Abstract
Background
Current treatment of Helicobacter pylori consists of three or four drugs for 7–14 days with important associated cost and adverse events.
Aims
This study compared efficacy and safety of standard dose vs. half-dose concomitant nonbismuth quadruple therapy (NBQT) for 7 days. The standard dose consisted of twice daily rabeprazole 20 mg, amoxicillin 1 g, metronidazole 500 mg, and clarithromycin 500 mg.
Methods
This was a prospective randomized trial. 14C-urea breath test was performed ≥4 weeks after treatment and ≥2 weeks off acid suppressive therapy. Compliance and adverse events were monitored during treatment.
Results
A total of 200 consecutive treatment-naïve patients were enrolled. Baseline characteristics were similar between groups, with 15.5% of subjects reporting prior macrolide use. Eradication occurred in 78% (95% CI 68.6–85.7%) in both groups on intention-to-treat analysis. Per-protocol rates were 82.1 vs. 83.9% for standard-dose patients vs. half-dose patients, respectively (p = NS). Adverse events (only mild) were reported in 57 vs. 41% of standard-dose patients vs. half-dose patients (p = 0.024), with metallic taste and nausea notably less frequent in the latter (36 vs. 12% and 18 vs. 7%, respectively; p < 0.05 for both). Overall, eradication failed in 38.7% of prior macrolide users vs. 18.9% without such exposure (p = 0.019). On multivariate logistic regression, prior macrolide exposure was the only factor associated with failed eradication (OR 2.60, 95% CI 1.06–6.39; p = 0.038). Treatment was cheaper with the half-dose regimen.
Interpretation
A 50% reduction in antibiotic dosage does not diminish efficacy of concomitant nonbismuth quadruple therapy but leads to significant reduction in cost and adverse events. Seven-day concomitant NBQT is suboptimal for H. pylori independent of prior macrolide exposure.
Keywords: Adverse events, dyspepsia, eradication, gastritis, pylori
Introduction
Helicobacter pylori is a common infection worldwide, particularly in developing countries.1 H. pylori infection is associated with chronic gastritis, peptic ulcer disease, and gastric adenocarcinoma and non-Hodgkin's mucosa-associated lymphoid tissue lymphoma of the stomach.2,3 Eradication of this organism has been shown to improve gastric inflammation, accelerate ulcer healing, eliminate ulcer diathesis, and even reverse early mucosa-associated lymphoid tissue lymphoma.4–6 Several treatment regimens for the eradication of H. pylori have been adopted in different regions of the world with varying results, but the search continues for a safe, tolerable, cheap, and highly effective regimen. To be considered effective, a treatment regimen should achieve at least an 80% eradication rate,7 with some experts suggesting that the target eradication rate should be closer to 90–95%.8 The most commonly used regimen worldwide is the so-called triple regimen consisting of a proton pump inhibitor (PPI) and clarithromycin in combination with amoxicillin or, in the case of penicillin allergy, metronidazole.6,7 Despite recent evidence that the efficacy of this ‘heritage’ clarithromycin-based triple therapy is waning – to unacceptably low levels in some populations – it remains the most commonly used first-line therapy for eradication of H. pylori.8
The addition of metronidazole to legacy triple therapy, whether part of the sequential or concomitant regimen, as so-called nonbismuth quadruple therapy (NBQT), has been shown to improve eradication rates of H. pylori, including clarithromycin-resistant strains.9,10 A meta-analysis by Essa et al.11 comparing all three aforementioned regimens concluded that sequential and concomitant quadruple therapy are equally effective and superior to standard triple therapy (STT). The concomitant regimen was found to be less complex than the sequential regimen and was therefore recommended in regions where STT was rendered ineffective. A recent study from Spain and Italy,12 regions of relatively high clarithromycin resistance (19–20%), suggested that more than 90% of H. pylori infections can be eradicated with a 14-d course of concomitant or hybrid (PPI and amoxicillin for 14 days with addition of clarithromycin and nitroimidazole for the final 7 days) NBQT including high-dose PPI (omeprazole 40 mg b.i.d.).
We have recently shown that a 10-day half-dose triple regimen – where the dose of all three components of the STT is reduced by 50% – can achieve similar eradication rate as its standard-dose counterpart but with significant reduction in adverse events and cost. The overall eradication rate was, however, suboptimal (77.6% with standard-dose therapy and 77.2% with half-dose triple therapy on ITT), signalling a 13.8% decline in eradication rate of the exact standard-dose regimen over a 6-year period within the same geographic area and institution.13,14 Based on the reported superiority of NBQT and the promising concept of reduced-dose combination therapy, this study was designed to investigate the efficacy and safety of 7-day standard-dose vs. half-dose concomitant NBQT regimen for the eradication of H. pylori infection.
