Abstract
Liposomes and liposome-derived nanovesicles such as archaeosomes and virosomes have become important carrier systems in vaccine development and the interest for liposome-based vaccines has markedly increased. A key advantage of liposomes, archaeosomes and virosomes in general, and liposome-based vaccine delivery systems in particular, is their versatility and plasticity. Liposome composition and preparation can be chosen to achieve desired features such as selection of lipid, charge, size, size distribution, entrapment and location of antigens or adjuvants. Depending on the chemical properties, water-soluble antigens (proteins, peptides, nucleic acids, carbohydrates, haptens) are entrapped within the aqueous inner space of liposomes, whereas lipophilic compounds (lipopeptides, antigens, adjuvants, linker molecules) are intercalated into the lipid bilayer and antigens or adjuvants can be attached to the liposome surface either by adsorption or stable chemical linking. Coformulations containing different types of antigens or adjuvants can be combined with the parameters mentioned to tailor liposomal vaccines for individual applications. Special emphasis is given in this review to cationic adjuvant liposome vaccine formulations. Examples of vaccines made with CAF01, an adjuvant composed of the synthetic immune-stimulating mycobacterial cordfactor glycolipid trehalose dibehenate as immunomodulator and the cationic membrane forming molecule dimethyl dioctadecylammonium are presented. Other vaccines such as cationic liposome–DNA complexes (CLDCs) and other adjuvants like muramyl dipeptide, monophosphoryl lipid A and listeriolysin O are mentioned as well. The field of liposomes and liposome-based vaccines is vast. Therefore, this review concentrates on recent and relevant studies emphasizing current reports dealing with the most studied antigens and adjuvants, and pertinent examples of vaccines. Studies on liposome-based veterinary vaccines and experimental therapeutic cancer vaccines are also summarized.
Keywords: adjuvants, antigens, archaeosomes, liposomes, therapeutic cancer vaccines, vaccines, veterinary vaccines, virosomes
Introduction
Classical vaccines rely on the use of whole killed or attenuated pathogens. Today, research is focused on the development of subunit vaccines because they are better defined, easier to produce and safer. Vaccines are manufactured on the basis of well characterized antigens, such as recombinant proteins and peptides. However, due to their synthetic nature, their immune response is often weak, which is largely related to the inability of the antigens to induce maturation of dendritic cells (DCs), the primary antigen-presenting cells (APCs) that react to foreign pathogens and trigger the immune response [Moser and Leo, 2010; Reed et al. 2013].
The immune system is composed of the innate and the adaptive systems. The first is responsible for first-line host defense, rapidly recognizing and responding to foreign pathogens. The complement system and phagocytic cells belong to this defense system which depends on pattern recognition receptors (PRRs) that recognize pathogen-associated molecular patterns (PAMPs). Toll-like receptors (TLRs) present on APCs are the receptors for pathogens containing PAMPs. TLR activation is the hallmark of innate immune response. The second defense line, the adaptive immune system, mounts specific responses against molecular determinants on pathogenic agents. These responses are initiated by antigen-mediated triggering of T cells, the CD4+ T-helper (TH) cells, the CD8+ cytotoxic T lymphocytes (CTLs) and B lymphocytes carrying antigen-specific surface receptors. TH cells have subpopulations, of which TH1 and TH2 are the most important [Nordly et al. 2009; Kawai and Akira, 2010].
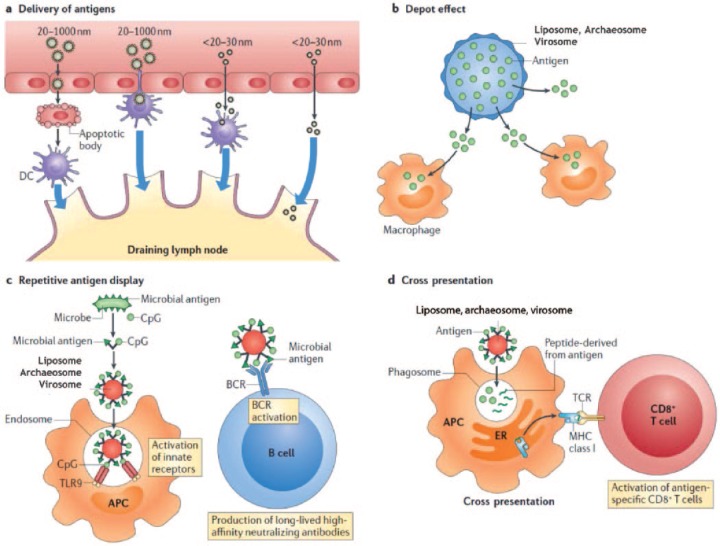
The diverse mechanisms by which nanoparticles induce immune responses are summarized in Figure 1. Activation of PRRs triggers the initiation of the innate immune response. Activated CTLs recognize peptides bound to the major histocompatibility complex class I and II molecules (MHC-I, MHC-II), which express antigenic peptides on APCs and bind to T cells via the T-cell receptor. A costimulatory signal is needed for full CTL and TH cell activation which differentiate into TH1 or TH2 and other T-helper lineages that produce cytokines. TH cells provide help to antigen-specific B cells, resulting in antibody production [Lin et al. 2010; Chen and Flies, 2013]. Each invasion of a foreign antigen requires activation of a specific type of adaptive immune response for efficient control and elimination. Thus, vaccine formulations should be designed rationally to induce specific protective responses. This includes the choice of antigen and adjuvant(s) and their pharmaceutical formulation.
Figure 1.
Mechanisms by which nanoparticles alter the induction of immune responses. The immunostimulatory activity of nanocarriers such as liposomes, archaeosomes and virosomes depends on diverse mechanisms: antigen delivery, particle size-dependent tissue penetration and access to the lymphatics (a); depot effects, promoting persistence, stability, conformational integrity and gradual release of vaccine antigens (b); and antigen display facilitating B-cell receptor (BCR) coaggregation, triggering and activation (c). Additional mechanisms include Toll-like receptor (TLR)-dependent and TLR-independent signal transduction (not shown); cross-presentation into major histocompatibility type I (MHC-I) pathways, caused by nanoparticle-mediated leakage of antigens into the cytosol after phagosome uptake (d); and release of cytokines, chemokines and immunomodulatory molecules that regulate the immune response (not shown). APC, antigen-presenting cell; CpG, cytosine–phosphorothioate–guanine oligodeoxynucleotide; DC, dendritic cell; ER, endoplasmic reticulum; TCR, T-cell receptor. Reproduced and modified with permission [Smith et al. 2013].
Adjuvants
The ability to enhance the immune response of vaccines by certain compounds was first demonstrated with aluminum salts, termed ‘adjuvants’, added to killed or attenuated pathogens. Their functions were related to the ability to form a depot which prolonged antigen exposure to APCs. However, efficient adjuvants also stimulate the immune system by direct interaction with APCs. The nature of immune adjuvants is large and heterogeneous. Adjuvants are divided into immunostimulants and delivery systems. Immunostimulants interact with specific receptors, like TLRs and others, while delivery systems increase the immune response by multiple mechanisms, depending on their particular characteristics [Leroux-Roels, 2010; Alving et al. 2012]. Thus, modern vaccines comprise adjuvants such as pathogen-derived subcellular components, recombinant proteins, peptides and nucleic acid sequences [Zepp, 2010; Perez et al. 2013; Reed et al. 2013]. In addition, due to better knowledge of the immune system and improvements in formulation technology, effective therapeutic cancer vaccines are developed [Joshi et al. 2012]. Today’s challenges in vaccine development are linked to complex pathogens [e.g. malaria, tuberculosis, human immunodeficiency virus (HIV)] and to antigens susceptible to genetic mutations (e.g. influenza) as well as to subjects with a compromised or dysfunctional immune system [Leroux-Roels, 2010].
Nanoparticulate carriers provide adjuvant activity by enhancing antigen delivery or by activating innate immune responses. Strength and mechanisms of immunostimulation induced by nanocarrier vaccines depend on various factors, such as chemical composition, particle size and homogeneity, charge, nature and location of antigens and/or adjuvants within the carrier and, last but not least, the site of administration (see Figure 2) [Watson et al. 2012; Brito et al. 2013; Gregory et al. 2013; Smith et al. 2013; Zaman et al. 2013].
Figure 2.
