Abstract
Stem cells from human exfoliated deciduous teeth (SHED) are a unique postnatal stem cell population, possessing multipotent differentiation capacity and immunomodulatory properties. However, the mechanism by which SHED treat immune diseases is not fully understood. Here we show that systemic transplantation of SHED via the tail vein ameliorates ovariectomy (OVX)-induced osteopenia by reducing T-helper 1 (Th1) and T-helper 17 (Th17) cell numbers in the recipient OVX mice. Mechanistically, SHED transplantation induces activated T-cell apoptosis in OVX mice via Fas ligand (FasL)-mediated Fas pathway activation, leading to up-regulation of regulatory T-cells (Tregs) and down-regulation of Th1 and Th17 cells. This SHED-mediated immunomodulation rescues OVX-induced impairment of bone marrow mesenchymal stem cells (BMMSCs) and activation of osteoclastogenesis, resulting in increased bone mass. In summary, SHED-mediated T-cell apoptosis via a FasL/Fas pathway results in immune tolerance and ameliorates the osteopenia phenotype in OVX mice.
Keywords: immunotherapy, deciduous teeth, mesenchymal stem cells, apoptosis, Fas ligand, regulatory T cells (Tregs)
Introduction
Osteoporosis is a disease characterized by systemic loss of bone mass and structure that results in fragility fractures (Raisz, 2005). Osteoporosis is associated with orofacial disorders, such as periodontal disease and alveolar bone loss (Hildebolt, 1997). Estrogen-deficient osteoporosis is the most common and significant form of postmenopausal osteoporosis. Multiple factors are implicated in postmenopausal osteoporosis, and the mechanisms involved are not fully understood (Weitzmann and Pacifici, 2006; Schett and David, 2010). Overactivated T-lymphocytes may secrete inflammatory cytokines to promote osteoclast differentiation in the bone remodeling process, accelerating bone resorption (Cenci et al., 2000; Roggia et al., 2001; Gao et al., 2007). Additionally, immune components may impair bone marrow mesenchymal stem cells (BMMSCs) and cause osteogenic deficiency (Yamaza et al., 2008). Thus, regulation of the immune system may be able to provide appropriate therapy for osteoporosis.
Stem cells from human exfoliated deciduous teeth (SHED) have been identified as a population of postnatal stem cells with the capacity to differentiate into osteogenic/odontogenic cells, adipocytes, and neural cells (Miura et al., 2003). SHED possess the capacity to regenerate bone and repair craniofacial defects, as has been demonstrated in an animal model (Seo et al., 2008; Zheng et al., 2009); furthermore, they can inhibit Th17 cells when transplanted systemically, thereby ameliorating systemic lupus erythematosus (SLE)-associated disorders in MRL/Lpr mice (Yamaza et al., 2010). Ovariectomized (OVX) mice represent a reliable estrogen-deficient osteoporosis animal model. The femur metaphysis is the area most sensitive to estrogen deficiency. Elevated osteoclast number and activity are responsible for the initial trabecular bone loss in the acute phase in OVX mice, while there is no apparent change in osteoclast activity in the chronic phase (Jee and Yao, 2001). Estrogen-deficiency-induced reduction of osteoclast apoptosis and acceleration of osteoclast activity were observed in postmenopausal osteoporosis conditions (Raisz, 2005). In addition, significantly reduced BMMSC function also contributes to OVX-induced osteoporosis (Yamaza et al., 2010; Wang et al., 2013). In this study, we attempted to test whether one-time transplantation of SHED could prevent the OVX-induced early osteoporotic phenotype. Our findings suggest that SHED-mediated T-cell apoptosis via a FasL/Fas pathway results in immune tolerance and ameliorates the osteopenia phenotype in OVX mice.
Materials & Methods
Animals
C3H/HeJ mice (females, 8 wk old) were purchased from Jackson Lab (Bar Harbor, ME, USA). The OVX procedure was performed on 10-week-old C3H/HeJ mice; age-matched C3H/HeJ mice receiving a sham operation served as the controls (n = 5) (Kitazawa et al., 1994). We injected 2×105 SHED or human BMMSCs into OVX mice via the tail vein at day 3 post-OVX, and the mice were sacrificed at 4 wk post-OVX for further examination. Beige nude/nude Xid (III) mice (females, 10 wk old) were purchased from Harlan (Indianapolis, IN, USA). All animal experiments were performed under institutionally approved protocols for the use of animal research (University of Southern California #10941, 11141, and 11327).
Antibodies and Reagents
All antibodies and reagents used in this study are described in the Appendix.
Enzyme-linked Immunosorbent (ELISA) Assay
Serum markers of bone turnover, including collagen X link-1 (CTX-1), tartrate-resistant acid phosphatase 5b (TRAP 5b), receptor activator of nuclear factor kappa-B ligand (RANKL), and osteoprotegerin (OPG), were measured with ELISA kits purchased from R&D Systems (Minneapolis, MN, USA) and IDS (Scottsdale, AZ, USA), according to the manufacturers’ instructions.
MicroCT Analysis
MicroCT analysis was performed as reported previously (Bouxsein et al., 2010). Detailed methods are described in the Appendix.
Histological and Histomorphometric Analysis
Femurs were fixed with 4% paraformaldehyde, followed by decalcification with 10% EDTA (pH 8.0), and embedded in paraffin. For histological and histomorphometric analysis, sections were deparaffinized and stained with hematoxylin and eosin (H&E), followed by calculation of trabecular bone volume percentage (Tb percentage, %) with ImageJ software (NIH, Bethesda, MD, USA). Tartrate-resistant acid phosphate (TRAP) staining was performed according to a previous report (Chiang et al., 1999). TRAP staining images were acquired from the metaphyseal region of the femur. For quantification of bone resorption, 5 representative images were analyzed with ImageJ software. The results were shown as the number of osteoclasts per mm of bone surface area (N.Oc/BS).
