Abstract
Selective serotonin reuptake inhibitors (SSRIs), the most widely used drugs for the treatment of depression, have been reported to reduce bone formation and increase the risk of bone fracture. Since osseointegration is influenced by bone metabolism, this study aimed to investigate the association between SSRIs and the risk of failures in osseointegrated implants. This retrospective cohort study was conducted on patients treated with dental implants from January 2007 to January 2013. A total of 916 dental implants in 490 patients (94 implants on 51 patients using SSRIs) were used to estimate the risk of failure associated with the use of SSRIs. Data analysis involved Cox proportional hazards, generalized estimating equation models, multilevel mixed effects parametric survival analysis, and Kaplan-Meier analysis. After 3 to 67 mo of follow-up, 38 dental implants failed and 784 succeeded in the nonusers group, while 10 failed and 84 succeeded in the SSRI-users group. The main limitation of this retrospective study was that drug compliance dose and treatment period could not be acquired from the files of the patients. The primary outcome was that compared with nonusers of SSRIs, SSRI usage was associated with an increased risk of dental implants failure (hazard ratio, 6.28; 95% confidence interval, 1.25-31.61; p = .03). The failure rates were 4.6% for SSRI nonusers and 10.6% for SSRI users. The secondary outcomes were that small implant diameters (≤4 mm; p = .02) and smoking habits (p = .01) also seemed to be associated with higher risk of implant failure. Our findings indicate that treatment with SSRIs is associated with an increased failure risk of osseointegrated implants, which might suggest a careful surgical treatment planning for SSRI users.
Keywords: medical devices, risk factors, dental implants, bone remodeling, osseointergration, epidemiology
Introduction
Depression—a state of low mood that affects a person’s thoughts, behavior, feelings, and sense of well-being—has become a threatening global disease because of its high prevalence and associative public health problems (Murray and Lopez, 1997; Krishnan and Nestler, 2008). The World Health Organization estimates that more than 350 million people worldwide suffer from depression. Serotonin (5-hydroxytryptamine [5-HT]) is a monoamine neurotransmitter in the brain that contributes to the feelings of well-being and happiness (Krishnan and Nestler, 2008). Lower levels of serotonin or obstacles for its utilization can lead to depression (Krishnan and Nestler, 2008). Selective serotonin reuptake inhibitors (SSRIs)—such as Celexa, Paxil, Lexapro, Prozac, and Zoloft—are drugs designed to inhibit the reuptake of serotonin and boost its levels to treat depression (Liu et al., 1998). Because of their unique effectiveness in depression treatment, SSRIs have become the most widely used antidepressants all over the world (Tsapakis et al., 2012).
Serotonin receptors can be found in not only the nervous tissue but also peripheral tissues such as the digestive tract, blood platelets, and bones; accordingly, SSRIs can affect the function of the digestive, cardiovascular, and skeletal systems (Tsapakis et al., 2012). In bone metabolism, serotonin regulates bone cells by acting on 5-HT1B, 5-HT2B, 5-HT2C receptors and serotonin transporters (5-HTTs), resulting in complex signal transmissions in osteoblasts and osteoclasts (Tsapakis et al., 2012). Therefore, SSRIs block 5-HTTs on bone cells, resulting in a direct negative effect in bone formation (Diem et al., 2007; Yadav et al., 2008) and metabolism (Tsapakis et al., 2012) by increasing osteoclast differentiations (Battaglino et al., 2004) and inhibiting osteoblast proliferation (Tsapakis et al., 2012). As a result, SSRIs decrease bone mass and bone mineral density (Battaglino et al., 2004; Diem et al., 2007), at an annual reduction rate of 0.60% to 0.93% (Diem et al., 2007), increasing the risk of osteoporosis (Verdel et al., 2010), bone fracture (Liu et al., 1998; Verdel et al., 2010), and osteoporotic fracture (Verdel et al., 2010).
Osseointegrated medical devices, mainly made of titanium, can create a firm and lasting connection with the recipient bone (Albrektsson et al., 1981), and these have been applied as bone-anchored craniofacial prostheses, joint replacements, and dental implants (Albrektsson et al., 1981; Del Valle et al., 1995; Esposito et al., 1998). They have become a revolutionary step in achieving soft or hard tissue replacement, and they have proven to be a routine and reliable treatment choice (Carlsson et al., 1986). Failure of osseointegration between the device and the host bone can cause treatment failure and need for reintervention and in some cases (e.g., hip replacement) can shorten patients’ life expectancy (Schep et al., 2004).