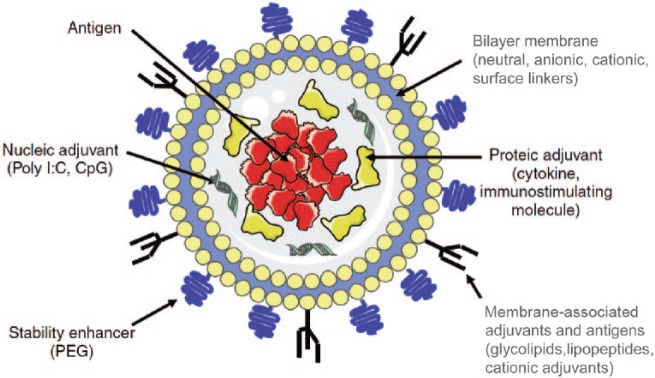
Schematic representation of a small unilamellar liposome showing the versatility of incorporation of various compounds either by encapsulation in the aqueous inner space or by integration in the bilayer or surface attachment on the lipid bilayer membrane. CpG, cytosine–phosphorothioate–guanine oligodeoxynucleotide; PEG, poly(ethyleneglycol). Reproduced and modified with permission [Heegaard et al. 2011].
Liposomes: ideal carriers for antigens and adjuvants
The ability of liposomes to induce immune responses to incorporated or associated antigens was first reported by Gregoriadis and Allison [Allison and Gregoriadis, 1974, 1976]. Since then, liposomes and liposome-derived nanovesicles such as archaeosomes and virosomes have become important carrier systems and the interest for liposome-based vaccines has markedly increased. The field of liposomes and liposome-based vaccines is vast. Therefore, this review concentrates on recent reports highlighting the most studied antigens and adjuvants in pertinent examples of vaccines, including summaries of veterinary and experimental therapeutic cancer vaccines. Other nanoparticulate vaccines based on lipoplexes, niosomes, virus-like particles, solid lipid nanoparticles and nanoemulsions are not covered in this review.
A key advantage of liposomes, archaeosomes and virosomes in general, and liposome-based delivery systems in particular, is their versatility and plasticity (see Table 1). Liposome composition and preparation can be chosen to achieve desired features such as lipid composition, charge, size, size distribution, entrapment and location of antigens or adjuvants. Depending on the chemical properties, water-soluble compounds (proteins, peptides, nucleic acids, carbohydrates, haptens) are entrapped within the aqueous inner space, whereas lipophilic compounds (lipopeptides, antigens, adjuvants, linker molecules) are intercalated into the lipid bilayer and antigens can be attached to the liposome surface either by adsorption or stable chemical linking [Torchilin, 2005; Watson et al. 2012]. Coformulations containing different types of antigens and adjuvants can be combined to tailor liposomal vaccines for individual applications (see Figure 2).
Table 1.
Characteristics of liposome, archaeosome, and virosome vaccines.
| Vesicle type | Composition | Characteristics | References |
|---|---|---|---|
|
Liposome Neutral or anionic |
Neutral and anionic lipids (PC, PG, PS, cholesterol) plus immunomodulators (MPLA, CpG, lipopeptides, glycolipids, etc.) and antigens (OVA, plasmids, mRNA, etc.) | Flexible compositions and antigen or adjuvant incorporation (encapsulation, adsorption, covalent surface attachment) TH1 and cell-mediated immune responses | Torchilin [2005]; Watson et al. [2012] |
|
Liposome Cationic |
Cationic lipids (DDA, DC-chol, DOTAP, etc.) plus neutral phospholipids, cholesterol plus immunomodulators (TDB, MPLA, CAF01, etc.) | Long depot effect at site of injection. Strong electrostatic interactions with APCs and strong TH1 and TH17 mediated immunostimulatory effects | Christensen et al. [2011]; Korsholm et al. [2012] |
| Archaeosome | Polar glycerolipids from Archaea and other bacteria plus phospholipids, cholesterol and antigens (OVA, plasmids) | Very stable formulations due to ether lipid bilayers. Archaeal glycerolipids are strong adjuvants mediating TH1 and cellular immune responses without need for TLR agonists | Krishnan and Sprott, [2008]; Patel and Chen [2010] |
| Virosome | Vesicles reconstituted from virus membranes (influenza, Semliki Forest, respiratory syncytial virus) and phospholipids. Hemaglutinin (HA), neuraminidase (NA) | Strong binding to cells and high immunogenicity induced by HA and NAHuman influenza and hepatitis A vaccines (Inflexal, Epaxal) | Gluck et al. [2005]; Herzog et al. [2009]; Kamphuis et al. [2013] |
CAF01 = DDA/TDB. APC, antigen-presenting cell; CpG, cytosine–phosphorothioate–guanine oligodeoxynucleotide; DC-chol, 3β-[N-(N’,N’-dimethylaminoethane) carbamoyl] cholesterol; DDA, dimethyl dioctadecylammonium; DOTAP, dioleoyl-3-trimethyl ammonium propane; MPLA, monophosphoryl lipid A; OVA, ovalbumin; PC, phosphatidylcholine; PG, phosphatidylglycerol; PS, phosphatidylserine;TDB, trehalose dibehenate; TH, T helper; TLR, Toll-like receptor.
Liposome-based antigens
Liposome-mediated effects of antigen uptake, trafficking, processing and presentation
As the majority of vaccines are administered by intramuscular or subcutaneous injection, liposome properties play a major role in local tissue distribution, retention, trafficking, uptake and processing by APCs. Earlier studies showed clear size-dependent, but not unambiguous charge or lipid composition dependent effects at the injection site [Oussoren et al. 1997]. Newer studies with the cationic liposome formulation dimethyl dioctadecylammonium (DDA) plus trehalose dibehenate (TDB) (DDA/TDB, CAF01) showed no differences in liposome draining or antigen release from the injection site. However, differences in movement to regional lymph nodes (LNs) were noted [Henriksen-Lacey et al. 2010, 2011]. A cationic liposome pDNA vaccine of 500 nm and 140 nm size with encapsulated ovalbumin (OVA) encoding pDNA as antigen showed strongest retention at large vesicle size. Addition of poly(ethyleneglycol) (peg) coating resulted in enhanced lymphatic drainage, without improved immune response [Carstens et al. 2011]. Other pegylated DDA/TDB liposomes reduced the depot effect and altered the immune response, confirming these results [Kaur et al. 2012]. Badiee and colleagues evaluated liposomes of different sizes containing the surface glycoprotein of Leishmania (rgp63). Immunization with small liposomes induced a TH2 response, whereas large liposomes induced a TH1 response, higher interferon γ (IFNγ) levels and immunoglobulin IgG2a/IgG1 ratios [Badiee et al. 2012]. Adjuvant effects of neutral, positive or negative liposomes were evaluated when admixed with OVA, cationized OVA (cOVA) or Bacillus anthracis antigen by Yanasarn and colleagues. Immunization with OVA admixed with different liposomes generated different antibody responses. Interestingly, OVA admixed with negative 1,2-dioleyl-sn-glycero-3-phosphatidic acid liposomes was as immunogenic as OVA admixed with positive 1,2-dioleoyl-3-trimethyl ammonium propane liposomes. The cOVA antigen showed comparable adjuvant activities in all liposomes [Yanasarn et al. 2011]. Neutral phosphatidylcholine (PC)/cholesterol small unilamellar vesicles (SUV) also proved to be effective vaccine carriers. We evaluated a vaccine with peptides derived from the glycoprotein of the lymphocytic choriomeningitis virus (LCMV). Liposome-encapsulated peptides were highly immunogenic and elicited protective antiviral immunity by in vivo antigen loading of DCs. Encapsulated cytosine–phosphorothioate–guanine oligodeoxynucleotides (CpGs) further enhanced immune activation [Ludewig et al. 2000]. We also used the vaccine to prime a CD8+ T-cell response against 10 different hepatitis C virus (HCV) epitopes, resulting in strong CTL responses. Challenge experiments with Vaccinia virus expressing HCV epitopes emphasized the utility of neutral liposomes as HCV vaccine [Engler et al. 2004; Schwendener et al. 2010]. Moon and colleagues describe novel interbilayer-crosslinked multilamellar vesicles (MLVs) formed by crosslinking adjacent lipid bilayers within MLVs. These vesicles entrapped protein antigens in their core and lipid-based immunostimulatory molecules in the bilayers, forming a potent vaccine, eliciting strong T-cell and antibody responses [Moon et al. 2011].
Investigation of hemagglutinin (HA) adsorption versus encapsulation and coencapsulation of CpGs in 3β-[N-(N’,N’-dimethylaminoethane)-carbamoyl] cholesterol (DC-chol) liposomes showed that adsorbed HA was more immunogenic than encapsulated HA. Cholesterol enhanced the adjuvant effect and CpG-loaded liposomes were highly efficient at enhancing HA-specific humoral responses [Barnier Quer et al. 2012, 2013]. Covalent attachment of protein antigens to nanocarriers can disrupt protein structure and mask epitopes, altering the antibody response. Watson and colleagues used metal chelation via nitrilotriacetic acid (NTA) to attach antigens to liposomes. OVA and a HIV-1 gp41 (N-MPR) peptide were attached via NTA or covalent linkage. Attachment of N-MPR, but not OVA, elicited stronger antibody responses than antigen admixed with liposomes and covalent attachment was superior to NTA-anchored antigens [Watson et al. 2011].