Materials and methods
Study design
From December 2011 to August 2012, 200 consecutive adult patients with H. pylori infection, documented by either urea breath test (UBT) or rapid urease assay, were enrolled in the study. Patients were excluded if they were allergic to any of the drugs used, had taken antibiotics recently (within 2 weeks of enrolment), had severe ulcers or bleeding, gastric perforation or obstruction, previous gastrectomy, or gastric cancer, were pregnant or lactating, had received prior eradication therapy for H. pylori, or had severe concomitant disease or a condition making the treatment unlikely to be effective (e.g. alcoholism or drug addiction). The study was approved by the Institutional Review Board at the American University of Beirut and registered at clinicaltrials.gov (ID: NCT01219764).
After informed consent, patients were randomized using a computer-generated random-numbers table into one of two treatment groups. Patients in the standard-dose group were assigned to receive rabeprazole 20 mg b.i.d. (before meals), amoxicillin 1 g b.i.d. (with or after meals), clarithromycin 500 mg b.i.d. (with or after meals), and metronidazole 500 mg b.i.d. (with or after meals) for 7 days. Patients in half-dose arm received all four drugs but at 50% posology. A brief questionnaire was filled out for each patient regarding demographics including age, sex, height, weight, smoking status, previous use of clarithromycin or azithromycin, and current use of PPIs. All study participants were contacted by telephone on days 3 and 7 of the treatment period to evaluate compliance and inquire about possible adverse events. Patients were specifically asked if they experienced nausea, diarrhoea, abdominal pain, or metallic taste during the course of treatment. 14C-UBT were performed a minimum of 4 weeks after therapy or use of any antibiotic and a minimum of 2 weeks off any acid-suppressive therapy.
Statistical analysis
The primary end point of the study was the rate of H. pylori eradication. The sample size calculation for a noninferiority trial, assuming an eradication rate in the standard and half-dose groups of 90% and a noninferiority limit of 10–11% with 80% power, was 93 to 112 patients per arm. Secondary end points included adverse events and overall cost, factoring in the cost of failed therapy (i.e. use of a second-line eradication regimen and repeat UBT testing). All data entry and statistical analysis were carried out using SPSS version 16.0 for Windows (SPSS, Chicago, IL, USA). Chi-squared and Fisher exact tests were used to compare the major outcomes between these groups. A p-value <0.05 was considered statistically significant.
Eradication rates were evaluated by both intention-to-treat (ITT) and per protocol (PP) analyses. The ITT analysis included all study participants who took at least one dose of the study medications. Patients who were subsequently lost to follow up after starting treatment or whose infection status was unknown after treatment were considered treatment failures for the purpose of the ITT analysis. The PP analysis included only patients who completed a full course of therapy according to study instructions and returned for a post-treatment UBT. Differences in adverse events were compared between groups using Chi-squared and Fisher exact tests. Univariate testing using Chi-squared and Fisher exact tests was also used to determine whether any of the patient’s characteristics obtained from the patient questionnaire were significant factors affecting the response to treatment in both groups.
Results
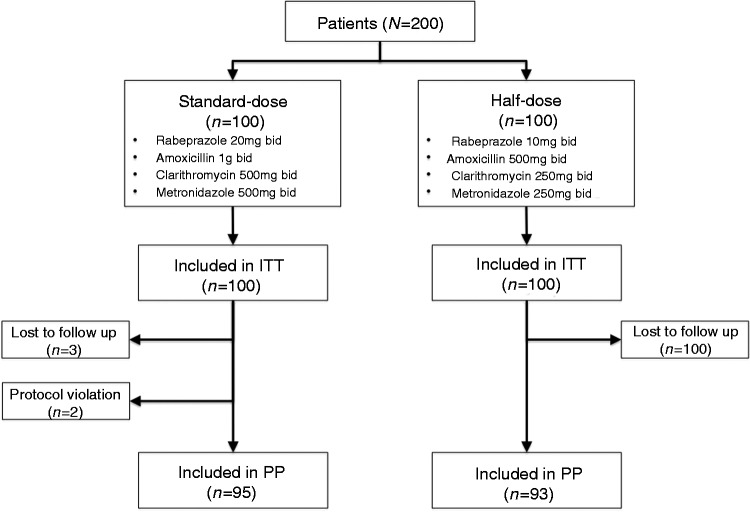
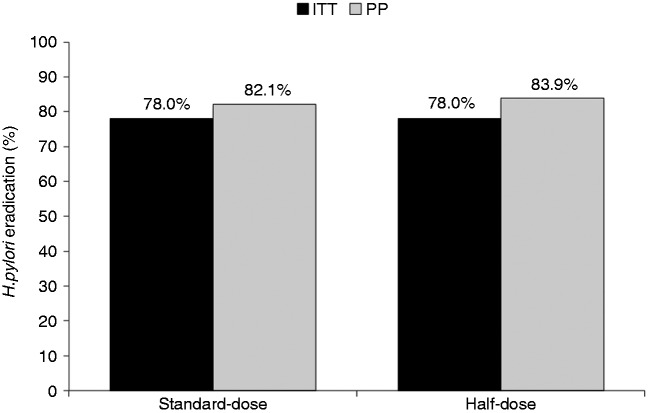
A total of 200 patients infected with H. pylori were randomized to either standard-dose (n = 100) or half-dose arm (n = 100). The clinical and demographic characteristics of the study subjects are summarized in Table 1. There was no significant difference in age, sex, body mass index, smoking status, previous use of macrolides, or current use of PPIs between the two groups. Of the study patients, 181 had oesophagogastroduodenoscopy and 43 (23.8%) had documented peptic ulcer disease: 21/92 (22.8%) in the standard-dose arm vs. 22/89 (24.7%) in the half-dose arm, respectively (p = 0.54). Overall, 10 patients (three in the standard-dose group and seven in the half-dose group) did not complete the study and/or return for UBT after treatment; two patients from the standard-dose group stopped treatment on day 3 and then resumed 2 days later because of severe nausea (Figure 1). On ITT analysis, the eradication rate for both groups was 78 out of 100 (78.0%, 95% CI 68.6–85.7%) and on PP analysis, the eradication rate was 78/95 (82.1%, 95% CI 72.9–89.2%) in the standard-dose group vs. 78/93 (83.9%, 95% CI 74.8–90.7%; p = 0.71) in the half-dose group (Figure 2). There was no difference in H. pylori eradication rates between those with or without endoscopically proven peptic ulcer disease.