Osseointegration of implants is highly dependent on the quality of the recipient bone (Wong et al., 1995), and since SSRIs seem to have a negative effect on bone formation (Battaglino et al., 2004; Gustafsson et al., 2006; Diem et al., 2007), we hypothesize that SSRI treatment might have a negative effect on titanium implant osseointegration and survival rate. Given the large portion of the population taking SSRIs, and the increased number of surgeries using osseointegated implants, it is vital to investigate whether SSRI treatment can affect osseointegated implant survival rate. In order to test our hypothesis, a cohort study was carried out on patients treated with one type of osseointegrated medical devices, titanium dental implants, to investigate whether the use of SSRIs is associated with higher risk of titanium implant failure.
Materials & Methods
Patients and Data Sources
Approval (12-321 GEN) was obtained from the Ethical Committee for Clinical Trials of McGill University to carry out a retrospective cohort study in the dental clinic East Coast Oral Surgery (Moncton, Canada). Written informed consent was granted from all subjects. Our study is a human observational study and has conformed to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Records of patients with dental osseointegrated prosthesis for this retrospective cohort study were identified in the clinic database, and the original hard copy files were retrieved for manual examination. The overall study period was 6 yr, between January 1, 2007, and September 8, 2013. Preoperative patient information, including medication, habits, and behavioral factors, was self-reported through a standardized questionnaire that was filled prior to the surgical intervention. Patients were excluded if they had a severe systemic disease (American Society of Anaesthesiology III or IV), were pregnant, or had a medical disorder known to substantially affect bone metabolism, such as osteoporosis, osteomalacia, Paget’s disease, vitamin D deficiency, hyperthyroidism, cancer (excluding nonmelanoma skin cancer), or alcoholism, as were those on corticosteroids, antiepileptic drugs, antihypertensive drugs, proton pump inhibitors, or bisphosphonates (Tamimi et al., 2012). Smoking habit was considered in our analysis; subjects who smoked more than 10 cigarettes per day were defined as smokers (Tonetti et al., 1995).
SSRI Medication Definition
SSRI usage was defined as filling a prescription for SSRIs at the time of implant placement (citalopram, dapoxetine, escitalopram, fluoxetine, fluvoxamine, indalpine, paroxetine, sertraline, venlafaxine, and zimelidine; Diem et al., 2007).
Surgical Protocol and Postoperative Treatment
In patients with sufficient native bone, implant (Nobel Biocare) surgery was performed under local anaesthesia, with or without intravenous sedation, according to the manufacturer’s recommended protocol (Finkemeier, 2002). In cases with inadequate bone volume for implant placement, bone augmentation (i.e., lateral bone grafting, sinus lifting) was performed 6 mo prior to implant placement via a mixture of autogenous and allogenic bone substitutes (allogeneic bone, Straumann, Andover, MA, USA; Finkemeier, 2002).
The use of antibiotics in implant dentistry is controversial, so postoperatively, patients were instructed to rinse 4 times per day for a period of 7 d with 0.2% chlorhexidine solution (Peridex, Periogard, Allentown, PA, USA) and to follow a soft diet. They also received prophylactically a prescription of antibiotics for a period of 7 d (amoxicillin, 500 mg, orally, 3 times per day [GlaxoSmithKline, Middlesex, UK], or clindamycin, 300 mg, orally, 4 times per day [Sandoz, Boucherville, Canada]).Analgesic agents were prescribed as needed (acetaminophen, 500 mg, 3 times per day [Tylenol, McNeil Consumer Healthcare, Fort Washington, PA, USA]; or ibuprofen, 400 mg, 3 times per day [Advil, Wyeth Consumer Healthcare, Madison, NJ, USA]).
Patients were seen for follow-up examinations 10 d after surgery; all sutures were removed; and hygiene instructions were reinforced. Before delivery of the final implant-supported prosthesis, osseointegration was evaluated clinically by assessing vertical, lateral, and rotational signs of mobility. Implants with at least one of the following complications were defined as failures: pain on function; mobility; radiographic bone loss equivalent to one-half of the implant length; uncontrolled exudate; or implant no longer in mouth (Misch et al., 2008).
Study Outcomes and Follow-up
The primary study endpoint was a binary dental implants outcome, comprising successful implants and failed implants. For either outcome, we followed patients until they experienced dental implant failure, died, or were censored for losing the track or reaching the end of the study period, whatever came first. The following parameters were retrieved from the patients’ files and standardized questionnaires: patient age, sex, implant dimensions, bone augmentation, smoking habit, physical condition, medicine undertaken, and follow-up time.
Sample Size Calculation and Statistical Analysis
This cohort study was designed to examine the association between dental implant failure and SSRI treatment along with other factors. Sample size calculation based on Cohen’s F test indicated that a minimum of 645 implants was required to achieve a power of 0.8 at an effect size (f 2) of 0.25 and a probability level of 0.05 with 8 covariates (Kemp, 2003). Accordingly, differences were considered of no clinical relevance if <1.1% based on Cohen’s F test with a 25% standard deviations difference between the 2 groups’ means (Pjetursson et al., 2012).