Mannose receptors (MRs) expressed on macrophages and APCs mediate endocytosis and cooperate in antigen capture and presentation. MRs recognize carbohydrate moieties of many pathogens. Thus, targeting of glycosylated antigens or carrier systems to MRs is a method to develop vaccines [Irache et al. 2008]. To establish a human papilloma virus 16 (HPV16) cancer vaccine, Mizuuchi and colleagues generated oligomannose liposomes containing HPV16-E6 plasmid antigens (OML-HPV). HPV16-E6-specific CTLs were generated from HPV16-positive cervical carcinoma patients with OML-HPV, but not with standard liposomes [Mizuuchi et al. 2012]. OMLs in combination with entrapped dsRNA to induce antihuman parainfluenza virus 3 (HPIV3) immunity were studied by Senchi and colleagues [Senchi et al. 2013]. Hemagglutinin neuraminidase antigen was coencapsulated with adjuvant poly(I:C) into OMLs. Systemic and mucosal immune responses were generated and immune sera suppressed viral infection in vitro. Finally, Li and colleagues constructed a mannosylated liposome/protamine/DNA (Man-LPD) vaccine. Man-LPD exhibited higher intracellular uptake and transfection in vitro and induction of costimulatory molecules on bone marrow DCs [Li et al. 2013].
Peptides and proteins as antigens
The antigen location in liposomes influences immunogenicity. Both, entrapped or surface-attached antigens induce T-cell responses, the latter having advantages of availability for antibody or B-cell recognition, whereas encapsulated antigens require vesicle disruption to be accessible. The necessity of CD4+ T cells to induce memory CD8+ T cells was investigated in mice immunized with liposome surface-coupled OVA peptides. CTL responses were induced and confirmed in mice lacking CD4+ T cells, suggesting that CD4+ T cells were not required for memory CD8+ T-cell generation [Taneichi et al. 2010]. Phosphatidylserine (PS)-liposome conjugated antigens were efficiently captured by APCs, resulting in TH cell stimulation, validating PS as adjuvant for peptide vaccines [Ichihashi et al. 2013]. Takagi and colleagues coupled several HCV peptides to liposomes. One Db-restricted and three HLA-A(*)0201-restricted peptides conferred complete protection to immunized mice and long-term memory [Takagi et al. 2013].
Liposome-encapsulated protein antigens have been used frequently in earlier work. More recently, Nagill and colleagues compared encapsulated 78 kDa antigen of Leishmania donovani with antigen plus monophosphoryl lipid A (MPLA), resulting in decreased parasite burden after challenge [Nagill and Kaur, 2010]. In another study, Bal and colleagues coencapsulated OVA and the TLR ligand Pam3CysSK4 or CpGs in dioleoyl-3-trimethyl ammonium propane (DOTAP) liposomes. Encapsulation of both ligands did not obstruct activation of TLR-transfected cells and OVA/CpG liposomes shifted the IgG1/IgG2a balance to IgG2a, whereas Pam3CysSK4 was less efficient [Bal et al. 2011]. Hepatitis B surface antigen (HBsAg) encapsulated liposomes coupled with Ulex europaeus agglutinin 1 were developed by Gupta and Vyas. Lectinized liposomes were predominantly targeted to M cells on intestinal Peyer’s patches after oral immunization, yielding high antibody titers in mucosal secretions [Gupta and Vyas, 2011]. Another mucosal vaccine was described by Figueiredo and colleagues who encapsulated Streptococcus equi antigens in PC/cholesterol/stearylamine liposomes or chitosan nanoparticles. Intranasal immunization of mice elicited mucosal, humoral and cellular responses with higher serum IgA levels of the chitosan nanoparticles, due to enhanced mucoadhesive properties [Figueiredo et al. 2012]. Liposomes modified with pH-sensitive 3-methyl-glutarylated hyperbranched poly(glycidol) (MGlu-HPG) were used to encapsulate OVA. MGlu-HPG liposomes induced a strong immune response which was suppressed with anti-MHC-I/MHC-II antibodies [Hebishima et al. 2012]. Ding and colleagues developed so-called RAFTsomes by isolating membrane microdomains containing MHC-I and I-Ab restricted epitopes from OVA-primed DCs and reconstituted them on liposome surfaces. RAFTsome immunization gave high anti-OVA IgG1 levels and protection against OVA-expressing EG.7 tumor challenge [Ding et al. 2013].
Liposomal DNA vaccines
Nucleic acid vaccines are an alternative to attenuated bacterial antigens or protein or peptide vaccines. MLVs as inexpensive carriers were used by Rodriguez and colleagues to deliver DNA to mice with plasmids encoding bovine herpesvirus type 1. Vaccinated mice developed specific IgG responses [Rodriguez et al. 2013]. The M1 gene of influenza A virus was used by Liu and colleagues to construct a cationic liposome/DNA vaccine with a M1-encoding plasmid for oral vaccination, resulting in M1 gene expression in intestines of vaccinated mice and strong immune responses and protection against challenge infection [Liu et al. 2014]. Liposomes were also used to deliver plasmid DNA encoding heat shock protein 65 (hsp65) to treat the pulmonary fungal infection paracoccidiomycosis, resulting in protective immune response and reduced fungal burden [Ribeiro et al. 2013]. Amidi and colleagues proposed liposomes as artificial microbes that can be programmed to produce specific antigens for vaccination. A bacterial transcription and translation system together with a gene construct encoding β-galactosidase or a luciferase–nucleoprotein (NP) fusion epitope as antigens were entrapped in liposomes. Vaccination of mice showed that such antigen-producing liposomes elicited higher specific immune responses against the produced antigen than control vaccines [Amidi et al. 2011, 2012].
Liposomal messenger RNA vaccines
The immune system is naturally activated by foreign nucleic acids by inducing specific immune responses. Lack of persistence, genome integration and auto-antibody induction are advantages of mRNA and siRNA vaccines. Currently, mRNA vaccines are developed to treat various diseases, including cancers. Pichon and Midoux loaded mannosylated nanoparticles with mRNA encoding a melanoma antigen [Pichon and Midoux, 2013]. The mRNA was formulated with histidylated liposomes promoting endosome destabilization, allowing cytosolic nucleic acid delivery which enhanced anti-B16F10 melanoma vaccination in mice. A liposome encapsulated double-stranded RNA (LE-PolyICLC) was tested in the influenza (H5N1-HPIV) model by Li and colleagues. Intranasal LE-PolyICLC inhibited virus replication, reduced viral titers, increased survival of infected mice and attenuated pulmonary fibrosis [Li et al. 2011].
The MUC1 (BLP25) antigen
The MUC1 glycoprotein is often overexpressed and hypoglycosylated in tumor cells of cancers, making it an attractive target for immunotherapy (for other examples, see Table 2) [Acres and Limacher, 2005; Roulois et al. 2013]. MUC1 variable number tandem repeats conjugated to tumor-associated carbohydrate antigens (TACAs) break self tolerance in humanized MUC1 transgenic mice. Sarkar and colleagues formulated an anticancer vaccine composed of a MUC1 glycopeptide containing a GalNAc-O-Thr (Tn) TACA conjugated to a TLR ligand. Additional surface-displayed l-rhamnose (Rha) epitopes were included in 1,2-dipalmitoyl-sn-glycero-3-phosphatidyl-choline (DPPC) liposomes. Mice were immunized with a Rha-Ficoll conjugate followed by the vaccine, resulting in an increase in anti-MUC1-Tn more than eightfold, anti-Tn antibody titers and increased T-cell proliferation [Sarkar et al. 2013]. Another liposome vaccine containing the immunoadjuvant Pam3CysSK4, a TH peptide epitope and a glycosylated MUC1 peptide was reported by Lakshminarayanan and colleagues. Covalent surface linkage of all three components was essential for maximum efficacy [Lakshminarayanan et al. 2012]. The BLP25 liposome (L-BLP25) vaccine which targets MUC1 extended survival of patients with non-small cell lung cancer (NSCLC) and showed promise in prostate cancer [North and Butts, 2005; North et al. 2006]. Butts and colleagues conducted phase II/IIB studies to evaluate L-BLP25 in patients with stage IIIA/IIIB NSCLC. Patients received either L-BLP25 plus best supportive care (BSC) or BSC alone. Survival time and rates were longer in patients receiving the combination compared with BSC alone [Butts et al. 2010, 2011]. Wu and colleagues are conducting an ongoing L-BLP25 study (INSPIRE) in patients with NSCLC of East Asian ethnicity, which is the first large therapeutic cancer vaccine study in an East-Asian population [Wu et al. 2011]. Accordingly, a L-BLP25 study was conducted in Japanese patients with NSCLC showing consistency with studies of white patients [Ohyanagi et al. 2011].