Table 1.
Patient characteristics
| Standard-dose (n = 100) | Half-dose (n = 100) | p-value | |
|---|---|---|---|
| Age | 47.3 ± 17.2 | 44.7 ± 15.5 | 0.264 |
| Body mass index | 26.8 ± 5.5 | 25.6 ± 4.3 | 0.086 |
| Male gender | 48 | 57 | 0.203 |
| Smoking | 48 | 51 | 0.671 |
| Prior macrolide use | 16 | 15 | 0.845 |
| Current PPI use | 42 | 34 | 0.221 |
Values are mean ± SD or n.
PPI, proton pump inhibitor.
Figure 1.
Study consort flow diagram.
Figure 2.
Rates of eradication according to group assignment. ITT, intention-to-treat; PP, per-protocol.
All patients were evaluated for compliance and adverse events on days 3 and 7 of treatment. Compliance, self-reported during phone interview on day 7 of treatment, was 98 and 100% in both groups (Figure 1). In total, 57.0% of patients in the standard-dose group vs. 41.0% in the half-dose group reported at least one adverse event during therapy (p = 0.024). Metallic taste was the most commonly reported adverse effect in both groups (36% in the standard-dose group vs. 12% in the half-dose group on day 7 of treatment, respectively; p < 0.001). Nausea was the second most common side effect (18% in standard-dose group vs. 7% in half-dose group on day 7 of treatment; p = 0.019) (Table 2).
Table 2.
Side effects reported during treatment
| Standard dose (n = 100) | Half-dose (n = 100) | p-value | |
|---|---|---|---|
| Day 3 | |||
| Nausea | 23 | 16 | 0.212 |
| Diarrhoea | 12 | 13 | 0.831 |
| Metallic taste | 45 | 19 | <0.001 |
| Abdominal pain | 12 | 10 | 0.651 |
| Day 7 | |||
| Nausea | 18 | 7 | 0.019 |
| Diarrhoea | 12 | 10 | 0.651 |
| Metallic taste | 36 | 12 | <0.001 |
| Abdominal pain | 10 | 7 | 0.447 |
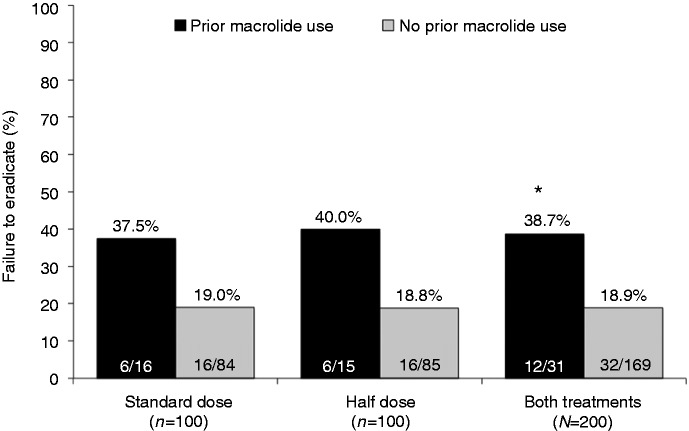
Treatment failure was noted in 12/31 patients (38.7%) who reported prior macrolide use compared to 32/169 (18.9%) patients who did not (ITT; p = 0.019) (Figure 3). On multivariate analysis, age, sex, body mass index, smoking status, peptic ulcer disease, and use of PPIs before treatment did not have any significant impact on the observed eradication rates. Previous macrolide use was the only variable significantly associated with an increased risk of ineffective eradication (OR 2.60, 95% CI 1.06–6.39; p = 0.038). Given absolute parity in the eradication rate in both groups, the cost of treatment (based on local retail price) was significantly less in patients receiving the half-dose regimen (ITT analysis; p < 0.05).
Figure 3.
Failure of eradication according to macrolide exposure history.