Comparison between SSRI users and nonusers in terms of demographic systemic conditions and other factors, as well as the healing period calculation, was done through the chi-square test. Cox proportional hazards model was performed to assess the association between potential risk factors, including SSRI usage, and dental implant failure rate, adjusting for potential confounders factors. In addition, we used generalized estimating equation (GEE) models and multilevel mixed effects parametric survival analysis (Stephenson et al., 2010) to account for cluster effects of multiple implants when placed and evaluated in a single patient (repeated observations; Zeger and Liang, 1986; Stephenson et al., 2010).
Analyses were adjusted to the following potential confounders: sex, age, implant diameter, implant length, bone augmentation, smoking habit. These covariates were selected because of their associations with bone status or dental implant survival rate and have been controlled for in studies of similar design (Verdel et al., 2010). Statistical analysis was performed with the software SPSS 19.0 and STATA13 for Windows. The results were considered statistically significant if the corresponding p value was < .05. Post hoc power calculation was done with Cohen’s F test. Kaplan-Meier survival curves were plotted for the primary outcome “dental implant failure.”
Results
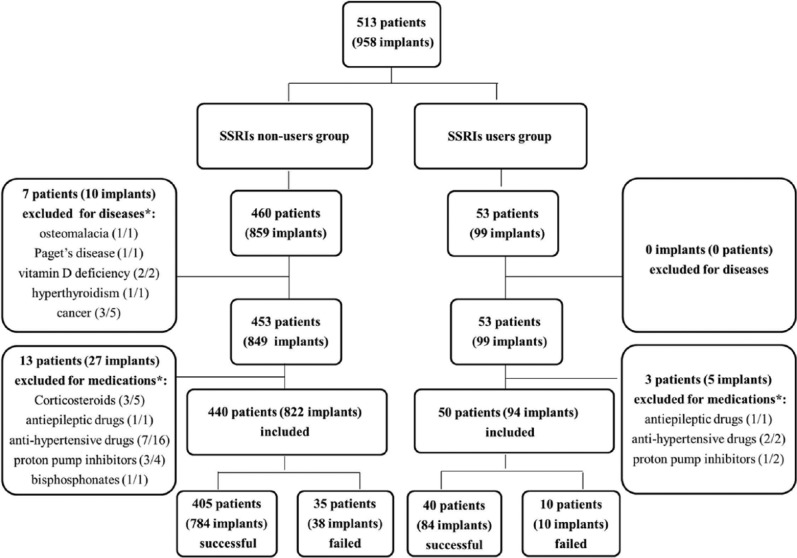
During the study period between 2007 and 2013, 42 implants in 23 patients were excluded for bone-related diseases and medications (Fig. 1). In sum, the 490 patients who met our inclusion consisted of 292 women and 198 men, with ages spanning 17 to 93 yr, averaging 56.4 ± 13.7. A total of 916 dental implants were placed in the included patients, out of which 94 were placed in SSRI users whereas 822 were placed in SSRI nonusers. Also, 436 implants were placed in nonsmokers, whereas 54 were placed in smokers. Implants had diameters ranging from 3.0 to 5.5 mm, lengths ranging from 7.0 to 42.0 mm, and torque at insertion from 10 to 65 N·cm (Appendix Table 2). The healing period for all implants was ranging from 0 to 8 mo (5.1 ± 1.6). Other relevant information is shown in Appendix Tables 1 and 3.
Figure 1.
Flow diagram of participants.
*Patients/implants.
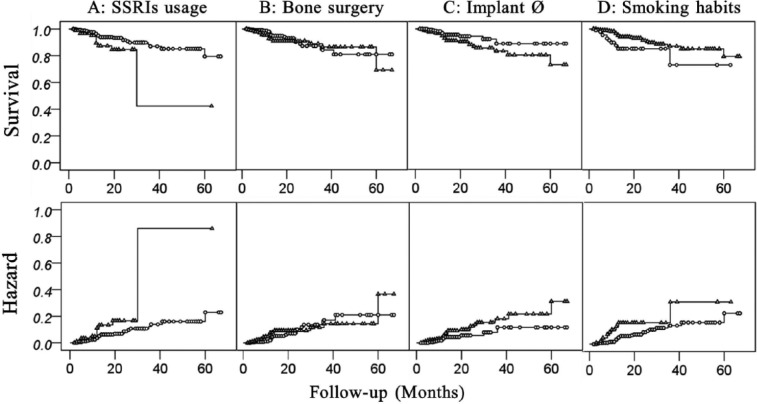
During the entire observation period, 868 implants survived and 48 failed. The failure rates were 4.6% for SSRI nonusers and 10.6% SSRI users. SSRI users and nonusers were comparable in terms of age, sex, bone augmentation, smoking habit, implant diameter, implant length, implant torque, and follow-up period (Table 1). Risk analysis confirmed our hypothesis by revealing that SSRI treatment (p = .03) was associated with an increased risk of implant failure (Table 2). Also, smoking habit (p = .01) and small (≤4 mm) implant diameter (p = .02) were associated with an increased risk of implant failure (Table 3). Multilevel survival analysis adjusted for potential confounding factors is shown in Table 1 and 2. Patient’s age, sex, bone augmentation, follow-up period, implant length, and torque had no significant association with implant survival rate (Table 3). The post hoc power was 0.93. Kaplan-Meier survival curves for dental implant failure in terms of SSRI use, bone augmentation, smoking habit, and implant diameter are shown in Figure 2.