Isolation and Culture of SHED and Human BMMSCs
SHED and human BMMSCs (hBMMSC) were isolated and cultured as described in the Appendix.
Isolation and Culture of Mouse BMMSCs
Mouse BMMSCs (mBMMSC) were isolated and cultured as described in the Appendix.
Implantation of mBMMSCs into Immunocompromised Mice
We mixed 4.0×106 mBMMSCs from OVX, OVX/SHED-treated, OVX/hBMMSC-treated, or sham-treated mice with 40 mg of hydroxyapatite/tricalcium phosphate (HA/TCP) ceramic powder (Zimmer Inc., Warsaw, IN, USA) and subsequently implanted into the dorsal surfaces of 10-week-old immunocompromised mice as previously described (Miura et al., 2004). These procedures were performed in accordance with specifications of an approved small-animal protocol (USC#10874). The implants were harvested at 8 wk post-transplantation, fixed in 4% paraformaldehyde, and then decalcified with 10% EDTA (pH 8.0) for paraffin embedding. Paraffin sections were deparaffinized, rehydrated, and stained with hematoxylin and eosin (H&E). NIH software Image J was used for semi-quantitative analysis of new bone formation.
Cell Proliferation Assay
Cell proliferation assay was performed as described in the Appendix.
In vitro Osteogenic Differentiation Assay
Detailed methods are described in the Appendix.
In vitro Adipogenic Culture Conditions
Detailed methods are described in the Appendix.
Western Blot Analysis
Western blot analysis was performed as described in the Appendix.
Flow Cytometric Analysis
The detailed method of flow cytometric analysis is described in the Appendix.
T-lymphocyte Apoptosis Assay
The T-lymphocyte apoptosis assay was performed as described in the Appendix.
Osteoclast Formation and Co-culture of SHED with Osteoclasts
Detailed methods are described in the Appendix.
Statistics
SPSS 13.0 was used to perform statistical analysis. Comparisons between 2 groups were analyzed by independent two-tailed Student’s t tests, and comparisons between more than 2 groups were analyzed by one-way ANOVA. P values < .05 were considered statistically significant.
Results
One-time Infusion of SHED Prevented OVX-induced Early Bone Loss
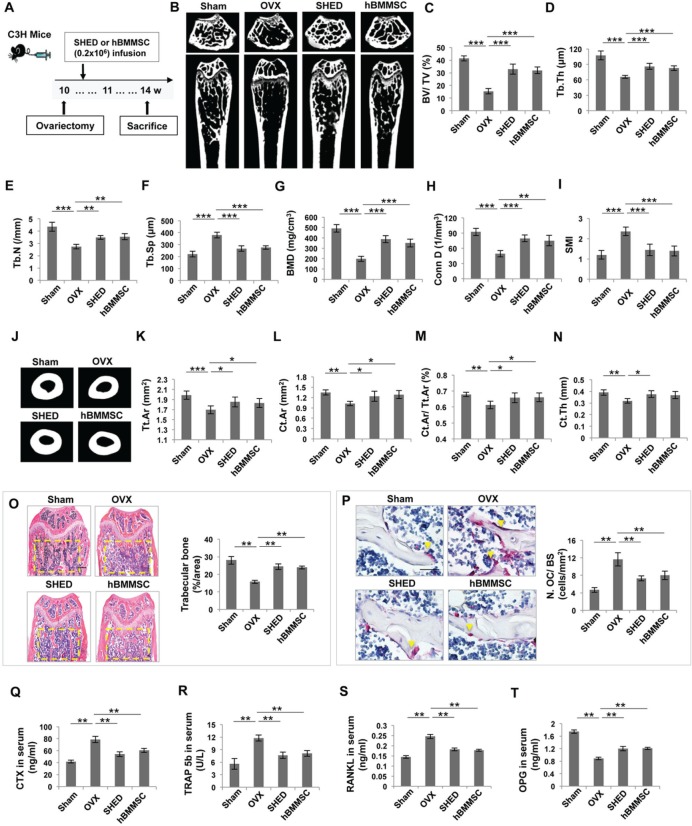
To determine whether transplantation of SHED ameliorates the osteoporotic phenotype, we infused SHED into OVX mice and analyzed the effects of treatment at 14 wk of age (Fig. 1A). It has been reported that the distal metaphysis of the femur is the area most responsive to estrogen deficiency (Jee and Yao, 2001). μCT analysis indicated that OVX induced significant bone loss in the trabecular bone of the distal femur metaphysis when compared with the sham group, as shown by decreased bone volume (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), bone mineral density (BMD), and connectivity density (Conn.D), along with increased trabecular space (Tb.Sp) and structure model index (SMI) in OVX mice (Fig. 1B-1I). In addition, we found that cortical bone mass was decreased in OVX mice, as shown by significantly decreased cortical bone parameters, including reduced total cross-sectional area (Tt.Ar), cortical bone area (Ct.Ar), cortical bone fraction (Ct.Ar/Tt.Ar), and cortical thickness (Ct.Th) (Figs. 1J-1N). μCT analysis also showed that SHED transplantation resulted in a marked increase in BV/TV (≈ 100%), Tb.Th (≈ 30%), Tb.N (≈ 25%), BMD (≈ 100%), and Conn.D (≈ 60%), along with decreased Tb.sp (≈ 30%) and SMI (≈ 40%) when compared with OVX mice (Figs. 1B-1I). Furthermore, SHED transplantation caused a slight, but significant, increase in the cortical bone parameters, including Tt.Ar (≈ 9%), Ct.Ar (≈ 20%), Ct.Ar/Tt.Ar (≈ 7%), and Ct.Th (≈ 20%) (Figs. 1J-1N). hBMMSC transplantation was similar to SHED transplantation in rescuing OVX-induced bone loss (Figs. 1B-1N). Histological analysis revealed that the trabecular bone volume in SHED- and hBMMSC-treated OVX mice was markedly elevated compared with that in OVX mice (Fig. 1O). Moreover, SHED and hBMMSC transplantation significantly reduced the number of osteoclasts in the distal femur metaphysis in OVX mice, as indicated by a decreased number of tartrate-resistant acid phosphatase (TRAP)-positive cells (Fig. 1P). To further assess the impact of SHED and hBMMSC transplantation on osteoclasts, we examined serum levels of CTX-1 and TRAP5b and found that SHED and hBMMSC transplantation significantly reduced the levels of CTX-1 (≈ 30%) and TRAP 5b (≈ 35%) in OVX mice (Figs. 1Q, 1R). Analysis of these data indicated that SHED and hBMMSC transplantation may inhibit osteoclast activity. Analysis of ELISA data also revealed that transplantation of SHED down-regulated the level of RANKL (≈ 25%) and up-regulated the level of OPG (≈ 35%) in OVX mouse serum (Figs. 1S, 1T). Analysis of these data suggested that systemic infusion of SHED is an effective therapeutic approach for OVX-induced bone loss.