Discussion
The ideal H. pylori treatment regimen should be effective, simple, affordable, and tolerable with minimal side effects. The recent Maastricht III consensus guidelines recommend the use of either triple or bismuth-based quadruple therapy as first-line therapy but caution that clarithromycin-based regimens should be abandoned when local resistance to this macrolide antibiotic exceeds 15–20%. Worldwide resistance data confirm a time-dependent increase in clarithromycin resistance and a parallel decline in the eradication rates of STT.15–19 Several randomized controlled trials have demonstrated that NBQT, involving the addition of metronidazole or tinidazole to STT, is equally well tolerated but more effective than STT, including for clarithromycin-resistant strains.9,10 In 2009, a meta-analysis of 15 studies involving 1723 patients calculated a mean ITT cure of 90% for NBQT. The concomitant version of NBQT was found to be as effective as sequential therapy, yet simpler11,20 and was recommended by some as first line therapy for H. pylori in regions where STT has been rendered relatively ineffective.11 More recently, multiple trials of concomitant NBQT have confirmed an ITT eradication rate of 75–96.4% (per-protocol rates consistently superior to 80%) with a possible advantage for longer treatment duration (Table 3).15,16,21–32
Table 3.
Trials published since 2010 investigating concomitant NBQT
| Authors | Year | No. of patients | Eradication ITT (%) | Eradication PP (%) | Course (days) | Region |
|---|---|---|---|---|---|---|
| Wu et al.21 | 2010 | 117 | 93 | 93 | 10 | Taiwan |
| Toros et al.22 | 2011 | 84 | 75 | 75 | 14 | Turkey |
| Greenberg et al.23 | 2011 | 489 | 73.6 | 76.4 | 5 | Latin America |
| Molina-Infante et al.16 | 2012 | 209 | 87 | 89 | 10 | Spain |
| Yanai et al.24 | 2012 | 59 | 94.9 | 98.3 | 7 | Japan |
| Kongchayanun et al.25 | 2012 | 55 | 89.1 | 89.1 | 5 | Thailand |
| Kongchayanun et al.25 | 2012 | 55 | 96.4 | 96.4 | 10 | Thailand |
| Huang et al.26 | 2012 | 84 | 88.1 | 94.6 | 10 | China |
| Kao et al.27 | 2012 | 319 | 93.7 | 96.4 | 7 | Taiwan |
| Georgopoulos et al.15 | 2013 | 127 | 90.5 | 93.3 | 10 | Greece |
| Kim et al.28 | 2013 | 135 | 80.7 | 91.4 | 5 | Korea |
| Lim et al.29 | 2013 | 78 | 80.8 | 81.3 | 14 | Korea |
| McNicholl et al.30 | 2013 | 168 | 87 | 91 | 10 | Spain |
| Molina-Infante et al.31 | 2013 | 172 | 91.7 | 96.1 | 14 | Spain and Italy |
| Zullo et al.32 | 2013 | 90 | 85.5 | 91.6 | 5 | Italy |
| Current study | 2013 | 200 | 78 | 82.9 | 7 | Lebanon |
ITT, intention-to-treat; PP, per-protocol.
The results of this randomized trial appear to confirm our earlier observation13 that reduced-dose regimens (down to 50% posology) performs similarly to standard-dose regimens with the added benefit of reduced cost and adverse events. To date, three randomized controlled trials, involving a total of 475 patients, have examined the value of 50% reduction in the dose of all administered antibiotics for H. pylori (two STT and the current concomitant NBQT) and have all confirmed similar eradication rates to the standard-dose comparator (Table 4), with a reduction in adverse events and cost.13,33 This concept is attractive and challenges the prevailing dogma in the treatment of H. pylori. One theoretical concern, however, is that these low-dose regimens may produce prolonged sub-minimum inhibitory concentration effects which may, in turn, contribute to development of resistant strains. However, the posology of antibiotics commonly used in H. pylori treatment regimens has been largely empirical and has relied traditionally on standard single-agent antibiotic dosing standards such as those used, for example, for respiratory tract infections in adults. In fact, there is very limited information on antibiotic penetration and tissue levels in the gastric mucus layer and crypts where H. pylori lives. McNulty et al.34 measured gastric mucosal concentrations of amoxicillin in patients at upper gastrointestinal endoscopy 38–480 min after a 500 mg oral dose of amoxicillin and found that high concentrations were achieved (range 15–322 mg/kg); the lowest gastric mucosal concentration after 2 hours was 100 times the MIC90 of 0.12 mg/l.34 Nakamura et al.35 measured plasma, gastric mucosa, and gastric juice concentrations following either low- or high-dose amoxicillin (750 or 1000 mg b.i.d.) and clarithromycin (400 or 500 mg b.i.d.) given in combination with omeprazole 20 mg b.i.d. to 12 male volunteers in an open crossover study. Amoxicillin and clarithromycin concentrations were highest in gastric juice followed by gastric mucosa, and were both superior to plasma levels. After 6 hours, antibiotics were no longer detectable in mucosa samples. However, plasma and gastric juice concentrations remained above the minimum inhibitory concentration for amoxicillin- and clarithromycin-susceptible bacteria, including in recipients of the low-dose therapy.35
Table 4.