Table 1.
Description of the Cohort by Implants (n = 916) among SSRI User and Nonusers
| SSRI Use, n (%) |
||||
|---|---|---|---|---|
| Variables | Yes | No | Odds Ratio (95% CI) | p |
| Age, yr | ||||
| ≤60 | 50 (53.2) | 480 (58.4) | 1 | |
| >60 | 40 (42.6) | 315 (38.3) | 1.22 (0.79-1.89) | .43 |
| Missing | 4 (4.2) | 27 (3.3) | 0.76 (0.26-2.23) | .62 |
| Sex | ||||
| Male | 32 (34.0) | 363 (44.2) | 1 | |
| Female | 62 (66.0) | 459 (55.8) | 0.65 (0.42-1.02) | .06 |
| Diabetes | ||||
| Yes | 5 (5.3) | 43 (2.8) | 1 | |
| No | 89 (94.7) | 779 (97.2) | 1.02 (0.39-2.64) | 1.00 |
| Smoking habits | ||||
| Yes | 12 (12.8) | 85 (10.3) | 1 | |
| No | 82 (86.2) | 737 (89.5) | 1.27 (0.67-2.42) | .48 |
| Bone regeneration | ||||
| Yes | 47 (50) | 339 (41.2) | 1 | |
| No | 47 (50) | 472 (57.4) | 1.39 (0.91-2.14) | .15 |
| Missing | 0 (0) | 11 (1.4) | 3.22 (0.19-55.50) | .42 |
| Implant diameter, mm | ||||
| >4 | 32 (34.0) | 326 (39.7) | 1 | |
| ≤4 | 61 (64.9) | 445 (54.1) | 0.72 (0.46-1.12) | .15 |
| Missing | 1 (1.1) | 51 (6.2) | 6.15 (0.84-45.04) | .07 |
| Implant length, mm | ||||
| >10 | 68 (72.3) | 586 (71.3) | 1 | |
| ≤10 | 25 (26.6) | 186 (22.6) | 0.86 (0.53-1.41) | .61 |
| Missing | 1 (1.1) | 50 (6.1) | 6.02 (0.82-44.11) | .07 |
| Implant torque, N·cm | ||||
| ≥35 | 36 (38.3) | 263 (32.0) | 1 | |
| <35 | 44 (46.8) | 423 (51.5) | 0.76 (0.48-1.21) | .28 |
| Missing | 14 (14.9) | 136 (16.5) | 1.13 (0.62-2.06) | .68 |
| Implant loading time | ||||
| Immediate | 3 (3.0) | 34 (4.1) | 1 | |
| Delayed | 91 (97.0) | 736 (89.5) | 0.71 (0.21-2.37) | .58 |
| Missing | 0 | 52 (6.4) | 10.65 (0.53-212.72) | .12 |
| Follow-up time, mo | ||||
| ≥12 | 42 (44.7) | 336 (40.4) | 1 | |
| <12 | 52 (55.3) | 485 (59.5) | 1.17 (0.76-1.79) | .51 |
| Missing | 0 (0) | 1 (0.1) | 0.35 (0.01-8.53) | .52 |
| Parafunctional habitsa | ||||
| No | 91 (97.0) | 801 (97.4) | 1 | |
| Yes | 3 (3.0) | 21 (2.6) | 1.26 (0.37-4.30) | .73 |
| Implant position | ||||
| Maxilla | 65 (69.1) | 571 (69.5) | 1 | |
| Mandibular | 29 (30.9) | 251 (30.5) | 1.02 (0.64-1.61) | .52 |
CI, confidence interval; SSRI, selective serotonin reuptake inhibitor.
Parafunctional habits include bruxism, attrition, and temporomandibular disorders.
Table 2.