Figure 1.
SHED transplantation blocked early osteoporotic phenotype development in OVX mice. (A) Schema indicating the experimental design for SHED or hBMMSC transplantation in OVX mice. (B) μCT images of trabecular bone structure in the femurs of sham, OVX, SHED-transplanted, and hBMMSC-transplanted mice. OVX mice showed a reduced trabecular bone volume compared with that in the sham group. SHED and hBMMSC transplantation partially rescued OVX-induced reduction of trabecular bone volume. (C-I) μCT analysis showed that OVX induced reduced bone volume (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), bone mineral density (BMD), and connectivity density (Conn.D), along with increased trabecular space (Tb.Sp) and structure model index (SMI) in the femurs. (J) μCT analysis showed that the cortical bone volume in the femur was reduced in OVX mice. SHED and hBMMSC transplantation increased the cortical bone volume in the femurs of OVX mice. (K-N) μCT analysis showed that OVX resulted in reduced total cross-sectional area (Tt.Ar), cortical bone area (Ct.Ar), cortical bone fraction (Ct.Ar/Tt.Ar), and cortical thickness (Ct.Th) in the femurs of OVX mice. SHED and hBMMSC transplantation significantly increased Tt.Ar, Ct.Ar, Ct.Ar/Tt.Ar, and Ct.Th in OVX mice. (O) Hematoxylin and eosin (H&E) staining showed that OVX resulted in reduced trabecular bone area (yellow circled area) compared with the sham mice, as analyzed by ImageJ software. SHED and hBMMSC transplantation significantly increased the trabecular bone area. (P) TRAP staining showed that OVX resulted in increased TRAP+ cells (yellow arrows) in the femurs of OVX mice. SHED and hBMMSC transplantation reduced the number of TRAP+ cells in the femurs of OVX mice. (Q-R) The levels of CTX-1, TRAP 5b, and RANKL in serum were markedly decreased in both SHED and hBMMSC transplantation groups compared with the “OVX without transplantation” group, whereas OPG levels were markedly increased in both treatment groups. n = 5 in each group. Scale bar = 200 μm; *p < .05; **p < .01; Error bars: mean ± SD.
SHED Transplantation Restored the Biphasic Changes of T-cells in OVX Mice
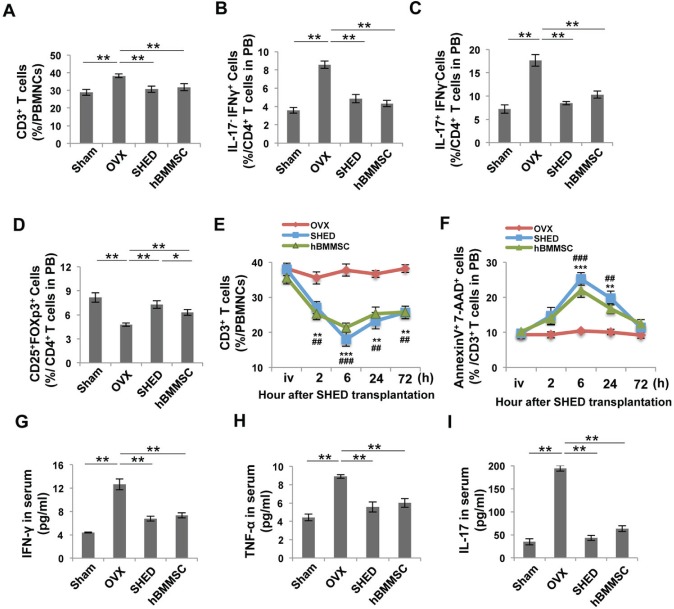
Recently, increasing evidence has indicated that immunomodulatory properties are important characteristics of BMMSCs, which make them an effective resource for treatment of immune disorders (Aggarwal and Pittenger, 2005; Nauta and Fibbe, 2007; Uccelli et al, 2008). BMMSC transplantation is able to inhibit T-cell activation and proliferation, leading to immune tolerance and thereby ameliorating autoimmune diseases (Ghannam et al., 2010; Akiyama et al., 2012). OVX mice are estrogen-deficient and provide an animal model for postmenopausal osteoporosis. The mechanism by which estrogen deficiency triggers osteoporosis is associated with T-cell overactivation and increased pro-inflammatory cytokines (Cenci et al., 2003; Yamaza et al., 2008; Li et al., 2011). Flow cytometric analysis confirmed that OVX increased the number of CD3+ T-cells (≈ 25%) compared with the sham-operated group (Fig. 2A). Moreover, OVX resulted in a biphasic change of T-subtype cells in which CD4+IL17-INFγ+ T-helper 1 (Th1) cells and CD4+IL17+INFγ- T-helper 17 (Th17) cells were up-regulated (≈ 140%) and CD4+CD25+Foxp3+ regulatory T-cells (Tregs) were down-regulated (≈ 40%) (Figs. 2B-2D). Th1 and Th17, subsets of CD4+ T-helper cells, are capable of producing pro-inflammatory cytokines, such as interferon-γ (IFN-γ), tumor necrosis factor α (TNF-α), and interleukin-17 (IL-17). Tregs, known as suppressor T-cells, play an important role in modulating immune response and maintaining tolerance. In this study, we investigated how the immunomodulatory properties of SHED may counteract this phenotype. To learn whether and how SHED transplantation modulates the immune system in OVX mice, we collected peripheral blood, bone marrow, and spleen samples. SHED transplantation significantly reduced the number of CD3+ T-cells and increased the number of apoptotic CD3+ T-cells starting at 2 h, reaching a peak at 6 h, and diminishing at 72 h post-transplantation (Figs. 2E, 2F). At 4 wk post-treatment, flow cytometric analysis revealed that SHED and hBMMSC transplantation significantly down-regulated CD3+ T-cells (≈ 20%), Th1 cells (≈ 45%), and Th17 cells (≈ 50%), and up-regulated Tregs (≈ 50%) in peripheral blood compared with the untreated OVX group (Figs. 2A-2D). The biphasic changes in T-subtype cells in the bone marrow and spleen were confirmed by flow cytometric analysis (Appendix Figs. 1A-1F). OVX mice showed significantly increased levels of IFN-γ (≈ 180%), TNF-α (≈ 100%), and IL-17 (≈ 450%), as assessed by ELISA (Figs. 2G-2I). SHED transplantation markedly down-regulated the levels of IFN-γ (≈ 50%), TNF-α (≈ 40%), and IL-17 (≈80%) in OVX mice (Figs. 2G-2I). Analysis of these data indicated that SHED transplantation re-establishes T-cell homeostasis in OVX mice.