Table 2.
Examples of liposomal veterinary vaccines.
| Type of liposome | Antigenvaccine | Adjuvant or challenge | Animal speciesapplication | Outcome | Reference |
|---|---|---|---|---|---|
| PC/cholesterol | Newcastle disease vaccine | Gypenoside-saponin (GPS)encapsulated in liposomes | Chickens, oral | Enhanced immune response with GPS liposomes; T-cell, B-cell proliferation | Yu et al. [2013] |
| PC/cholesterol, sonicated SUV | Newcastle disease vaccine | Glycyrrhetinic acid (GA) encapsulated in liposomes | Chickens | Enhanced immune response with GA liposomes; Higher IgG, IgM antibody titers; T-cell, B-cell proliferation | Zhao et al. [2011] |
| DPPC/cholesterol, MLV | Newcastle disease (La Sota) encapsulated in MLV | Newcastle disease, Sato strain | Chickens, intranasal | High mucosal anti-NDV IgA, high serum IgG; high HA inhibition and survival rate; macrophage activation via ERK 1/2 and NFκB | Lin et al. [2011] |
| DOTAP/cholesterol, catioinic MLV | Newcastle disease (La Sota) encapsulated in MLV | Newcastle virus (Herts 33) | Chickens, oral | Higher antibody titers, 100% survival | Onuigbo et al. [2012] |
| DPPC/cholesterol, DPPS/cholesterol, SA/cholesterol, MLV | Newcastle disease (La Sota) encapsulated in MLV | Newcastle disease, Sato strain | Chickens, intranasal | DPPC/Chol-liposomes 320-fold higher HA inhibition than SA/Chol liposomes; 90% survival with DPPC/Chol vaccine | Tseng et al. [2010] |
| PC/cholesterol/ α-tocopherol SUV | Newcastle disease vaccine | Epimedium polysaccharide propolis flavone (EP), EP liposomes; challenge: NDV F48E9 strain | Chickens, intramuscular | High protection, high T-cell, B-cell proliferation; low mortality | Fan et al. [2012] |
| PC/bovine brain PS/cholesterol, MLV | Nonendotoxic LPS core types R1–R4 | Nonendotoxic LPS core types R1–R4 encaps. in MLV challenge: virulent Escherichia coli strain O78 | Chickens, intramuscular | Increased antibody response and lower lesion scores with MLV | Dissanayake et al. [2010] |
| PC/cholesterol/DDA cationic SUV | Inactivated avian pathogenic E. coli vaccine (APEC) | Inactivated APEC adsorbed to SUV Challenge: APEC (PDI-386 strain O78) | Chickens, eye drops or coarse spraying | Higher mucosal and serum antibodies, reduced bacteria in blood | Yaguchi et al. [2009] |
| Liposome-associated SEF14 | Salmonella enteritidis fimbrial protein, SEF14 | Live S. enteritidis | Chickens, oral | High IgG, IgA in intestinal mucus and serum. Low bacterial and low excretion of S. enteritidis in feces | Pang et al. [2013] |
| DOTAP/DOPE cationic MLV | n.a | Plasmid DNA of microneme Toxoplasma gondii protein, MIC3 adsorbed to MLV | Sheep, intramuscular | High IgG2 and IgG1 serum levels, high anti-MIC3 antibodies | Hiszczynska-Sawicka et al. [2012] |
| DSPC/DOPE/DOTAP cationic SUV | Recombinant foot and mouth disease protein vaccine (IgG FMDV) | Porcine IFNα DNA adsorbed to SUV Challenge: FMDV isolate HKY 2002 | Swine, intramuscular | Strong inflammatory cytokine production and TH1 response, high IFNα, no viremia or lesions | Cheng et al. [2007] |
| DPPC/cholesterol/ Mannotriose DPPE liposomes | M3-NcGRA7 | Dense granule protein 7 of Neospora caninum (M3-NcGRA7 ) encapsulated in liposomes Challenge: tachyzoites of N. caninum | Cattle, subcutaneous | Decreased parasite load | Nishimura et al. [2013] |
| DDA/TDB liposomes (CAF01) | rec. Mycobacterium avium paratuberculosis proteins | CAF01, rec. M. avium paratuberculosis proteins | Calves of different age, subcutaneous | Age-dependent IFNγ response, humoral response age independent | Thakur et al. [2013] |
DCPC, 1,2-dihexanoyl-sn-glycero-3-phosphocholine; DDA, dimethyl dioctadecylammonium; DOPE, 1,2-dioleyl-sn-glycero-3-phosphatidyl ethanolamine; DOTAP, dioleoyl-3-trimethyl ammonium propane; DPPC, 1,2-dipalmitoyl-sn-glycero-3-phosphatidyl choline; DPPE, 1,2-dipalmitoyl-sn-glycero-3-phosphatidyl ethanolamine; DPPS, 1,2-dipalmitoyl phosphatidylserine; DSPC, 1,2-distearoyl-sn-glycero-3-phosphocholine; IFN, interferon; Ig, immunoglobulin; LPS, lipopolysaccharide; MLV, multilamellar vesicle; n.a., not applicable; NDV, Newcastle disease virus; NFκB, nuclear factor κB; PC, phosphatidylcholine; PG, phosphatidyl glycerol; PS, phosphatidylserine; SA, starylamine; SUV, small unilamellar vesicle; TDP, trehalose dibehenate; TH, T helper.
Liposomes as carriers for adjuvants
Liposomal DNA as adjuvant
CpGs are adjuvants composed of unmethylated CpG dinucleotide sequences similar to those found in bacterial DNA. They trigger TLR9, activate DC maturation, increase antigen expression and induce TH1 immune responses [Shirota and Klinman, 2014]. Antigens and CpGs must be colocalized in one APC to generate optimal immune responses [Krishnamachari and Salem, 2009]. CpG encapsulation in liposomes of different properties altered antigen encapsulation efficiency, release and delivery rates, thus influencing the immune response. OVA/CpG coencapsulation augmented TH1 and cell-mediated immune response [Erikci et al. 2011]. Coencapsulation of OVA and Pam3CysSK4 or CpGs in cationic liposomes shifted the IgG1/IgG2a balance to IgG2a, showing that antigen/adjuvant coencapsulation shapes the type of immune response [Bal et al. 2011]. Nuclease-resistant phosphorothioate CpGs (PS-CpGs) or sensitive phosphodiester CpGs (PO-CpGs) were used by Shargh and colleagues in a leishmaniasis model. PO-CpGs or PS-CpGs were encapsulated in DOTAP liposomes for protection against nuclease degradation. Mice immunized with liposomal soluble Leishmania antigens (SLA) coincorporated with PO-CpGs or PS-CpGs showed no significant difference in immune response. Thus, nuclease-sensitive PO-CpGs can be used as adjuvants [Shargh et al. 2012]. Finally, CpGs incorporated in cationic DOTAP liposomes but not in neutral 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) liposomes provided complete protection against challenge with Burkholderia pseudomallei in a mouse model [Puangpetch et al. 2012].
Cationic liposome adjuvant vaccines
The introduction of positively charged compounds is a common method used to alter liposome properties. Cationic liposomes are frequently used as cell transfection reagents and vaccine adjuvants. Most cationic lipids form bilayer liposomes but often additional lipids are needed. The high surface density of positive charges increases liposome adsorption on negatively charged cell surfaces. Cationic liposomes penetrate into cells through specific mechanisms and activate different cellular pathways depending on cell type, cationic lipid nature, but also on formulation types and liposome size [Korsholm et al. 2012; Lonez et al. 2012].