Implant-based Comparison between SSRI Group and Nonuser Group
| Implants |
p
|
|||||
|---|---|---|---|---|---|---|
| SSRI | Successful | Failed | Failure Rate, % | GEE | Multilevel | HRa (95% CI) |
| No | 784 | 38 | 4.6 | — | — | — |
| Yes | 84 | 10 | 10.6 | .004* | 0.03* | 6.28 (1.25-31.61) |
CI, confidence interval; GEE, generalized estimating equation; HR, hazard ratio; multilevel, multilevel mixed effects parametric survival analysis; SSRI, selective serotonin reuptake inhibitor.
HRs were performed with multilevel mixed effects parametric survival analysis adjusted to the following factors: sex, age, implant diameter, implant length, bone augmentation, smoking.
Statistically significant.
Table 3.
Risk Analysis for Dental Implant Failure in Terms of Different Factors
| Implants, n (%) |
p
|
|||||
|---|---|---|---|---|---|---|
| Factor | Successful | Failed | Failure Rate, % | GEE | Multilevel | HRa (95% CI) |
| Sex | ||||||
| Male | 375 (43.1) | 20 (42.9) | 5.1 | |||
| Female | 493 (56.9) | 28 (57.1) | 5.4 | .98 | .42 | 1.62 (0.50-5.28) |
| Age, yr | ||||||
| >60 | 338 (40.0) | 17 (34.7) | 4.8 | |||
| ≤60 | 500 (57.6) | 30 (63.3) | 5.7 | .57 | .88 | 0.91 (0.27-3.12) |
| Missing | 30 (2.4) | 1 (2.0) | 3.2 | |||
| Implant diameter, mm | ||||||
| ≤4 | 472 (54.4) | 34 (69.4) | 6.7 | |||
| >4 | 345 (39.7) | 13 (28.6) | 3.6 | .01* | .02* | 0.24 (0.07-0.78) |
| Missing | 51 (5.9) | 1 (2.0) | 1.9 | |||
| Implant length, mm | ||||||
| ≤10 | 201 (23.2) | 10 (20.4) | 4.7 | |||
| >10 | 617 (71.0) | 37 (77.6) | 5.7 | .58 | .97 | 0.98 (0.34-2.80) |
| Missing | 50 (5.8) | 1 (2.0) | 2.0 | |||
| Implant torque, N·cm | ||||||
| <35 | 283 (32.2) | 16 (32.7) | 5.4 | |||
| ≥35 | 442 (51.3) | 25 (53.1) | 5.4 | .81 | NA | NA |
| Missing | 143 (16.5) | 7 (14.2) | 4.7 | |||
| Implant loading | ||||||
| Immediate | 33 (3.8) | 4 (8.2) | 10.8 | |||
| Delayed | 783 (90.2) | 44 (91.8) | 5.3 | .74 | NA | NA |
| Missing | 52 (6.0) | 0 (0) | 0 | |||
| Bone augmentation | ||||||
| No | 498 (57.4) | 21 (42.9) | 4.0 | |||
| Yes | 359 (41.3) | 27 (57.1) | 7.0 | .04* | .05 | 2.73 (0.99-7.51) |
| Missing | 11 (1.3) | 0 (0) | 0 | |||
| Smoking habits | ||||||
| No | 782 (90.1) | 37 (77.1) | 4.3 | |||
| Yes | 86 (9.9) | 11 (22.9) | 11.3 | .004* | .01* | 7.66 (1.67-35.09) |
| Follow-up time, mo | ||||||
| <12 | 508 (58.5) | 29 (60.4) | 5.4 | |||
| ≥12 | 359 (41.4) | 19 (39.6) | 5.0 | .71 | NA | NA |
| Missing | 1 (0.1) | 0 (0) | 0 | |||
CI, confidence interval; GEE, generalized estimating equation; HR, hazard ratio; multilevel: multilevel mixed effects parametric survival analysis; NA, not applicable.
HRs were performed with multilevel mixed effects parametric survival analysis adjusted to the following factors: sex, age, implant diameter, implant length, bone augmentation, smoking.
Statistically significant.
Figure 2.
Kaplan-Meier hazard curves and survival curves for dental implant failure in terms of (A) SSRI (selective serotonin reuptake inhibitor) usage (Δ: usage, ο: nonusage), (B) bone surgery (Δ: yes, ο: no), (C) implant ∅ (Δ: <4 mm, ο: ≥4 mm), and (D) smoking habits (Δ: smoker, ο: nonsmoker).
Discussion
Our hypothesis was confirmed by the present study, showing through a multivariate analysis that SSRI usage, as well as other factors, increases the risk of osseointegrated dental implant failure. Each of these factors is discussed in detail.