Randomized controlled trials examining standard-dose vs. half-dose antibiotic therapy for H. pylori infection
| Authors | Year | No. of patients | Standard dose | Half-dose | Eradication rate (%: ITT/PP) |
|---|---|---|---|---|---|
| Keshavarz et al.33 | 2007 | 160 | Omeprazole 20 mg b.i.d. | Omeprazole 20 mg b.i.d. | Standard: 83.8/89 |
| Amoxicillin 1000 mg b.i.d. | Amoxicillin 500 mg b.i.d. | Half: 85/88 | |||
| Clarithromycin 500 mg b.i.d. | Clarithromycin 250 mg b.i.d. | ||||
| 14 days | 14 days | ||||
| Mansour et al.13 | 2011 | 115 | Rabeprazole 20 mg b.i.d. | Rabeprazole 10 mg b.i.d. | Standard: 77.6/78.9 |
| Amoxicillin 1000 mg b.i.d. | Amoxicillin 500 mg b.i.d. | Half: 77.2/81.5 | |||
| Clarithromycin 500 mg b.i.d. | Clarithromycin 250 mg b.i.d. | ||||
| 10 days | 10 days | ||||
| Current study | 2013 | 200 | Rabeprazole 20 mg b.i.d. | Rabeprazole 10 mg b.i.d. | Standard 78/82.1 |
| Amoxicillin 1000 mg b.i.d. | Amoxicillin 500 mg b.i.d. | Half 78/83.9 | |||
| Clarithromycin 500 mg b.i.d. | Clarithromycin 250 mg b.i.d. | ||||
| Metronidazole 500 mg b.i.d. | Metronidazole 250 mg b.i.d. | ||||
| 7 days | 7 days |
An important result of this study is the significant reduction in cost and adverse events with the half-dose concomitant NBQT. Although adverse events are often mild with first-line H. pylori regimens, they remain frequent and are an important cause for noncompliance and suboptimal adherence with detrimental clinical outcomes. In H. pylori clinical trials, approximately 5–10% of enrolled subjects drop out or are excluded from per-protocol analysis because of adverse events, poor compliance, or adherence36 and this number increases with longer duration of treatment.37 In the recent trial by Moline-Infante et al.,12 patients receiving the hybrid version of the 14-day NBQT (diminished pill burden by reduced exposure to clarithromycin and metronidazole to the last 7 days) had less adverse events (47 vs. 56%; p = 0.06) and greater compliance (98.8 vs. 95.2%; p = 0.05) than the concomitant NBQT version. Despite the long duration of therapy in that study, a heavy pill burden (112 tablets) and frequent, albeit mild, adverse effects, a remarkable 93.5% of 343 patients were ‘fully compliant’ with the prescribed concomitant NBQT and only 6% withdrew because of adverse events.12 The real-world scenario is, however, different and is likely to be worse. Drug therapies often possess different performance characteristics in controlled clinical trials compared to the real world (effectiveness vs. efficacy). Outcomes in clinical trials are often favoured because of the homogeneous nature of the study population, close patient follow up, the presence of well-defined protocols, adherence controls (e.g. pill counts, phone calls), financial incentive (free drug supplies, tests, and follow up), and patient motivation. Patient adherence is thus a critical variable that influences the ultimate effectiveness of treatment and adverse events are an important determinant to such adherence.
In our study, both standard-dose and half-dose concomitant NBQT for 7 days resulted in suboptimal eradication rates (<80%). Despite limited direct comparative data, a longer duration of NBQT is likely to result in improved eradication (Table 3). Prolonging the duration of therapy may add what amounts to 0.5–1%-per-day benefit in eradication rates but is expected to result in significant increase in adverse events and cost, as well as diminished compliance and adherence.
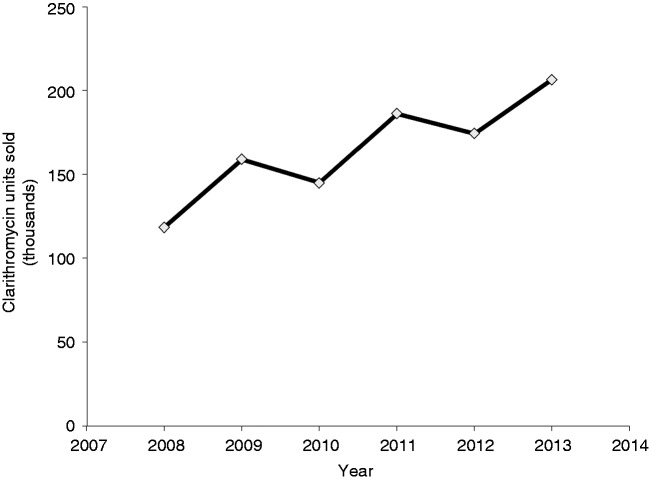
Whether the added benefit with respect to eradication rates is consistent in the case of clarithromycin-resistant strains is largely untested. In the absence of shorter, simpler, and safer alternatives, the use of 14-day NBQT may be an acceptable option in the era of increased resistance to clarithromycin. However, in our study, 38.7% of patients with a history of prior macrolide use failed to eradicate H. pylori compared to 18.9% of those with no such history. A history of prior macrolide use leads to more complex resistant strains and the rates of resistance are directly proportional to the courses of macrolides used.38,39 A recent multicentre European survey study showed a steady increase in clarithromycin resistance, with almost doubling over a 10-year period from 9.8 to 17.5%.40,41 Studies have suggested that this global increase in clarithromycin resistance appears to correlate with increase prescription usage of clarithromycin, roxithromycin, and azithromycin.41,42 Although we did not perform in vitro susceptibility testing, the high rate of treatment failure noted in prior macrolide users in our study appears to support this conclusion. Data from IMS Health show a steady increase in the usage of clarithromycin in Lebanon, with approximately 75% growth over a period of 5 years (Figure 4).