The cationic adjuvant CAF01
CAF01 is a novel adjuvant composed of the synthetic immunostimulating mycobacterial cordfactor glycolipid TDB and the cationic membrane forming molecule DDA. TDB induces strong TH1 and TH17 immune responses and the C-type lectin Mincle is the receptor for APC activation. The adjuvant effect also requires MyD88 and Schweneker and colleagues identified the Nlrp3 inflammasome as mediator for TDB-triggered induction of immune response [Werninghaus et al. 2009; Desel et al. 2013; Schweneker et al. 2013]. Properties of cationic liposome-forming lipids were studied with rigid DDA or fluid dimethyldioleoylammonium (DODA) liposomes. When the antigen Ag85B-ESAT-6 was mixed with DDA/TDB or DODA/TDB liposomes, DDA liposomes formed a depot, resulting in continuous activation of APCs, whereas DODA liposomes were rapidly cleared [Christensen et al. 2012]. Milicic and colleagues explored modifications of DDA/TDB liposomes such as size, antigen association and addition of TLR agonists to assess their activity using OVA as antigen. SUV without TLR agonists showed higher antigen-specific antibody responses than MLVs. Addition of TLR3 and TLR9 agonists increased the adjuvant effects of MLVs but not of SUVs. The ability of DDA/TDB SUVs to induce CD8+ CTL responses without immunostimulators could avoid safety risks associated with TLR agonists [Milicic et al. 2012]. Yu and colleagues tested several adjuvants, including DDA-monophosphoryl lipid A (DDA-MPLA), DDA-TDB (CAF01) and DDA-monomycolyl glycerol (DDA-MMG, CAF04). Chlamydia antigens were used in a mouse genital tract infection model. DDA-MPLA and DDA-TDB elicited the best protective immune responses, characterized by CD4+ T cells coexpressing IFNγ and tumor necrosis factor α and by significantly reduced infection [Yu et al. 2012]. Ingvarsson and colleagues studied the parameters of CAF01 spray dried powder formulations using lactose, mannitol or trehalose as stabilizers. Immunization of mice with the tuberculosis antigen H56 demonstrated that spray drying with trehalose resulted in the best preservation of adjuvant activity [Ingvarsson et al. 2011, 2013]. Lindenstrom and colleagues showed that CAF01 vaccination in mice led to establishment of TH17 memory cells by retaining phenotypic and functional properties for 2 years. Challenge with Mycobacterium tuberculosis (MTB) 2 years later induced TH17 memory cells at levels comparable to TH1 memory cells [Lindenstrom et al. 2012]. A trivalent influenza vaccine (TIV) with CAF01 enhanced the immune response determined by HA inhibition and antibody titers, promoting strong TH1 responses. Maintenance of the TH1/TH17 cytokine profile over 20 weeks resulted in complete survival of H1N1 challenged mice [Rosenkrands et al. 2011]. A commercially available TIV was compared with the same vaccine mixed with CAF01 in ferrets. CAF01 induced increased influenza-specific IgA and IgG levels and promoted immunity and protection against challenge with H1N1 [Martel et al. 2011]. The combination of cationic liposomes and immunopotentiators such as MPL with DDA/TDB liposomes was tested in mice using OVA as antigen. DDA/TDB/MPL liposomes induced antigen-specific CD8+ T-cell and humoral responses [Nordly et al. 2011]. CAF01 was also used in a phase I trial with a therapeutic HIV-1 peptide vaccine. Safety and immunogenicity were assessed in individuals with untreated HIV-1 infection. Vaccine-specific T-cell responses were induced in 6 of 14 individuals, showing that therapeutic immunization with CAF01-adjuvanted HIV-1 peptide in humans is feasible [Roman et al. 2013]. In another clinical trial the potential of inducing T-cell immunity during chronic HIV-1 infection was investigated. Treatment-naive individuals with HIV-1 infection were immunized with peptides/CAF01. Specific CD4+ and CD8+ T-cell responses were induced in all individuals [Karlsson et al. 2013]. Kamath and colleagues reported that physical linkage between antigens and immunomodulators is required to elicit TH1/TH17 responses. Separate same-site administration of a mycobacterial fusion antigen and CAF01 failed to elicit TH1/TH17 responses. Tracking experiments showed that separate same-site administration elicited an early antigen-positive/adjuvant-negative DC population. Antigen targeting of LN DCs prior to their activation generated nonactivated antigen-pulsed DCs that recruited antigen-specific T cells and triggered proliferation, but interfered with TH1 induction in a dose-dependent manner. Thus, synchronization of DC targeting and activation is a critical determinant for TH1/TH17 adjuvanticity [Kamath et al. 2012].
In summary, CAF01-adjuvant liposomes prove to be a valuable vaccine formulation for different antigens.
CLDC adjuvant liposomes
Another widely studied cationic liposome complex contains the cationic lipid 1-[2-(oleoyloxy)-ethyl]-2-oleyl-3-(2-hydroxyethyl)imidazolinium-chloride and cholesterol. CLDCs are prepared by mixing liposomes with DNA. CLDC (JVRS-100, Juvaris BioTherapeutics, Burlingame, CA, USA) is a lyophilized powder composed of selected plasmid DNA complexed with liposomes. CLDCs facilitate APC uptake, activate TLRs and IFN production and stimulate the adaptive immune response. Several CLDC vaccines have been tested in various models. Gowen and colleagues analyzed liposomal delivery and CpG content of plasmid DNA with CLDCs. CpG-free or CpG-containing plasmids with and without liposomes, and poly(I:C) were evaluated to elicit protection against lethal Punta Toro virus challenge in hamsters. CLDC-containing CpG plasmid significantly improved survival, decreased viral loads and reduced liver damage [Gowen et al. 2009]. CLDC enhanced anti-simian immunodeficiency virus (SIV) immune responses induced by SIV vaccines. CLDC immunized rhesus macaques developed stronger SIV-specific T- and B-cell responses compared with controls, resulting in persistence and better memory responses [Fairman et al. 2009]. As no vaccines are available for common herpes simplex virus (HSV) infections CLDCs were evaluated for a HSV gD2 vaccine in a genital herpes guinea pig model. The CLDC/gD2 vaccine significantly decreased duration of acute and recurrent disease compared with gD2 alone. However, when evaluated as therapeutic vaccines they were ineffective, suggesting that such HSV-2 vaccines need improvement [Bernstein et al. 2010, 2011]. The protective effects of CLDCs against encephalitic arboviral infection were investigated in a Western equine encephalitis virus (WEEV) model. CLDC-vaccinated mice were challenged with virulent WEEV. CLDC pretreatment provided increased survival and higher cytokine levels, strong TH1 activation and protective immunity against lethal WEEF [Logue et al. 2010]. An influenza A virus vaccine adjuvanted with CLDC or alum was tested by Hong and colleagues. CLDC induced more robust adaptive immune responses with higher levels of virus-specific IgG2a/c and CD4+ and CD8+ T cells plus cross protection from lethal viral challenges [Hong et al. 2010]. In another influenza A vaccine study, Dong and colleagues showed that addition of CLDC (JVRS-100) to a H5N1 split vaccine induced higher virus-specific responses than adjuvant-free formulations. CLDC-vaccinated mice challenged with H5N1 had mild illness, very low viral titers, 100% survival and long-lasting protective immunity [Dong et al. 2012]. Strategies to improve influenza vaccine efficacy in older individuals are needed. Thus, Carroll and colleagues determined whether CLDC (JVRS-100) could improve the efficacy of the influenza vaccine Fluzone (Sanofi Pasteur, Lyon, France) in older rhesus macaques. Vaccination with Fluzone with or without CLDC and challenge with human H1N1 influenza virus showed that only the Fluzone/CLDC-vaccinated animals had lower virus replication. Thus, CLDC enhances immunogenicity and efficacy of a licensed vaccine in immunosenescent monkeys [Lay et al. 2009; Carroll et al. 2014]. CLDC (JVRS-100) was also evaluated as adjuvant for HBsAg in mice expressing hepatitis B virus (HBV). HBsAg+JVRS-100 elicited T- and B-cell responses, whereas HBsAg elicited only a B-cell response. However, the response by HBsAg+JVRS-100 was not sufficient to cause destruction of infected liver cells, but it suppressed HBV DNA noncytolytically [Morrey et al. 2011]. Similar results were obtained using the woodchuck model of HBV. HBV infection induced T-cell responses to Woodchuck hepatitis surface antigen (WHsAg) and selected WH peptides and expression of CD8+ CTL and TH1 cytokines. WHsAg plus CLDCs elicited antibodies earlier, in more woodchucks and with higher titers than WHsAg and alum [Cote et al. 2009]. There is a need for mucosal vaccines for pulmonary Yersinia pestis infections. The ability of an oral CLDC-adjuvanted vaccine against lethal pneumonic plague was investigated by Jones and colleagues. Oral immunization with Y. pestis F1 antigen combined with CLDC produced high titers of anti-F1 antibodies and long-lasting CD4+ T-cell-dependent protection from lethal pulmonary challenge with Y. pestis [Jones et al. 2010].