SSRIs and Dental Implant Failure
Our study focused on the possible association of SSRI treatment with increased dental implant failure. In Table 2, we show that SSRIs have a significant association with higher risk of dental implant failure. Despite the fact that there were no significant differences between the group of SSRI users and nonusers in terms of systemic and demographic conditions (Table 1), SSRI users were more susceptible (hazard ratio, 6.28; 95% confidence interval, 1.25-31.61) to implant failures than nonusers.
Osseointegrated implant failure is usually caused by failed osseointegration, peri-implantitis, mechanical overloading (Esposito et al., 1998), or a combination of these factors (Tonetti and Schmid, 1994). Early failures, occurring weeks to a few months after implant placement (Tonetti and Schmid, 1994), often result from impaired healing (Esposito et al., 1998), implant contamination, or lack of mechanical stability (Tonetti and Schmid, 1994). Late failures are frequently caused by peri-implantitis (plaque-induced progressive marginal bone loss) mainly occurring after two-year follow-up (Charalampakis et al., 2012). The failures caused by mechanical overloading usually occur after the loading time of 4 and 6 mo (Esposito et al., 1998). In our study, the stratification of the follow-up period in Kaplan-Maier curves showed that failures occurred mostly between 4 and 14 mo (8 failed cases out of 10) after implant placement. Implants placed in SSRI users had favorable primary mechanical stability (torque: 29.6 ± 8.8 N·cm), acceptable bone quality and quantity, appropriate implant dimensions, and good early healing (all implants were loaded; Table 1). Therefore, the main reason causing implant failure by SSRIs was probably associated with problems in the mechanical loading of the implants. This is in agreement with previous in vivo studies demonstrating that serotonin plays an important role in the anabolic response of bone to mechanical loading (Sibilia et al., 2013). This study indicates that SSRIs might cause bone mass loss by inhibiting the bone-remodeling processes triggered by mechanical loading. Accordingly, SSRIs might also be impairing bone remodeling around functional implants, although this hypothesis will require further mechanistic experiments to be confirmed.
Inappropriate response to mechanical loading can be the possible cause of “the after-loading failures.” Future studies are needed to confirm the hypothesis. However, the effect of SSRIs on the risk of implants failures in our study can to some extent lead to careful surgical planning in SSRI users.
Bone Augmentation and Dental Implant Failure
In our study, bone augmentation seemed to be associated with higher dental implant failure in GEE analysis, but the association was not significant on the basis of multilevel mixed effects parametric survival analysis in STATA. Bone augmentation is essential for placement of implants when bone volume is insufficient. However, previous studies (Yamazaki et al., 2012) indicated that higher implant survival rate can be expected when there is no need for bone regeneration procedures. The negative impact might indicate that the quality and quantity of regenerated bone are often deficient (Yamazaki et al., 2012). Moreover, bone surgeries may require more maintenance of bone integrity and more firm immobilization after surgeries (Yamazaki et al., 2012).
Smoking Habit and Dental Implant Failure
In this study, we observed a significant increased risk of dental implant failure associated with smoking habits. This was in agreement with previous studies recognizing a higher rate of dental implant failure in smokers (odds ratios ranging from 3.6 to 4.6; Alsaadi et al., 2008), probably because smoking impairs bone healing after dental implant surgical treatment. The adverse effect during the early stage of osseointegration may be explained by the influence of smoking on the wound-healing process (Alsaadi et al., 2008) through a direct toxic effect (Krall and Dawson-Hughes, 1991) on the bone around implants. Smoking, especially nicotine, impairs new bone formation, reduces calcium absorption, and decreases bone mineral density transiently (Riebel et al., 1995).
Implant Dimensions and Dental Implant Failure
We demonstrated that smaller implant diameters were associated with higher risk of implant failure, which was confirmed by other studies (Davarpanah et al., 2000). The use of narrow-diameter implants has been proposed to avoid bone augmentation procedures and reduce surgical complexity (Davarpanah et al., 2000). However, they have less surface area for interaction and anchorage, which may lead to insufficient bone integration, as well as unfavorable distribution of biomechanical forces, causing reduced resistance to fracture (Davarpanah et al., 2000). In our study, we did not observe a significant association between short implant length and increased failure. The implant length may be a factor in survival (Porter and von Fraunhofer, 2004), but in our study, it does not appear to be as critical as SSRI treatment, bone quality, smoking habits, and implant diameters.
Superiority and Limitations
To avoid bias, we had comparable control and experimental groups (Table 1) with sufficient sample size. We performed a comprehensive statistical analysis adjusted to multiple confounders with sufficient power and used GEE models and multilevel mixed effects parametric survival analysis to solve data cluster. Furthermore, the surgeries for all included patients were carried out by a single surgeon, avoiding most of the personal bias and operation variances.