Figure 4.
Clarithromycin sales in Lebanon 2008–2013 (data with permission from IMS Health Lebanon).
In the recent multicentre Spanish–Italian study, 14-day of concomitant NBQT was successful at eradicating all clarithromycin-resistant strains of H. pylori. However, this was applicable to only eight patients (of 68 undergoing in vitro culture and susceptibility). Based on the above and in the absence of stronger evidence to the contrary, we believe that all clarithromycin-based regimens should be avoided in patients with known or documented history of prior macrolide use. Such an important variable should be sought in every patient considered for H. pylori treatment.
There are a few limitations to our study. Culture and in vitro susceptibility testing were not performed and hence the exact role of antibiotic resistance (namely to clarithromycin and metronidazole) in eradication failure cannot be evaluated. Moreover, information on prior macrolide use was collected from patients using a questionnaire and may therefore be subject to recall bias. Although this is one of the largest controlled trials on concomitant NBQT (Table 3), it was powered at 80% with a delta of 10% for noninferiority assuming an eradication rate of 90% in both study arms. Eradication rates in this study were, however, suboptimal in both arms. This notwithstanding, given absolute parity on ITT analysis, it is somewhat unlikely that we missed a true or clinically significant difference between both regimens. Lastly, the potential effect of CYP2C19 genetic polymorphism on PPI metabolism and, consequently, the stability of the acid-labile antibiotics amoxicillin and clarithromycin was not investigated. This may conceivably be of added importance when one uses reduced-dose PPIs in CYP2C19 ultra-metabolizers, a common phenotype in Western populations. It is important to note, however, that rabeprazole undergoes primarily a nonenzymic hepatic metabolism and that multiple studies have confirmed equivalent eradication rates between standard-dose and reduced-dose rabeprazole in clarithromycin-based triple therapy and independent of CYP2C19 genetic polymorphism.43 The equivalent eradication rates in our study in a population with matching CYP2C19 profile to people of European origin44 appears to fall in line with this observation.
In conclusion, a 7-day half-dose concomitant NBQT is equally effective but better tolerated and cheaper than its standard-dose equivalent in the treatment of H. pylori. The concept of reduced-dose antimicrobials in multidrug H. pylori regimens seems partially validated and deserves further evaluation in large randomized controlled trials. Such a strategy can lead to significant cost savings, reduced adverse events, and enhanced compliance, adherence and treatment uptake and may consitute a paradigm shift in the treatment of H. pylori. Although likely, it remains unproven whether the half-dose strategy remains effective with extended concomitant NBQT for 10 or 14 days and leads to acceptable eradication rates.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that there is no conflict of interest.
Presentation
This work was presented in part at the American College of Gastroenterology Annual Meeting, Las Vegas, NV, USA, 19–24 October 2012 and in full as an oral presentation at the 28th International Congress of Chemotherapy and Infection, Yokohama, Japan, 5–8 June 2013.