Other cationic lipid complexes
Several other cationic lipid adjuvant complexes were evaluated in various vaccine models. Phillips and colleagues tested an alphavirus vaccine composed of cationic lipid nucleic acid complexes (CLNCs) and the ectodomain (E1ecto) of WEEV. Interestingly, CLNC alone had therapeutic efficacy, as it increased survival of mice following lethal WEEV infection. Immunization with the CLNC/WEEV/E1ecto mixture provided full protection after challenge. Passive serum transfer from immunized to naïve mice conferred protection to challenge, indicating that antibody is sufficient for protection [Phillips et al. 2014]. Liposomes containing different cationic compounds and neutral DPPC were loaded with influenza HA by adsorption. DC-chol/DPPC liposomes with a high amount of DC-chol had stronger immunogenicity compared with less DC-chol and elicited higher antibody titers compared with the other compounds and nonadjuvanted HA. Liposome-adsorbed HA was more immunogenic than encapsulated HA and incorporation of cholesterol in DC-chol liposomes as well as CpGs enhanced adjuvancy [Barnier-Quer et al. 2013]. A similar study by Ma and colleagues showed that DOTAP/DOPC liposome regulated immune responses relied on surface charge density and might occur through reactive oxygen species (ROS) signaling. High charge density liposomes potently enhanced DC maturation, ROS generation, antigen uptake and production of IgG2a and IFNγ, whereas low-charge density liposomes failed to promote immune responses [Ma et al. 2011]. Lipid assemblies composed of a polycationic sphingolipid [ceramide carbamoyl spermine (CCS)] are effective adjuvants/carriers for several vaccines when complexed with cholesterol (CCS/C, VaxiSome, NasVax, Tel Aviv, Israel). Ferrets immunized intranasally with CCS/C-influenza vaccine produced higher HI antibody titers compared with controls. Following viral challenge, the vaccine reduced the severity of infection. Biodistribution studies showed that lipids and antigens are retained in nose and lung, increasing cytokine levels and expression of costimulatory molecules [Even-Or et al. 2011]. Chen and colleagues developed a cationic lipopolymer, the liposome–polyethyleneglycol–polyethyleneimine complex (LPPC) adjuvant for surface adsorption of antigens or immunomodulators. LPPC enhanced presentation on APCs, surface marker expression, cytokine release and activated TH1 immunity. With lipopolysaccharide (LPS) or CpGs, LPPC dramatically enhanced the IgA or IgG2A proportion of total Ig, demonstrating host immunity modulation [Chen et al. 2012]. Effects of pegylation of cationic DOTAP liposome vaccines on LN targeting and immunogenicity were studied by Zhuang and colleagues. Peg-DOTAP liposomes accelerated drainage into LNs, prolonged retention and APC uptake, increased anti-OVA antibody responses and modulated their biodistribution, which improved vaccine efficiency [Zhuang et al. 2012]. The activity of cationic vaccines can be hampered by immobilization in the extracellular matrix caused by electrostatic interactions. Thus, Van den Berg and colleagues found that surface shielding of DOTAP liposomes by pegylation improved antigen expression drastically. Mice vaccinated with pegylated pVAX/Luc-NP antigen containing liposomes elicited T-cell responses comparable to naked DNA, suggesting that charge shielding improves dermally applied vaccines [Van Den Berg et al. 2010].
Other adjuvants
Muramyl dipeptide
Muramyl dipeptide (MDP) originates from a bacterial peptidoglycan cell-wall fragment and is responsible for the activity of Freund’s complete adjuvant (FCA). After phagocytosis by APCs, MDP is detected by the NOD2 receptor that activates the immune response. Numerous MDP derivatives have been synthesized to evaluate their immunostimulatory effects and adjuvant activity [Traub et al. 2006; Ogawa et al. 2011]. It was recognized early on that liposomes were ideal carriers for MDP and its derivatives [Alving, 1991]. For example, the lipophilic MDP analogs muramyl dipeptide phosphatidyl ethanolamin (MDP-PE) and MDP glycerol dipalmitate were added to liposomal HBsAg formulations, both of which induced higher antibody titers, TH1 response and IFNγ levels [Jain et al. 2009]. Masek and colleagues used small Ni-chelating liposomes to attach His-tagged Candida albicans hsp (hsp90-CA) as antigen and to coincorporate the MDP derivative C18-O-6-norAbuMDP as adjuvant. The immune response was of TH1 and TH2 type, comparable to FCA, but without side effects [Masek et al. 2011]. Liposomes formulated as MDP with 1,2-dipalmitoyl-sn-glycero-3-phosphatidyl-ethanolamine are called mifamurtide (Mepact, Takeda, Osaka, Japan), which is an adjuvant to standard chemotherapy for osteosarcoma. A study by Anderson and colleagues in patients with metastatic osteosarcoma showed that mifamurtide had a manageable safety profile but that a randomized clinical trial is required to further determine its utility [Anderson et al. 2014].
Monophosphoryl lipid A
MPLA is the active moiety of the bacterial endotoxin LPS. TLR4 is the receptor for LPS forming a complex with MD2, representing the main LPS binding component. LPS supports the development of diverse TH cell types, depending on the tissue microenvironment. For instance, peripheral immunization with LPS drives TH1 priming in lymphoid tissue and TH17 priming in the intestinal mucosa [McAleer and Vella, 2010]. Several lipopeptides derived from microbial origin convey self-adjuvanting activity by TLR2 signaling, recognizing many PAMPs [Kawai and Akira, 2010]. MPLA liposomes have considerable potency and safety with a variety of candidate vaccines, including malaria, HIV-1 and several types of cancer [Alving et al. 2012; Zaman et al. 2013]. Combination of MPLA with the saponin QS-21 and immunostimulants in various adjuvant formulations forms the basis of Adjuvant Systems (AS; GlaxoSmithKline-Biologicals, Rixensart, Belgium) to promote protective immune responses. MPLA and aluminum salts are present in AS04, and both MPLA and QS-21 are present in AS01 and AS02, which are liposome- and emulsion-based formulations [Garcon and Van Mechelen, 2011]. Rizwan and colleagues analyzed the immunological activity of cubosomes containing MPLA and imiquimod. Cubosomes, a novel variation of MLVs, are composed of highly twisted lipid bilayers and nonintersecting water channels, providing increased encapsulation rates of lipophilic compounds. In MPLA cubosomes, sustained release of OVA was observed with induction of OVA-specific antibodies and CD8+ and CD4+ T-cell proliferation at higher efficiency than liposomes plus adjuvants and alum [Rizwan et al. 2013]. Immune response and protection induced by liposomal SLA formulated with lipid A trehalose dicorynomycolate (MPLA-TDM) was evaluated by Ravindran and colleagues. This vaccine induced strong and long-lasting protection against experimental visceral leishmaniasis [Ravindran et al. 2012]. An influenza vaccine with MPLA was developed by Adler-Moore and colleagues using the ectodomain of the M2e channel protein as a universal vaccine candidate. A liposomal M2e vaccine elicited anti-M2e antibodies that inhibited viral cell lysis and conferred complete protection to mice challenged with H1N1. Lymphocyte depletion markedly decreased protection, suggesting a primarily TH2-mediated immune response [Adler-Moore et al. 2011].
Listeriolysin
Cytolysins are virulence factors of various pathogenic bacteria. They form pores in target cell membranes, degrade membrane lipids or solubilize cell membranes. Bacteria use cytolysins to either inhibit functions of host immune cells or to gain access to intracellular niches. The bacterium Listeria monocytogenes can escape host immune defenses by lysis of the phagosomal membrane by use of listeriolysin O (LLO). LLO is used as vaccine adjuvant to provide cytosolic access for antigens in APCs [Dietrich et al. 2001]. LLO-based vaccines were reported by Mandal and Lee, who prepared OVA/LLO liposomes. OVA immunization resulted in higher CTL activity and high IFNγ production. The vaccine also conferred protection to mice from lethal challenges with antigen-expressing tumor cells [Mandal and Lee, 2002]. LLO liposomes were also used to deliver the LCMV NP to stimulate a NP-specific CTL response. Immunized mice generated high frequencies of NP-specific CD8+ T cells and full protection against a lethal intracerebral challenge with virulent LCMV [Mandal et al. 2004]. An anionic liposome–polycation–DNA complex combined with LLO was used as vaccine by Sun and colleagues to deliver OVA-cDNA. This formulation produced an enhanced CD8+ T-cell response, higher CTL frequency and IFNγ production after stimulation by an OVA-specific peptide [Sun et al. 2010]. Andrews and colleagues analyzed whether encapsulating CpGs in LLO liposomes would enhance cell-mediated immune response and skew TH1-type responses in a protein antigen-based vaccine utilizing LLO liposomes. Coencapsulation of CpGs in LLO liposomes activated the nuclear factor κB pathway, maintaining cytosolic delivery of antigen mediated by coencapsulated LLO. Immunization with OVA and CpG-LLO liposomes showed enhanced TH1 immune responses [Andrews et al. 2012].
Currently, 26 clinical trials are registered at ClinicalTrials.gov, a service of the US National Institutes of Health (see ClinicalTrials.gov with the search terms liposome AND vaccine).
Veterinary vaccines
Knowledge of molecular details of immune mechanisms is relatively scarce for veterinary and pet animals and special concerns regarding the use of vaccine adjuvants must be considered. Demands such as compatibility with human consumption, animal production, costs, challenges met by different species, vaccine administration for large numbers of animals and others must be evaluated [Heegaard et al. 2011; Underwood and Van Eps, 2012]. Table 2 summarizes some of the most recent experimental studies of liposome-based veterinary vaccines.