However, there were still several factors that could not be assessed in the study. Because of the lack of detailed information, we were not able to adjust to the degree of depression (Verdel et al., 2010), which might be a predictor for implant success rate. Within the limit of our knowledge, there is no evidence in the literature on whether depression is a risk factor for implant failure or oral complications. Lack of information about oral hygiene as dental implant maintenance was one of our limitations (Porter and von Fraunhofer, 2004). Moreover, drug compliance dose and treatment period could not be acquired from the files of the patients. Further studies investigating the dose-effect relationship and the influence of the treatment duration should be carried out to analyze this phenomenon in more depth. Moreover, the aspect of dose-relevant effects on bone metabolism could be of interest for prospective investigations. Randomized clinical trials should also be carried out in the future to confirm our results, since there is selection bias, such as confounding by indication, missing clinical data, and the risk of underreporting data in cohort studies.
Nevertheless, our study indeed, for the first time, indicated an association between SSRI treatment and higher risk of dental implant failure. Thus, this study might suggest careful surgical treatment planning for SSRI users.
Conclusion
Within the limits of our study, we can conclude that SSRI treatment is associated with higher risk of osseointegrated implant failure. Implant survival rate could also be significantly influenced by other factors, such as implant diameter, bone augmentation, and smoking habits.
Footnotes
The authors acknowledge the financial support from the China Scholarship Council, Clifford Wong Fellowship, Canadian Institutes of Health Research, Institute of Musculoskeletal Health and Arthritis Bridge Funding , and Le Réseau de recherche en santé buccodentaire et osseuse, as well as the staff at East Coast Oral Surgery for their help and support.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Details of Contributors: All authors substantially contributed to: study design (FT, XW), data collection (SAN, NGD, XW, KA, ER), data analysis and interpretation (XW, FT, BN, KA), manuscript writing (XW, FT, BN), and approval of the final version of the manuscript (XW, FT, BN, SAN, NGD, KA, ER). The authors take responsibility for the integrity of the data analysis.
References
- Albrektsson T, Brånemark P-I, Hansson H-A, Lindström J. (1981). Osseointegrated titanium implants: requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop 52:155-170. [DOI] [PubMed] [Google Scholar]
- Alsaadi G, Quirynen M, Komárek A, Van Steenberghe D. (2008). Impact of local and systemic factors on the incidence of late oral implant loss. Clin Oral Implants Res 19:670-676. [DOI] [PubMed] [Google Scholar]
- Battaglino R, Fu J, Späte U, Ersoy U, Joe M, Sedaghat L, et al. (2004). Serotonin regulates osteoclast differentiation through its transporter. J Bone Miner Res 19:1420-1431. [DOI] [PubMed] [Google Scholar]
- Carlsson L, Röstlund T, Albrektsson B, Albrektsson T, Brånemark PI. (1986). Osseointegration of titanium implants. Acta Orthop 57:285-289. [DOI] [PubMed] [Google Scholar]
- Charalampakis G, Leonhardt Å, Rabe P, Dahlén G. (2012). Clinical and microbiological characteristics of peri-implantitis cases: a retrospective multicentre study. Clin Oral Implants Res 23:1045-1054. [DOI] [PubMed] [Google Scholar]
- Davarpanah M, Martinez H, Tecucianu JF, Celletti R, Lazzara R. (2000). Small diameter implants: indications and contraindications. J Esthet Restor Dent 12:186-194. [DOI] [PubMed] [Google Scholar]
- Del Valle V, Faulkner G, Wolfaardt J, Rangert B, Tan HK. (1995). Mechanical evaluation of craniofacial osseointegration retention systems. Int J Oral Maxillofac Implants 10:491-498. [PubMed] [Google Scholar]
- Diem SJ, Blackwell TL, Stone KL, Yaffe K, Haney EM, Bliziotes MM, et al. (2007). Use of antidepressants and rates of hip bone loss in older women: the study of osteoporotic fractures. Arch Intern Med 167:1240-1245. [DOI] [PubMed] [Google Scholar]
- Esposito M, Hirsch JM, Lekholm U, Thomsen P. (1998). Biological factors contributing to failures of osseointegrated oral implants (II): etiopathogenesis. Eur J Oral Sci 106:721-764. [DOI] [PubMed] [Google Scholar]
- Finkemeier CG. (2002). Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am 84:454-464. [DOI] [PubMed] [Google Scholar]