Figure 2.
SHED transplantation impeded changes in the T-cell subset in OVX mice. (A-D) Flow cytometric analysis showed that the percentage of CD3+ T-cells and the percentage of Th1 and Th17 cells in CD4+ T-cells were increased in peripheral blood of OVX mice. However, the percentage of Tregs in CD4+ T-cells was reduced. SHED and hBMMSC transplantation reduced the levels of CD3+ T-cells, Th1, and Th17 cells in OVX mice at 4 wk post-transplantation. SHED transplantation exhibited a stronger capacity to up-regulate the levels of Tregs in OVX mice compared with the hBMMSC transplantation group. (E, F) The percentage of CD3+ T-cells in peripheral blood was examined in a time-course by flow cytometric analysis. SHED or hBMMSC transplantation reduced the number of CD3+ T-cells and increased AnnexinV+7AAD+ apoptotic CD3+ T-cells in OVX mice, reaching a peak at 6 h. (G-I) ELISA analysis showed that OVX mice had elevated levels of IFN-γ, TNF-α, and IL-17 in peripheral blood when compared with the sham-operated control group. After SHED or hBMMSC transplantation, the levels of IFN-γ, TNF-α, and IL-17 were markedly reduced. N = 5 in each group; *p < .05; **p < .01; ***p < .005. Error bars: mean ± SD.
SHED Transplantation Rescued BMMSC Deficiency in OVX Mice
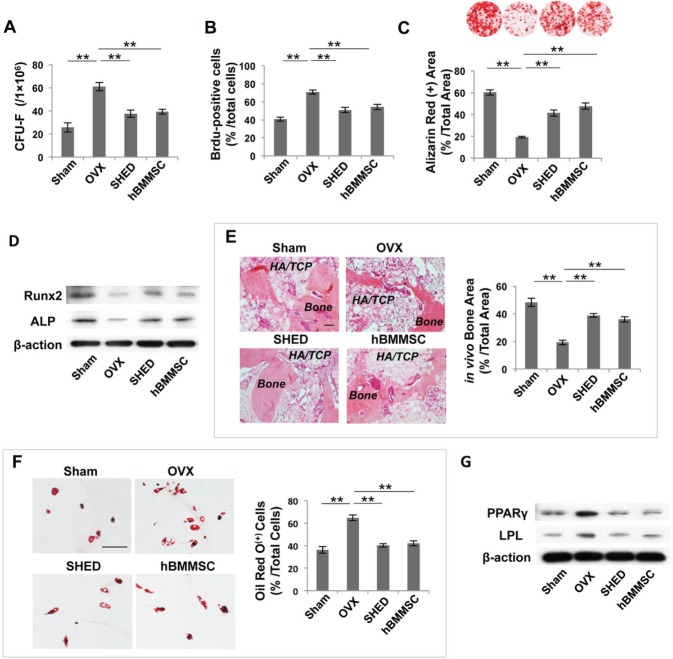
Our previous studies revealed that OVX-activated T-cells produced IFN-γ and TNF-α, which synergistically induced BMMSC deficiency via NFκB/SMAD7 signaling, partially accounting for reduced bone mineral density (Wang et al., 2013). To examine how OVX affects BMMSC function, we isolated mBMMSCs after transplantation at 14 wk of age and found that OVX resulted in a significantly increased number of CFU-F (≈ 140%) and Brdu-positive cells (≈ 70%), suggesting that the proliferation of mBMMSCs accelerated strongly in OVX mice (Figs. 3A, 3B). Investigating this deficiency further, we found that mBMMSCs derived from OVX mice showed a significant reduction in osteogenic differentiation, as indicated by decreased mineralized nodule formation (≈ 65%), assessed by Alizarin Red staining, and decreased expression of Runx2 and ALP, assessed by Western blot analysis (Figs. 3C, 3D). When implanted into immunocompromised mice, mBMMSCs derived from OVX mice exhibited reduced capacities to generate new bone (≈ 60%) (Fig. 3E). We hypothesized that SHED transplantation could rescue recipient BMMSC function by re-establishing T-cell homeostasis in OVX mice. Importantly, we revealed that SHED and hBMMSC transplantation reduced the number of CFU-F (≈ 40%) and proliferation rate (≈ 30%) of mBMMSCs in OVX mice (Figs. 3A, 3B). SHED and hBMMSC transplantation each significantly increased mineralized nodule formation (≈ 100%) and expression of Runx2 and ALP in recipient OVX-BMMSCs when cultured under osteogenic-inductive conditions (Figs. 3C, 3D). SHED and hBMMSC transplantation also elevated the new bone-forming capacity of recipient OVX-mBMMSCs, as assessed by increased bone formation area (≈ 100%), when implanted subcutaneously into immunocompromised mice (Fig. 3E). In addition, SHED and hBMMSC transplantation markedly reduced the adipogenic differentiation capacity of BMMSCs derived from OVX mice (Figs. 3F, 3G), as assessed by decreased Oil Red-positive cells (≈ 70%), and down-regulation of PPAPγ and LPL, as assessed by Western blot. The experimental evidence suggested that SHED transplantation is capable of rescuing the mBMMSC deficiency in OVX mice.