Therapeutic cancer vaccines
Although most cancers modify host proteins that can function as antigens, the development of effective vaccines against such antigens is hampered by the weak immune response and the immunosuppressive effects induced by cancers. Tumor-associated antigens include viral proteins (e.g. HPV), chromosomal translocation products (e.g. bcr/abl), overexpressed proteins likeHER2/neu, telomerase, MUC1 and others [Kozako et al. 2012]. In Table 3 some recent examples of experimental liposome-based cancer vaccines are listed.
Table 3.
Examples of liposomal therapeutic cancer vaccines.
| Type of liposome | Antigen | Adjuvant/ treatment |
Tumor model | Outcome | Reference |
|---|---|---|---|---|---|
| DC-chol/PC/pegDSPE/ protamine SUV, surface-linked anti-CD20 (rituximab) | Encapsulated Bcl-2 antisense oligonucleotide (G3139) | Bcl-2-targeted antisense G3139 as antisense therapeutic | Selective B-cell targeting in Raji B-cell lymphoma in NOD-SCID mice | Reduced adverse immunostimulatory effects of G3139.80% survival due to inhibition of TLR9-driven immunostimulation and Bcl-2 downregulation | Yu et al. [2013] |
| Pegylated PC/cholesterol liposomes with octa-arginine-SA + α-GC | α-galactosyl ceramide (α-GC), lipid antigen | Activation of NK T cells by α-GC, spleen targeting | B16 melanoma lung metastases | 65% inhibition of lung metastases | Nakamura et al. [2013] |
| Nanoliposomes | Encapsulated tumor associated antigen ESO-1 + tetanus toxoid helper peptide | Coadministration of palm-IL-1 and MAP-IFN-γ as adjuvants | In vitro targeting to Fcγ receptors on DCs | Highest immune response with nanoliposomes compared with soluble antigens | Cruz et al. [2014] |
| DOTAP-PIC-liposome complex (200–500 nm) | Hepa 1–6 cell lysates | polyriboinosinic: polyribocytidylic acid (poly I:C, PIC) | Hepa 1-6, subcutaneous | High tumor-specific CTL response and IFNγ levels; significant tumor growth inhibition with DOTAP-PIC + antigen | Wang et al. [2012] |
| Cationic TDB/DDA liposomes (CAF01) | Ovalbumin and HP16 E7 protein | Prophylactic and therapeutic vaccinations with CAF01 + poly I:C (= CAF05) | Lung B16-OVATC-1 expressing HP16 E7 protein, subcutaneously | Significant reduction of tumor growth with CAF05 vaccine by CD8 T-cell induced target cell lysis | Hansen et al. [2012] |
| PC/PG/cholesterol + DOG(Man)2 liposomes + mannosylated ligands | ErbB2 CTL and influenza virus HATH-peptide epitopes | TLR2/1 (Pam3CAG), TLR2/6 (Pam2CAG, Pam2CGD) agonists | Mice bearingRenCa-lacZ/ErbB2 tumors | 100% cures after vaccination with mannosylated liposomes + TLR2 ligand Pam3CAG | Thomann et al. [2011] |
| Mannosylated and histidylated lipopolyplexes with MART-1 mRNA(Man(11)-LPR100) | MART-1 (MelanA) mRNA | Immunization with Man(11)-LPR100 followed by challenge with B16F10 cells. | Delivery of mRNA to splenic DCs in vivo and anti-B16F10 melanoma vaccination in mice | Immunization with Man(11)-LPR100 gave better transfection of DCs and increased survival | Perche et al. [2011] |
| PC/cholesterol/octaarginine SUV liposomes with cell wall skeleton extracts of BCG (R8-liposome-BCG-CWS) | M. bovis bacillus Calmette-Guerin (BCG) liposome-incorporating cell wall skeleton (BCG-CWS) | Rats treated with BCG-CW or R8-liposome-BCG-CWS intravesically once a week for 8 weeks | Fisher-344 rats with nitrosamine-induced bladder cancer | R8-liposome-BCG-CWS-treated rats had significantly lower numbers of tumors | Miyazaki et al. [2011] |
| DOTAP/DOPE (SUV) + MPLA and surface-adsorbed bFGF | bFGF as angiogenesis stimulator | Human bFGF plus MPLA | C57 mice vaccinated with liposomal bFGF and challenged with Lewis lung carcinoma cells (LL-2) | Significant Inhibition of lung metastases in vaccinated mice | Zhong et al. [2010] |
| Anti-GD2-targeted CpG encapsulated in stealth liposomes (TL-CpG) | Neuroblastoma (NB) growth inhibiting CpGs | TLR9-dependent growth inhibition of NB induced by TL-CpG | HTLA-230 neuroblastoma expressing high levels of GD2 and TLR9 | TL-CpG inhibited proliferation of TLR9-expressing NB cells, induced apoptosis and prolonged survival of NB tumor xenografts. | Brignole et al. [2010] |
bFGF, basic fibroblast growth factor; BCG, Bacille Calmette-Guerin; CpG, cytosine–phosphorothioate–guanine oligodeoxynucleotide; CTL, cytotoxic T lymphocyte; DC, dendritic cell; DDA, dimethyl dioctadecylammonium; DOG(Man)2, mannosylated dioleylglycerol; DOPE, 1,2-dioleyl-sn-glycero-3-phosphatidyl ethanolamine; DOTAP, 1,2-dioleoyl-3-trimethyl-ammonium-propane; HP16, human papilloma virus 16; IL, interleukin; MAP, multiple antigen peptide; MPLA, monophosphoryl lipid A; NK, natural killer; OVA, ovalbumin; PC, phosphatidylcholine; pegDSPE, pegylated 1,2-distearoyl-sn-glycero-3-phosphatidylethanolamine; PG, phosphatidyl glycerol; PIC, polyriboinosinic: polyribocytidylic acid; SA, stearylamine; SUV, small unilamellar vesicle; TC-1, type 1 T cell; TDB, trehalose dibehenate; TLR, Toll-like receptor.
Archaeosomes
Archaebacteria (Archaea) were discovered and classified by Woese and Fox as a new group of prokaryotes, besides the Eubacteria (Bacteria) [Woese and Fox, 1977]. Archaea contain DNA-dependent RNA polymerases and proteinaceous cell walls that lack peptidoglycan. Their cell membranes are composed of L-glycerol ether lipids with isoprenoid chains instead of D-glycerol ester lipids with fatty acid chains [Spang et al. 2013]. Archaeal lipids are uniquely constituted of ether-linked isoprenoid phytanyl archaeol (diether) or caldarchaeol (tetraether) cores conferring high membrane stability. Archaeosomes are liposomes prepared with archaeal glycerolipids. The head groups displayed on the glycerol lipid cores of archaeosomes interact with APCs and induce TH1, TH2 and CD8+ T-cell responses to the entrapped antigen. The immune responses are persistent and subject to strong memory responses [Krishnan and Sprott, 2008; Benvegnu et al. 2009]. The polar lipid from the archaeon, Methanobrevibacter smithii, has been well characterized for its adjuvant potential. It contains archaetidyl serine, promoting interaction with a PS receptor on APCs. These archaeosomes mediate MHC-I cross priming and promote costimulation by APCs without inflammatory cytokine production [Krishnan et al. 2000]. Patel and colleagues showed that archaesomes prepared from M. smithii lipids were suitable adjuvants for multivalent mucosal vaccines. Archaeosomes containing the encapsulated antigens OVA, bovine serum albumin and hen egg lysozyme conferred strong and sustained specific antibody responses to all three antigens [Patel et al. 2004]. Intranasal immunization of mice with the archaeal lipid mucosal vaccine adjuvant and delivery (AMVAD) system, obtained by interaction of archaeosomes/antigens with multivalent cations, induced robust mucosal antigen-specific IgA responses. AMVAD formulations are stable, safe and show protective efficacy in murine models of infection/challenge [Patel and Chen, 2010]. Archaeosomes prepared from lipids of the nonpathogenic bacteria Leptospira biflexa (leptosomes) and Mycobacterium smegmatis (smegmosomes) were used as adjuvants. Both vesicles caused strong APC activation, cytokine release and expression of costimulatory signals, which was significantly higher for smegmosomes compared with leptosomes. APC activation by both formulations induced immune responses in mice to entrapped OVA [Faisal et al. 2009, 2011]. Borrero and colleagues studied immune response and cross reactivity against MTB with archaeosomes containing a mixture of cell wall glycolipids such as phosphatidylinositol mannosides of M. smegmatis (Ms) and 1,2-distearoyl-sn-glycero-3-phosphocholine/cholesterol. Ms-containing liposomes induced a specific IgG response and recognition of MTB surface antigens, showing that immunogenic Ms glycolipids could enhance subunit vaccines against tuberculosis [Borrero et al. 2013]. The relation between archaeal lipid structures and their activity was explored by synthesizing novel head groups linked to archaeol. Archaeosomes consisting of various combinations of synthesized lipids with entrapped OVA antigen were used to immunize mice. Addition of the glycolipids gentio-triosyl archaeol, mannotriosyl archaeol or maltotriosyl archaeol to archaetidylglycero-phosphate-O-methyl (AOM) archaeosomes significantly enhanced CD8+ T-cell responses, but diminished antibody titers. All three triglycosyl archaeols combined with AOM resulted in additive CD8+ T-cell responses [Sprott et al. 2012]. Ansari and colleagues showed that archaeosome-entrapped secretory antigens (SAgs) of L. monocytogenes resulted in upregulation of TH1 cytokines and boosted protective effects by reducing listerial burden in infected mice. Archaeosome-entrapped SAgs enhanced CTL response and increased survival of immunized animals [Ansari et al. 2012]. Finally, Singha and colleagues used E. coli lipid liposome (escheriosome) based DNA delivery to induce superoxide dismutase (SOD) and interleukin (IL)-18-specific immune responses in murine Brucellosis. Escheriosome-mediated delivery of SOD- and IL-18-encoding DNA induced specific immune responses in immunized mice. Coexpression of SOD + IL-18 resulted in stronger IgG2a-type response compared with free SOD DNA [Singha et al. 2011].