- Gustafsson B, Thommesen L, Stunes AK, Tommeras K, Westbroek I, Waldum H, et al. (2006). Serotonin and fluoxetine modulate bone cell function in vitro. J Cell Biochem 98:139-151. [DOI] [PubMed] [Google Scholar]
- Kemp F. (2003). Applied multiple regression/correlation analysis for the behavioral sciences. J R Stat Soc Series B Stat 52:691. [Google Scholar]
- Krall EA, Dawson-Hughes B. (1991). Smoking and bone loss among postmenopausal women. J Bone Miner Res 6:331-338. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. (2008). The molecular neurobiology of depression. Nature 455:894-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Anderson G, Mittmann N, To T, Axcell T, Shear N. (1998). Use of selective serotonin-reuptake inhibitors or tricyclic antidepressants and risk of hip fractures in elderly people. Lancet 351:1303-1307. [DOI] [PubMed] [Google Scholar]
- Misch CE, Perel ML, Wang HL, Sammartino G, Galindo-Moreno P, Trisi P, et al. (2008). Implant success, survival, and failure: the International Congress of Oral Implantologists (ICOI) pisa consensus conference. Implant Dent 17:5-15. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. (1997). Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet 349:1436-1442. [DOI] [PubMed] [Google Scholar]
- Pjetursson BE, Thoma D, Jung R, Zwahlen M, Zembic A. (2012). A systematic review of the survival and complication rates of implant-supported fixed dental prostheses (FDPs) after a mean observation period of at least 5 years. Clin Oral Implants Res 23(Suppl 6):22-38. [DOI] [PubMed] [Google Scholar]
- Porter JA, von Fraunhofer JA. (2004). Success or failure of dental implants? A literature review with treatment considerations. Gen Dent 53:423-432. [PubMed] [Google Scholar]
- Riebel GD, Boden SD, Whitesides TE, Hutton WC. (1995). The effect of nicotine on incorporation of cancellous bone graft in an animal model. Spine (Phila Pa 1976) 20:2198-2202. [DOI] [PubMed] [Google Scholar]
- Schep N, Heintjes R, Martens E, van Dortmont L, Van Vugt A. (2004). Retrospective analysis of factors influencing the operative result after percutaneous osteosynthesis of intracapsular femoral neck fractures. Injury 35:1003-1009. [DOI] [PubMed] [Google Scholar]
- Sibilia V, Pagani F, Dieci E, Mrak E, Marchese M, Zarattini G, et al. (2013). Dietary tryptophan manipulation reveals a central role for serotonin in the anabolic response of appendicular skeleton to physical activity in rats. Endocrine 44:790-802. [DOI] [PubMed] [Google Scholar]
- Stephenson J, Chadwick B, Playle R, Treasure E. (2010). Modelling childhood caries using parametric competing risks survival analysis methods for clustered data. Caries Res 44:69-80. [DOI] [PubMed] [Google Scholar]
- Tamimi I, Ojea T, Sanchez-Siles JM, Rojas F, Martin I, Gormaz I, et al. (2012). Acetylcholinesterase inhibitors and the risk of hip fracture in Alzheimer’s disease patients: a case-control study. J Bone Miner Res 27:1518-1527. [DOI] [PubMed] [Google Scholar]
- Tonetti MS, Schmid J. (1994). Pathogenesis of implant failures. Periodontol 2000. 4: 127-138. [DOI] [PubMed] [Google Scholar]
- Tonetti MS, Pini-Prato G, Cortellini P. (1995). Effect of cigarette smoking on periodontal healing following GTR in infrabony defects. J Clin Periodontol 22:229-234. [DOI] [PubMed] [Google Scholar]
- Tsapakis E, Gamie Z, Tran G, Adshead S, Lampard A, Mantalaris A, et al. (2012). The adverse skeletal effects of selective serotonin reuptake inhibitors. Eur Psychiatry 27:156-169. [DOI] [PubMed] [Google Scholar]
- Verdel BM, Souverein PC, Egberts TC, Van Staa TP, Leufkens HG, de Vries F. (2010). Use of antidepressant drugs and risk of osteoporotic and non-osteoporotic fractures. Bone 47:604-609. [DOI] [PubMed] [Google Scholar]
- Wong M, Eulenberger J, Schenk R, Hunziker E. (1995). Effect of surface topology on the osseointegration of implant materials in trabecular bone. J Biomed Mater Res 29:1567-1575. [DOI] [PubMed] [Google Scholar]
- Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, Schütz G, et al. (2008). Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell 135:825-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki M, Kanzaki S, Tominaga K, Miyamoto I, Yamauchi K, Fukuda M, et al. (2012). Evaluation of secondary bone grafting of the alveolar cleft in adult cleft lip and palate patients. J Oral Maxillofac Surg Med Pathol 24:86-89. [Google Scholar]
- Zeger SL, Liang KY. (1986). Longitudinal data analysis for discrete and continuous outcomes. Biometrics 42:121-130. [PubMed] [Google Scholar]