Figure 3.
SHED transplantation rescued BMMSC deficiency in OVX mice. (A) The number of CFU-F was markedly increased in OVX mice when compared with sham-operated control mice. SHED or hBMMSC transplantation markedly decreased the number of CFU-F. (B) The proliferation rate of mBMMSCs from the OVX group was significantly increased compared with that in the sham-operated control group, and SHED or hBMMSC transplantation reduced the number of Brdu-positive cells. (C) Alizarin Red staining showed that both SHED and hBMMSC transplantation increased mineralized nodule formation when compared with that in untreated OVX mice. (D) Western blot analysis showed that mBMMSCs derived from SHED-treated or hBMMSC-treated OVX mice expressed higher levels of Runx2 and ALP when cultured under osteogenic-inductive conditions. (E) When implanted into immunocompromised mice with HA/TCP as a carrier, mBMMSCs derived from OVX mice exhibited reduced capacities to generate new bone. After SHED or hBMMSC transplantation, mBMMSCs from the recipient mice showed increased capacity to form new bone. (F) SHED or hBMMSC transplantation decreased the adipogenic differentiation of mBMMSCs derived from recipient OVX mice when compared with mBMMSCs from untreated OVX mice, assessed by the decreased number of Oil Red O-positive cells. (G) Western blot analysis showed that the expression of adipogenic genes PPARγ and LPL was markedly decreased in mBMMSCs derived from SHED- or hBMMSC-treated OVX mice. n = 5 in each group. Scale bar = 200 μm; *p < .05; **p < .01; ***p < .005. Error bars: mean ± SD.
FasL/Fas Pathway Played a Vital Role in SHED-mediated T-cell Responses in OVX Mice
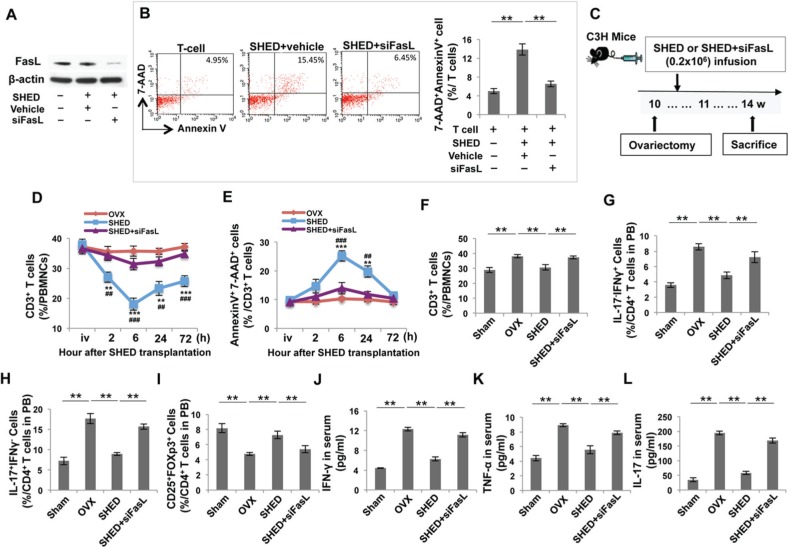
FasL is a type-II transmembrane protein that binds with Fas to form the death-inducing signaling complex (DISC). The FasL/Fas-mediated cell death pathway represents typical apoptotic signaling in many cell types (Hohlbaum et al., 2000; Pluchino et al., 2005; Zhang et al., 2008). BMMSCs express FasL and can induce T-cell apoptosis via the FasL/Fas pathway, which may play a crucial role in BMMSC-based immunomodulation (Akiyama et al., 2012; Chen et al., 2014). Thus, we hypothesized that FasL may be required for SHED-mediated immunotherapy in OVX mice. To test this hypothesis, we used the siRNA approach to knock down FasL expression in SHED (Fig. 4A). FasL knockdown resulted in a reduced capacity to induce activated T-cell apoptosis in a SHED/T-cell co-culture system, when compared with a control siRNA group (Fig. 4B). Next, we infused either SHED or FasL-knockdown SHED into OVX mice (Fig. 4C) and found that FasL-knockdown SHED had no significant effect in reducing the number of CD3+ T-cells and increasing the number of apoptotic CD3+ T-cells in OVX mice (Figs. 4D, 4E). FasL-knockdown SHED failed to down-regulate the levels of CD3+ T-Cells, Th1, and Th17 cells and also failed to up-regulate the levels of Tregs, as assessed by flow cytometric analysis (Figs. 4F-4I). Transplantation of FasL-knockdown SHED failed to reduce the levels of IFN-γ, TNF-α, and IL-17 in OVX mice in comparison with the untreated OVX group (Figs. 4J-4L).
Figure 4.
