Although the signaling pathways involved in the specification and differentiation of endoderm-derived organs in vertebrates have been studied extensively, no gene or set of genes has been found to exclusively regulate the specification of the lung.1 In the mouse, the liver, thyroid, lungs and ventral pancreas originate from the ventral foregut.2 The lung primordium is specified in the ventral aspect of the anterior foregut around embryonic day 9 (E9) in the mouse, and subsequently evaginates ventrally from the foregut distinguishing the tracheal primordium from the esophageal primordium around embryonic day E10–10.5.3 The developing tracheal and lung epithelium is encompassed by splanchnic mesoderm, which will go on to differentiate into several cell lineages including airway and vascular smooth muscle and cartilage surrounding the upper airways. Thereafter, the lung undergoes branching morphogenesis to form the three-dimensional arborized network of airways required for respiration.
Wnts, Fgfs, Bmps, TGFβ and Shh have all been implicated in lung development.1 Transcription factors including the GATA, Forkheads (Fox) and Nkx-2 families also regulate differentiation of the lung from the foregut endoderm.4 Nkx2.1 (also known as thyroid transcription factor 1) is the earliest known gene whose expression demarcates the presumptive lung endoderm. Targeted disruption of Nkx2.1 has demonstrated that it is not required for lung specification.5 Thus, the specific signaling events and transcriptional pathways that commit the multipotent foregut endoderm to a lung cell fate prior to morphogenesis have, up to this point, been poorly understood.
Wnt ligands produced in the mesoderm surrounding the ventral foregut have been shown to regulate the development of several vertebrate endoderm derivatives. In zebrafish, wnt2b has been shown to be essential for early liver development.6 In contrast, repression of Wnt/β-catenin signaling in foregut endoderm has been shown to be necessary for initial hepatic and pancreatic specification, as well as promoting hindgut identity in Xenopus.7 Transgenic reporter mice have demonstrated canonical Wnt/β-catenin signaling activity in the primitive lung field of the anterior foregut endoderm.8 Several Wnt genes are expressed in the developing lung including Wnt2, Wnt2b, Wnt7b and Wnt5a and all have been reported to be activators of the β-catenin pathway with the exception of Wnt5a, which has been found to activate non-canonical Wnt pathway.9–11
The contributions of Wnt2 and Wnt2b to lung development were previously unexplored and their unique overlapping spatiotemporal expression pattern prompted us to investigate whether these ligands played a role in early lung specification. Wnt2 and Wnt2b exhibit a complex pattern of expression beginning at around E8.0–E9.5 in the precardiac mesoderm surrounding the anterior foregut endoderm where the trachea will form. The ligands are then expressed throughout the developing lung mesenchyme with Wnt2 persisting through the later stages of embryonic development and into the adult.9–12
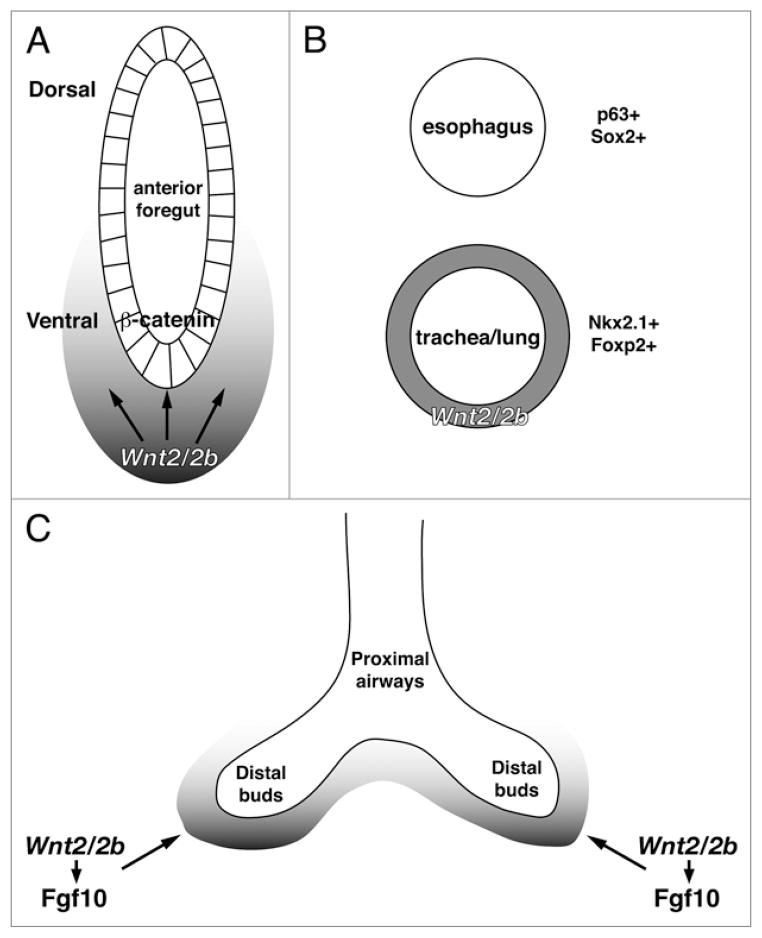
Using newly generated Wnt2 and Wnt2b mouse knockout models, we assessed the overall contribution of Wnt2/2b signaling in early foregut patterning. Wnt2 knockout mice exhibit lung hypoplasia and loss of smooth muscle development whereas Wnt2b knockout mice did not display an overt lung phenotype.9 Wnt2/Wnt2b double mutant embryos displayed a dramatic phenotype: complete lung agenesis. Moreover, we found a complete loss of Nkx2.1 expression in the anterior foregut region at the time of lung specification (E9.5) and a complete absence of tracheal septation. The striking phenotype and loss of the earliest primitive lung marker suggest that paracrine Wnt2/2b signaling regulates lung specification in the anterior foregut endoderm (Fig. 1).