References
- 1.Everhart JE. Recent developments in the epidemiology of Helicobacter pylori. Gastroenterol Clin North Am 2000; 29: 559–578 [DOI] [PubMed] [Google Scholar]
- 2.Labenz J, Borsch G. Evidence for the essential role of Helicobacter pylori in gastric ulcer disease. Gut 1994; 35: 19–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bechi P, Balzi M, Becciolini A, et al. Helicobacter pylori and cell proliferation of the gastric mucosa: possible implications for gastric carcinogenesis. Am J Gastroenterol 1996; 91: 271–276 [PubMed] [Google Scholar]
- 4.Tytgat GN. Peptic ulcer and Helicobacter pylori: eradication and relapse. Scand J Gastroenterol Suppl 1995; 210: 70–72 [DOI] [PubMed] [Google Scholar]
- 5.Solcia E, Villani L, Fiocca R, et al. Effects of eradication of Helicobacter pylori on gastritis in duodenal ulcer patients. Scand J Gastroenterol Suppl 1994; 201: 28–34 [PubMed] [Google Scholar]
- 6.Chey WD, Wong BC. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol 2007; 102: 1808–1825 [DOI] [PubMed] [Google Scholar]
- 7.Malfertheiner P, Megraud F, O’Morain C, et al. Current concepts in the management of Helicobacter pylori infection – the Maastricht 2-2000 Consensus Report. Aliment Pharmacol Ther 2002; 16: 167–180 [DOI] [PubMed] [Google Scholar]
- 8.Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 2010; 59: 1143–1153 [DOI] [PubMed] [Google Scholar]
- 9.Moayyedi P. Sequential regimens for Helicobacter pylori eradication. Lancet 2007; 370: 1010–1012 [DOI] [PubMed] [Google Scholar]
- 10.Zullo A, De Francesco V, Hassan C, et al. The sequential therapy regimen for Helicobacter pylori eradication: a pooled-data analysis. Gut 2007; 56: 1353–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Essa AS, Kramer JR, Graham DY, et al. Meta-analysis: four-drug, three-antibiotic, non-bismuth-containing ‘concomitant therapy’ versus triple therapy for Helicobacter pylori eradication. Helicobacter 2009; 14: 109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molina-Infante J, Romano M, Fernandez-Bermejo M, et al. Optimized nonbismuth quadruple therapies cure most patients with Helicobacter pylori infection in populations with high rates of antibiotic resistance. Gastroenterology 2013; 145: 121–128, e1 [DOI] [PubMed] [Google Scholar]
- 13. Mansour NM, Hashash JG, El-Halabi M, et al. A randomized trial of standard-dose versus half-dose rabeprazole, clarithromycin, and amoxicillin in the treatment of Helicobacter pylori infection. Eur J Gastroenterol Hepatol 2011; 23: 865–870. [DOI] [PubMed]
- 14. Soweid A, Chedid M, Araj GF, et al. Efficacy and safety of a ten-day Helicobacter pylori eradication regimen with rabeprazole, amoxicillin and clarithromycin. Am J Gastroenterol 2003; 98(9s): S39–S39.
- 15.Georgopoulos S, Papastergiou V, Xirouchakis E, et al. Nonbismuth quadruple ‘concomitant’ therapy versus standard triple therapy, both of the duration of 10 days, for first-line H. pylori eradication: a randomized trial. J Clin Gastroenterol 2013; 47: 228–232 [DOI] [PubMed] [Google Scholar]
- 16.Molina-Infante J, Pazos-Pacheco C, Vinagre-Rodriguez G, et al. Nonbismuth quadruple (concomitant) therapy: empirical and tailored efficacy versus standard triple therapy for clarithromycin-susceptible Helicobacter pylori and versus sequential therapy for clarithromycin-resistant strains. Helicobacter 2012; 17: 269–276 [DOI] [PubMed] [Google Scholar]
- 17.Chen LW, Chien RN, Chang JJ, et al. Comparison of the once-daily levofloxacin-containing triple therapy with the twice-daily standard triple therapy for first-line Helicobacter pylori eradication: a prospective randomised study. Int J Clin Pract 2010; 64: 1530–1534 [DOI] [PubMed] [Google Scholar]
- 18.Basu PP, Rayapudi K, Pacana T, et al. A randomized study comparing levofloxacin, omeprazole, nitazoxanide, and doxycycline versus triple therapy for the eradication of Helicobacter pylori. Am J Gastroenterol 2011; 106: 1970–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sasaki M, Ogasawara N, Utsumi K, et al. Changes in 12-year first-line eradication rate of Helicobacter pylori based on triple therapy with proton pump inhibitor, amoxicillin and clarithromycin. J Clin Biochem Nutr 2010; 47: 53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu W, Yang Y, Sun G. Recent insights into antibiotic resistance in Helicobacter pylori eradication. Gastroenterol Res Pract 2012; 2012: 723183–723183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu DC, Hsu PI, Wu JY, et al. Sequential and concomitant therapy with four drugs is equally effective for eradication of H pylori infection. Clin Gastroenterol Hepatol 2010; 8: 36–41, e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toros AB, Ince AT, Kesici B, et al. A new modified concomitant therapy for Helicobacter pylori eradication in Turkey. Helicobacter 2011; 16: 225–228 [DOI] [PubMed] [Google Scholar]