The FasL signaling pathway was required for SHED-mediated immunomodulation in OVX mice. (A) Western blot analysis showed that FasL siRNA knocked down FasL expression in SHED. (B) When co-cultured with T-cells, FasL knockdown SHED showed a reduced capacity to induce Annexin+ 7AAD+ double-positive apoptotic T-cells when compared with the control siRNA group. (C) Schema indicating the experimental design for SHED or FasL knockdown SHED transplantation and OVX procedure. (D, E) Flow cytometric analysis showed that SHED transplantation reduced the percentage of CD3+ T-cells in peripheral blood. FasL knockdown SHED failed to reduce the number of CD3+ T-cells and increased AnnexinV+7AAD+ apoptotic CD3+ T-cells at in OVX mice. (F-I) Flow cytometric analysis showed that SHED transplantation reduced the percentage of CD3+ T-cells and the percentage of Th1 and Th17 in CD4+ T-cells in peripheral blood at 4 wk post-transplantation. However, SHED transplantation increased the percentage of Tregs in CD4+ T-cells in peripheral blood. FasL knockdown SHED failed to reduce the levels of CD3+ T-cells, Th1, and Th17 cells and also failed to increase the levels of Tregs in OVX mice when compared with the SHED-treated OVX group. (J-L) ELISA analysis showed that FasL knockdown SHED failed to decrease the levels of IFN-γ, TNF-α, and IL-17 in the serum of peripheral blood when compared with the SHED-treated OVX group. n = 5 in each group; *p < .05; **p < .01; ***p < .005. Error bars: mean ± SD.
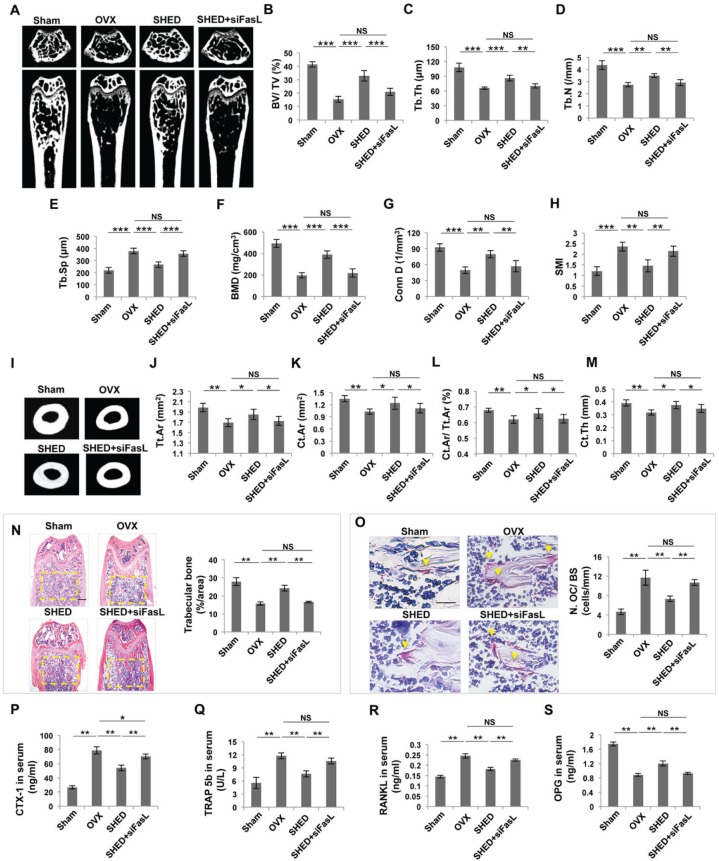
It has been reported that estrogen induces osteoclast apoptosis through the FasL/Fas pathway (Nakamura et al., 2007). We found that SHED, but not FasL knockdown SHED, were able to induce osteoclast apoptosis in a SHED/osteoclast co-culture system (Appendix Fig. 2). However, most of the transplanted SHED underwent apoptosis and became undetectable at 24 h post-transplantation (Appendix Fig. 3). μCT analysis showed that transplantation of FasL-knockdown SHED failed to prevent bone loss in OVX mouse femurs, as assessed by trabecular bone parameters (Figs. 5A-5H) and cortical bone parameters (Figs. 5I-5M). Histological analysis also confirmed that trabecular bone volume was not markedly increased in the FasL-knockdown SHED group (Fig. 5N). Furthermore, we found that FasL-knockdown blocked SHED infusion-mediated reduction of the number of TRAP-positive cells and the levels of CTX-1 and TRAP 5b in OVX mouse serum (Figs. 5O-5Q). Analysis of ELISA data also revealed that transplantation of FasL-knockdown SHED failed to down-regulate the level of RANKL or up-regulate the level of OPG in OVX mouse serum (Figs. 5R, 5S). Analysis of these data suggested that FasL is required for SHED-mediated immune therapy in OVX mice.
Figure 5.
Transplantation of FasL knockdown SHED failed to ameliorate the osteoporotic phenotype in OVX mice. (A) μCT image showed that OVX mice had reduced trabecular bone volume in the femurs compared with the sham mice. SHED, but not FasL knockdown SHED, transplantation increased trabecular bone volume in the femurs of OVX mice. (B-H) μCT analysis showed that SHED, but not FasL knockdown SHED, transplantation increased bone volume (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), bone mineral density (BMD), and connectivity density (Conn.D), along with reduced trabecular space (Tb.Sp) and structure model index (SMI) in the femurs of OVX mice. (I) μCT images showed that SHED, but not FasL knockdown SHED, transplantation increased cortical bone volume in the femurs of OVX mice. (J-M) μCT analysis showed that SHED, but not FasL knockdown SHED, transplantation improved total cross-sectional area (Tt.Ar), cortical bone area (Ct.Ar), cortical bone fraction (Ct.Ar/Tt.Ar), and cortical thickness (Ct.Th) in the femurs of OVX mice. (N) Hematoxylin and eosin (H&E) staining showed that SHED, but not FasL knockdown SHED, transplantation increased trabecular bone area (yellow circled area) in distal femurs of OVX mice, as analyzed by ImageJ software. (O) TRAP staining showed that SHED, but not FasL knockdown SHED, transplantation reduced the number of TRAP+ cells (yellow arrows) in the femurs of OVX mice. (P-S) ELISA analysis showed that FasL knockdown SHED transplantation failed to regulate the serum levels of CTX-1, TRAP 5b, RANKL, and OPG in OVX mice in comparison with the SHED transplantation group. n = 5 in each group. Scale bar = 200 μm; *p < .05; **p < .01; ***p < .005. Error bars: mean ± SD.