Figure 1.
(A) A model highlighting the requirements for Wnt2/2b signaling in development of the mouse anterior foregut endoderm. Wnt2/2b ligands are expressed in the ventral mesoderm surrounding the anterior foregut and signal in a paracrine fashion to the adjacent endoderm to specify lung progenitors. Fgf10, a potent and necessary inducer of lung bud formation is regulated in an autocrine manner in the ventral mesoderm by Wnt2/2b signaling. In Wnt2/2b double mutant embryos Fgf10 expression is significantly downregulated. (B) Several markers including p63, Sox2, Foxp2 and Nkx2.1 demonstrate successful esophageal and tracheal endoderm septation and specification. Notably, in Wnt2/2b double mutant embryos, tracheoesophageal septation is disrupted and the resulting foregut endoderm tube expresses only the esophageal markers, p63 and Sox2, indicating loss of tracheal identity. (C) Later in lung development, Wnt2 promotes lung growth in part through its regulation of Fgf10 expression.
Based on published data demonstrating Wnt/β-catenin activity in the early foregut,8 we hypothesized that Wnt2/2b signal through β-catenin in the foregut to initiate lung specification. Loss of Wnt/β-catenin transgenic reporter line activity confirmed that Wnt2 and Wnt2b signal through the β-catenin pathway in the mouse foregut endoderm.
To further explore the requirement for β-catenin signaling activity in early foregut patterning, β-catenin was conditionally deleted or activated in the foregut endoderm using the Shh-cre line. Deletion of β-catenin recapitulated the Wnt2/2b double knockout lung agenesis phenotype, suggesting that β-catenin signaling regulated by Wnt2 and Wnt2b is required for lung specification. Conversely, stabilized β-catenin activity in the foregut expanded the presumptive lung progenitor (Nkx2.1+) domain suggesting that β-catenin positively modulates lung progenitor specification and/or expansion in the foregut endoderm. The Wnt2/2b knockout and β-catenin experiments demonstrate an intriguing and previously undiscovered mechanism for Wnt2/2b and β-catenin signaling in the mouse foregut. Our results offer important evidence that Wnt2/2b signaling is a critical upstream regulator of lung progenitor specification and patterning in the foregut endoderm (Fig. 1).
The studies investigating the implications of loss- and gain-of-function of β-catenin signaling in the foregut endoderm have also posited a chronologic role for the requirement of β-catenin signaling in the mouse endoderm. Future studies will focus on temporal requirements for β-catenin activity in the foregut and bring new knowledge to the precise requirements for Wnt/β-catenin signaling in early foregut endoderm patterning.
References
- 1.Cardoso WV, et al. Development. 2006;133:1611–24. doi: 10.1242/dev.02310. [DOI] [PubMed] [Google Scholar]
- 2.Wells JM, et al. Annu Rev Cell Dev Biol. 1999;15:393–410. doi: 10.1146/annurev.cellbio.15.1.393. [DOI] [PubMed] [Google Scholar]
- 3.Que J, et al. Differentiation. 2006;74:422–37. doi: 10.1111/j.1432-0436.2006.00096.x. [DOI] [PubMed] [Google Scholar]
- 4.Maeda Y, et al. Physiol Rev. 2007;87:219–44. doi: 10.1152/physrev.00028.2006. [DOI] [PubMed] [Google Scholar]
- 5.Minoo P, et al. Dev Biol. 1999;209:60–71. doi: 10.1006/dbio.1999.9234. [DOI] [PubMed] [Google Scholar]
- 6.Ober EA, et al. Nature. 2006;442:688–91. doi: 10.1038/nature04888. [DOI] [PubMed] [Google Scholar]
- 7.McLin VA, et al. Development. 2007;134:2207–17. doi: 10.1242/dev.001230. [DOI] [PubMed] [Google Scholar]
- 8.Okubo T, et al. J Biol. 2004;3:11–9. doi: 10.1186/jbiol3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goss AM, et al. Dev Cell. 2009;17:290–8. doi: 10.1016/j.devcel.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishikawa T, et al. Development. 2001;128:25–33. doi: 10.1242/dev.128.1.25. [DOI] [PubMed] [Google Scholar]
- 11.Wallingford JB, et al. Int J Dev Biol. 2001;45:225–7. [PubMed] [Google Scholar]
- 12.Zakin LD, et al. Mech Dev. 1998;73:107–16. doi: 10.1016/s0925-4773(98)00040-9. [DOI] [PubMed] [Google Scholar]