D [Google Scholar]
- The Standard (2012) News and Information about the United States Pharmacopeial Convention 2012, USP Looks at Drug Impurities Across Multiple Fronts. Available at: http://www.usp.org/sites/default/files/usp_pdf/EN/aboutUSP/standard_summer2012-web.pdf (accessed 10 June 2014).
- Tiefer L. (2006) The Viagra phenomenon. Sexualities 9: 273-294 [Google Scholar]
- United States Pharmacopeial Convention (2003) The United States Pharmacopeia/The National Formulary USP 26/NF 21. Rockville, MD: United States Pharmacopeial Convention, Inc. [Google Scholar]
- US Food and Drug Administration (2004) Recent FDA/U.S. Customs Import Blitz Exams Continue to Reveal Potentially Dangerous Illegally Imported Drug Shipments. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2004/ucm108232.htm (accessed 10 June 2014).
- US Food and Drug Administration (2010) Importing prescription drugs. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm170594.htm (accessed 10 June 2014).
- Veronin M. (2011) Packaging and labeling of pharmaceutical products obtained from the Internet. J Med Internet Res 13: e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronin M., Youan B. (2004) Magic bullet gone astray: medications and the Internet. Science 305: 481. [DOI] [PubMed] [Google Scholar]
- Watson D. (2005) Control of the quality of analytical methods. In Pharmaceutical Analysis. A Textbook for Pharmacy Students and Pharmaceutical Chemists, 2nd edn. London: Elsevier [Google Scholar]
- Webb D., Freestone S., Allen M., Muirhead G. (1999) Sildenafil citrate and blood-pressure-lowering drugs: results of drug interaction studies with an organic nitrate and a calcium antagonist. Am J Cardiol 83(5A): 21C-28C [DOI] [PubMed] [Google Scholar]
- Westenberger B., Ellison C., Fussner A., Jenney S., Kolinski R., Lipe T., et al. (2005) Quality assessment of Internet pharmaceutical products using traditional and non-traditional analytical techniques. Int J Pharm 306: 56-70 [DOI] [PubMed] [Google Scholar]
- Wikipedia (2014) Cultural icon. Available at: http://en.wikipedia.org/wiki/Cultural_icon (accessed 10 June 2014).
- Wilson J. (2013) The little blue pill that could, CNN Health. Available at: http://www.cnn.com/2013/03/27/health/viagra-anniversary-timeline/index.html (accessed 10 June 2014).
- Young C. (2013) Pfizer launches website for purchasing Viagra safely. Available at: http://www.pharmacist.com/pfizer-launches-website-purchasing-viagra-safely (accessed 10 June 2014).