Discussion
Although it is well-known that estrogen deficiency is associated with bone loss, the detailed mechanisms underlying this bone loss are not fully understood. Postmenopausal osteoporosis is the most common and significant form of osteoporosis, leading to a high risk of fragility fractures. Current osteoporosis treatments focus on preventing bone loss or increasing bone mass. Clinically, the most frequently used treatments include hormone replacement therapy, selective estrogen receptor modulators, bisphosphonates, and parathyroid hormone (Delmas, 2002). The treatments are able to prevent osteoporosis and decrease the risk of fragility fractures, but they have marked side effects, such as increased risk of cancer, heart disease, and bisphosphonate-related osteonecrosis of the jaw (Delmas, 2002; Kushner and Alpert, 2011). In this study, we pursued a cell-based therapy, finding inspiration from techniques that were originally used in the clinic more than 50 years ago (Copelan, 2006; Uccelli et al., 2008). More recently, systemic infusion of MSCs has been used to treat a variety of autoimmune and other diseases (Aggarwal and Pittenger, 2005; Nauta and Fibbe, 2007; Uccelli et al, 2008). Multiple mechanisms, including paracrine secretion of cytokines and cell-cell interactions, contribute to MSC-based immunotherapies (Ren et al., 2008; Nemeth et al., 2009; Choi et al., 2011). Here we used SHED, which are postnatal mesenchymal stem cells from human exfoliated deciduous teeth, because of their numerous advantages, including easy access, elevated proliferation potential, and multipotential differentiation. SHED have been used to repair critical craniofacial defects and to treat SLE in animal models. In this study, we reveal that SHED are an appropriate cell source for ameliorating the estrogen-deficient osteoporotic phenotype by rescuing the recipient’s T-cell homeostasis in OVX mice. OVX mice are excellent animal models for studying estrogen-deficiency-induced osteoporosis.
Bone provides an ideal microenvironment for immune cell self-renewal and function, in which a variety of cytokines influence osteogenic cell proliferation and differentiation. T-lymphocytes produce cytokines that have been identified as stimulators of bone resorption, such as IL-1, IL-6, IL-15, and IL-17 (Martin et al., 1998). It has been reported that activated T-cells play a key role in the regulation of osteoclast formation through the production of IFN-γ and TNF-α, augmenting RANKL-induced osteoclastogenesis (Kong et al., 1999; Takayanagi et al., 2000; Weitzmann et al., 2001). We found that the levels of Th1 and Th17 in CD4+ T-cells were elevated in OVX mice, along with reduced levels of Tregs. Th17 cells stimulate osteoclast differentiation indirectly via IL17 production (Kotake et al., 1999; Sato et al., 2006). Our previous study has revealed that IFN-γ and TNF-α synergistically induce MSC impairment (Wang et al., 2013). Therefore, OVX-induced activation of osteoclasts and impairment of the BMMSC/osteoblast lineage lead to significantly reduced bone mineral density and bone volume. In this study, we revealed a potential mechanism by which SHED transplantation ameliorated OVX-induced osteoporosis by inducing over-activated T-cell apoptosis and down-regulating serum levels of IFN-γ, TNF-α, and IL-17, leading to the reduction of osteoclasts and rescue of BMMSC function.
The FasL/Fas-mediated cell death pathway represents typical apoptotic signaling in many cell types (Hohlbaum et al., 2000; Pluchino et al., 2005; Zhang et al., 2008). Systemic infusion of BMMSCs can induce T-cell apoptosis via this pathway, leading to up-regulation of Tregs and immune tolerance (Akiyama et al., 2012). SHED possess immunomodulatory properties and inhibit Th17 cells significantly in comparison with BMMSCs (Yamaza et al., 2010). In this study, we found that SHED are able to induce T-cell apoptosis via producing FASL, thereby ameliorating estrogen-deficiency-induced osteoporosis in OVX mice. FasL knockdown SHED failed to ameliorate the osteoporotic phenotype in OVX mice because of their failure to elevate Treg levels and concomitant failure to reduce Th1 and Th17 cell levels. Therefore, FasL-induced T-cell apoptosis is required for SHED-based immune therapy for OVX-induced osteoporosis.
In summary, this study shows that systemic infusion of SHED ameliorates the osteoporotic phenotype in OVX mice by rescuing the recipient’s BMMSC deficiency and inhibiting osteoclastogenesis. FasL plays a key role in SHED-based immune therapy in OVX mice.