- 23.Greenberg ER, Anderson GL, Morgan DR, et al. 14-day triple, 5-day concomitant, and 10-day sequential therapies for Helicobacter pylori infection in seven Latin American sites: a randomised trial. Lancet 2011; 378: 507–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yanai A, Sakamoto K, Akanuma M, et al. Non-bismuth quadruple therapy for first-line Helicobacter pylori eradication: a randomized study in Japan. World J Gastrointest Pharmacol Ther 2012; 3: 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kongchayanun C, Vilaichone RK, Pornthisarn B, Amornsawadwattana S, and Mahachai V. Pilot studies to identify the optimum duration of concomitant Helicobacter pylori eradication therapy in Thailand. Helicobacter 2012; 17: 282–285 [DOI] [PubMed] [Google Scholar]
- 26.Huang YK, Wu MC, Wang SS, et al. Lansoprazole-based sequential and concomitant therapy for the first-line Helicobacter pylori eradication. J Dig Dis 2012; 13: 232–238 [DOI] [PubMed] [Google Scholar]
- 27.Kao SS, Chen WC, Hsu PI, et al. 7-day nonbismuth-containing concomitant therapy achieves a high eradication rate for Helicobacter pylori in Taiwan. Gastroenterol Res Pract 2012; 2012: 463985–463985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim SY, Lee SW, Hyun JJ, et al. Comparative study of Helicobacter pylori eradication rates with 5-day quadruple ‘concomitant’ therapy and 7-day standard triple therapy. J Clin Gastroenterol 2013; 47: 21–24 [DOI] [PubMed] [Google Scholar]
- 29.Lim JH, Lee DH, Choi C, et al. Clinical outcomes of two-week sequential and concomitant therapies for Helicobacter pylori eradication: a randomized pilot study. Helicobacter 2013; 18: 180–186 [DOI] [PubMed] [Google Scholar]
- 30.McNicholl AG, Marin AC, Molina-Infante J, et al. Randomised clinical trial comparing sequential and concomitant therapies for Helicobacter pylori eradication in routine clinical practice. Gut 2014; 63: 224–229 [DOI] [PubMed] [Google Scholar]
- 31.Molina-Infante J, Romano M, Fernandez-Bermejo M, et al. Optimized nonbismuth quadruple therapies cure most patients with Helicobacter pylori infection in populations with high rates of antibiotic resistance. Gastroenterology 2013; 145: 121–128 [DOI] [PubMed] [Google Scholar]
- 32.Zullo A, Scaccianoce G, De Francesco V, et al. Concomitant, sequential, and hybrid therapy for H. pylori eradication: a pilot study. Clin Res Hepatol Gastroenterol 2013; 37: 647–650 [DOI] [PubMed] [Google Scholar]
- 33.Keshavarz AA, Bashiri H, Rahbar M. Omeprazole-based triple therapy with low-versus high-dose of clarithromycin plus amoxicillin for H pylori eradication in Iranian population. World J Gastroenterol 2007; 13: 930–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McNulty CA, Dent JC, Ford GA, et al. Inhibitory antimicrobial concentrations against Campylobacter pylori in gastric mucosa. J Antimicrob Chemother 1988; 22: 729–738 [DOI] [PubMed] [Google Scholar]
- 35.Nakamura M, Spiller RC, Barrett DA, et al. Gastric juice, gastric tissue and blood antibiotic concentrations following omeprazole, amoxicillin and clarithromycin triple therapy. Helicobacter 2003; 8: 294–299 [DOI] [PubMed] [Google Scholar]
- 36.Malfertheiner P, Bazzoli F, Delchier JC, et al. Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: a randomised, open-label, non-inferiority, phase 3 trial. Lancet 2011; 377: 905–913 [DOI] [PubMed] [Google Scholar]
- 37.Laine L, Estrada R, Trujillo M, et al. Randomized comparison of differing periods of twice-a-day triple therapy for the eradication of Helicobacter pylori. Aliment Pharmacol Ther 1996; 10: 1029–1033 [DOI] [PubMed] [Google Scholar]
- 38.Kim JM, Kim JS, Kim N, et al. Gene mutations of 23S rRNA associated with clarithromycin resistance in Helicobacter pylori strains isolated from Korean patients. J Microbiol Biotechnol 2008; 18: 1584–1589 [PubMed] [Google Scholar]
- 39.McMahon BJ, Hennessy TW, Bensler JM, et al. The relationship among previous antimicrobial use, antimicrobial resistance, and treatment outcomes for Helicobacter pylori infections. Ann Intern Med 2003; 139: 463–469 [DOI] [PubMed] [Google Scholar]
- 40.Glupczynski Y, Megraud F, Lopez-Brea M, et al. European multicentre survey of in vitro antimicrobial resistance in Helicobacter pylori. Eur J Clin Microbiol Infect Dis 2001; 20: 820–823 [DOI] [PubMed] [Google Scholar]
- 41.Megraud F, Coenen S, Versporten A, et al. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut 2013; 62: 34–42 [DOI] [PubMed] [Google Scholar]
- 42.Grove DI, Koutsouridis G. Increasing resistance of Helicobacter pylori to clarithromycin: is the horse bolting? Pathology 2002; 34: 71–73 [DOI] [PubMed] [Google Scholar]
- 43.Sharara AI. Rabeprazole: the role of proton pump inhibitors in Helicobacter pylori eradication. Expert Rev Anti Infect Ther 2005; 3: 863–870 [DOI] [PubMed] [Google Scholar]
- 44.El-Halabi MM, Zgheib N, Mansour NM, et al. CYP2C19 genetic polymorphism, rabeprazole and esomeprazole have no effect on the antiplatelet action of clopidogrel. J Cardiovasc Pharmacol 2013; 62: 41–49 [DOI] [PubMed] [Google Scholar]