Supplementary Material
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This work was supported by grants from the US National Institute of Dental and Craniofacial Research (NIDCR), National Institutes of Health (NIH), and Department of Health and Human Services (R01DE017449 and R01DE019932 to S.S.).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Aggarwal S, Pittenger MF. (2005). Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105:1815-1822. [DOI] [PubMed] [Google Scholar]
- Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T, et al. (2012). Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell 10:544-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci S, Weitzmann MN, Roggia C, Namba N, Novack D, Woodring J, et al. (2000). Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J Clin Invest 106:1229-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci S, Toraldo G, Weitzmann MN, Roggia C, Gao Y, Qian WP, et al. (2003). Estrogen deficiency induces bone loss by increasing T cell proliferation and lifespan through IFN-gamma-induced class II transactivator. Proc Natl Acad Sci USA 100:10405-10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Akiyama K, Yamaza T, You YO, Xu X, Li B, et al. (2014). Telomerase governs immunomodulatory properties of mesenchymal stem cells by regulating FAS ligand expression. EMBO Mol Med 6:322-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang CY, Kyritsis G, Graves DT, Amar S. (1999). Interleukin-1 and tumor necrosis factor activities partially account for calvarial bone resorption induced by local injection of lipopolysaccharide. Infect Immun 67: 4231-4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ. (2011). Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-kappaB signaling in resident macrophages. Blood 118:330-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copelan EA. (2006). Hematopoietic stem cell transplantation. N Engl J Med 354:1813-1826. [DOI] [PubMed] [Google Scholar]
- Delmas PD. (2002). Treatment of postmenopausal osteoporosis. Lancet 359:2018-2026. [DOI] [PubMed] [Google Scholar]
- Gao Y, Grassi F, Ryan MR, Terauchi M, Page K, Yang X, et al. (2007). IFN-gamma stimulates osteoclast formation and bone loss in vivo via antigen-driven T cell activation. J Clin Invest 117:122-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghannam S, Pene J, Moquet-Torcy G, Jorgensen C, Yssel H. (2010). Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J Immunol 185:302-312. [DOI] [PubMed] [Google Scholar]
- Hildebolt CF. (1997). Osteoporosis and oral bone loss. Dentomaxillofac Radiol 26:3-15. [DOI] [PubMed] [Google Scholar]
- Hohlbaum AM, Moe S, Marshak-Rothstein A. (2000). Opposing effects of transmembrane and soluble Fas ligand expression on inflammation and tumor cell survival. J Exp Med 191:1209-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jee WS, Yao W. (2001). Overview: animal models of osteopenia and osteoporosis. J Musculoskel Neuron Interact 1:193-207. [PubMed] [Google Scholar]
- Kitazawa R, Kimble RB, Vannice JL, Kung VT, Pacifici R. (1994). Interleukin-1 receptor antagonist and tumor necrosis factor binding protein decrease osteoclast formation and bone resorption in ovariectomized mice. J Clin Invest 94:2397-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, et al. (1999). Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 402:304-309. [DOI] [PubMed] [Google Scholar]
- Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, et al. (1999). IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest 109:1345-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner GM, Alpert B. (2011). Bisphosphonate-related osteonecrosis of the jaws. Curr Opin Otolaryngol Head Neck Surg 19:302-306. [DOI] [PubMed] [Google Scholar]
- Li JY, Tawfeek H, Bedi B, Yang X, Adams J, Gao KY, et al. (2011). Ovariectomy disregulates osteoblast and osteoclast formation through the T-cell receptor CD40 ligand. Proc Natl Acad Sci USA 108:768-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TJ, Romas E, Gillespie MT. (1998). Interleukins in the control of osteoclast differentiation. Crit Rev Eukaryot Gene Expr 8:107-123. [DOI] [PubMed] [Google Scholar]
- Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. (2003). SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A 100:5807-5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, et al. (2007). Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell 130:811-823. [DOI] [PubMed] [Google Scholar]
- Nauta AJ, Fibbe WE. (2007). Immunomodulatory properties of mesenchymal stromal cells. Blood 110: 3499-3506. [DOI] [PubMed] [Google Scholar]
- Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, et al. (2009). Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 15:42-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, et al. (2005). Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature 436:266-271. [DOI] [PubMed] [Google Scholar]
- Raisz LG. (2005). Pathogenesis of osteoporosis: concepts, conflict, and prospects. J Clin Invest 115:3318-3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, et al. (2008). Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2:141-150. [DOI] [PubMed] [Google Scholar]
- Roggia C, Gao Y, Cenci S, Weitzmann MN, Toraldo G, Isaia G, et al. (2001). Up-regulation of TNF-producing T cells in the bone marrow: a key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci USA 98:13960-13965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, et al. (2006). Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med 203:2673-2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schett G, David JP. (2010). The multiple faces of autoimmune mediated bone loss. Nat Rev Endocrinol 6:698-706. [DOI] [PubMed] [Google Scholar]
- Seo BM, Sonoyama W, Yamaza T, Coppe C, Kikuiri T, Akiyama K, et al. (2008). SHED repair critical-size calvarial defects in mice. Oral Dis 14:428-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K, et al. (2000). T-cell-mediated regulation of osteoclastogenesis by signaling cross-talk between RANKL and IFN-gamma. Nature 408:600-605. [DOI] [PubMed] [Google Scholar]
- Uccelli A, Moretta L, Pistoia V. (2008). Mesenchymal stem cells in health and disease. Nat Rev Immunol 8:726-736. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhao Y, Liu Y, Akiyama K, Chen C, Qu C, et al. (2013). IFN-γ and TNF-α synergistically induce mesenchymal stem cell impairment and tumorigenesis via NFκB signaling. Stem Cells 31:1383-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzmann MN, Pacifici R. (2006). Estrogen deficiency and bone loss: an inflammatory tale. J Clin Invest 116:1186-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzmann MN, Cenci S, Rifas L, Haug J, Dipersio J, Pacifici R, et al. (2001). T cell activation induces human osteoclast formation via receptor activator of nuclear factor kappaB ligand dependent and independent mechanisms. J Bone Miner Res 16:328-337. [DOI] [PubMed] [Google Scholar]
- Yamaza T, Miura Y, Bi Y, Liu Y, Akiyama K, Sonoyama W, et al. (2008). Pharmacologic stem cell based intervention as a new approach to osteoporosis treatment in rodents. PLoS One 3:e2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaza T, Kentaro A, Chen C, Liu Y, Shi Y, Gronthos S, et al. (2010). Immunomodulatory properties of stem cells from human exfoliated deciduous teeth. Stem Cell Res Ther 1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xu G, Zhang L, Roberts AI, Shi Y. (2008). Th17 cells undergo Fas-mediated activation-induced cell death independent of IFN-gamma. J Immunol 181:190-196. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Liu Y, Zhang CM, Zhang HY, Li WH, Shi S, et al. (2009). Stem cells from deciduous tooth repair mandibular defect in swine. J Dent Res 88:249-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.