Currently, no clinical trials with archaeosomal vaccines are registered at ClinicalTrials.gov (see ClinicalTrials.gov, search terms archaeosome AND vaccine).
In summary, vaccines prepared with archaeal lipids, the archaeosomes, represent a new interesting and promising alternative to classical liposomes and virosomes.
Virosomes
Virosomes are liposomes prepared by combining natural or synthetic phospholipids with virus envelope phospholipids, viral spike glycoproteins and other viral proteins. The first virosomes were prepared and characterized by Almeida and colleagues [Almeida et al. 1975], followed by Helenius and colleagues who incorporated Semliki Forest virus glycoproteins in liposomes [Helenius et al. 1977; Balcarova et al. 1981]. Significant progress was made with virosomes termed ‘immunopotentiating reconstituted influenza virosomes’ (IRIVs). IRIVs are SUVs with spike projections of the influenza surface glycoproteins HA and neuraminidase. The fusogenic properties of HA are their primary features. IRIVs allow antigen presentation in the context of MHC-I and MHC-II and induce B- and T-cell responses [Gluck, 1992, Gluck et al. 2005]. The first virosome-based influenza vaccine used in humans was Inflexal V (Crucell NV, Leiden, The Netherlands), which has an excellent tolerability profile and immunogenicity in healthy and immunocompromised people [Herzog et al. 2009]. Another virosome vaccine containing inactivated hepatitis A virus (HAV), Epaxal (Crucell NV, Leiden, The Netherlands), was developed as hepatitis A vaccine. It is excellently tolerable and highly immunogenic, conferring protection of at least 9–11 years in vaccinated individuals [Ambrosch et al. 1997; Gluck and Walti, 2000; Bovier et al. 2010]. Immunogenicity and safety of Epaxal was evaluated in Thai children with HIV infection. Prevalence of HAV protective antibodies was 100% after vaccination, showing that Epaxal is an effective HAV vaccine for HIV-infected children [Saksawad et al. 2011]. Another vaccine contains an aspartyl proteinase 2 (Sap2) of Candida albicans incorporated into IRIVs. Following intravaginal administration, anti-Sap2 antibodies were detected in vaginal fluids of rats, inducing long-lasting protection [De Bernardis et al. 2012]. Walczak and colleagues demonstrated that a heterologous prime boost with Semliki Forest virus encoding a fusion protein of E6 and E7 of HPV16 and virosomes containing the HPV16-E7 protein resulted in higher numbers of antigen-specific CTL in mice than homologous protocols [Walczak et al. 2011]. Today, a second generation of influenza virosomes has evolved for various preclinical and clinical stage vaccine candidates. Additional components are included to optimize particle assembly and stability and to enhance immunostimulatory effects [Moser et al. 2013]. GPI-0100, a saponin derivative, enhanced immunogenicity and protective efficacy of a virosomal influenza vaccine, providing full protection of infected mice at extremely low antigen doses [Liu et al. 2013]. A combination of reconstituted respiratory syncytial virus (RSV) envelopes with incorporated MPLA (RSV-MPLA) virosomes was studied by Kamphuis and colleagues in enhanced respiratory disease prone rats. Vaccination with RSV-MPLA induced higher antibody levels and protection against infection [Kamphuis et al. 2013]. Jamali and colleagues developed a DNA vaccine using cationic influenza virosomes (CIV). CIV-delivered epitope-encoding DNA induced equal numbers of IFNγ and granzyme B-producing T cells than a 10-fold higher dose of naked pDNA [Jamali et al. 2012]. Another DNA/virosome vaccine was reported by Kheiri and colleagues, who prepared a vaccine complex containing an influenza NP-encoding plasmid that induced much higher T-cell responses and protection than plasmid alone [Kheiri et al. 2012]. In clinical trials, IRIVs have shown vast potential for delivery of peptides derived from Plasmodium falciparum antigens [Peduzzi et al. 2008]. An IRIV-formulated fusion protein composed of two malaria antigens was described by Tamborrini and colleagues. Compared with other vaccines, the adjuvant-free formulation elicited specific IgG1 antibody profiles in mice and cross reactivity with blood-stage parasites [Tamborrini et al. 2011]. Virosomes containing surface HIV-1 gp41-derived P1 lipid conjugated peptides (MYM-V101) as prophylactic HIV-1 vaccine were prepared. MYM-V101 was safe and well tolerated when administered by intramuscular and intranasal routes in healthy women. P1-specific serum IgGs and IgAs were detected in all recipients but P1-specific TH1 responses were not found [Leroux-Roels et al. 2013].
Currently, several clinical trials with virosome vaccines are registered at ClinicalTrials.gov (see ClinicalTrials.gov, search terms virosome AND vaccine).
Conclusion
The enormous versatility of liposomes and the related archaeosomes and virosomes endows them as highly valuable carrier systems for vaccines. Besides improving antigen stability and presentation to immunocompetent cells, depending on their specific properties including composition, size and surface properties, these nanocarriers also possess the ability to overcome biological barriers, such as skin and mucosa, and provide controlled and slow release of antigens. Together with the ability to induce strong immune responses provided by coformulated adjuvants, liposome-based vaccines provide properties that are fundamental for the development of modern vaccine formulations. It is predictable that these delivery systems will be increasingly applied in the near future with success, leading to major improvements in vaccine development.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The author declares that there is no conflict of interest.
References
- Acres B., Limacher J. (2005) Muc1 as a target antigen for cancer immunotherapy. Expert Rev Vaccines 4: 493–502 [DOI] [PubMed] [Google Scholar]
- Adler-Moore J., Munoz M., Kim H., Romero J., Tumpey T., Zeng H., et al. (2011) Characterization of the murine Th2 response to immunization with liposomal M2e influenza vaccine. Vaccine 29: 4460–4468 [DOI] [PubMed] [Google Scholar]
- Allison A., Gregoriadis G. (1974) Liposomes as immunological adjuvants. Nature 252: 252. [DOI] [PubMed] [Google Scholar]
- Allison A., Gregoriadis G. (1976) Liposomes as immunological adjuvants. Recent Results Cancer Res: 58–64 [DOI] [PubMed] [Google Scholar]
- Almeida J., Edwards D., Brand C., Heath T.D. (1975) Formation of virosomes from influenza subunits and liposomes. Lancet 2: 899–901 [DOI] [PubMed] [Google Scholar]
- Alving C. (1991) Liposomes as carriers of antigens and adjuvants. J Immunol Methods 140: 1–13 [DOI] [PubMed] [Google Scholar]
- Alving C., Peachman K., Rao M., Reed S. (2012) Adjuvants for human vaccines. Curr Opin Immunol 24: 310–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alving C., Rao M., Steers N., Matyas G., Mayorov A. (2012) Liposomes containing lipid A: an effective, safe, generic adjuvant system for synthetic vaccines. Expert Rev Vaccines 11: 733–744 [DOI] [PubMed] [Google Scholar]
- Ambrosch F., Wiedermann G., Jonas S., Althaus B., Finkel B., Gluck R., et al. (1997) Immunogenicity and protectivity of a new liposomal hepatitis A vaccine. Vaccine 15: 1209–1213 [DOI] [PubMed] [Google Scholar]
- Amidi M., De Raad M., Crommelin D., Hennink W., Mastrobattista E. (2011) Antigen-expressing immunostimulatory liposomes as a genetically programmable synthetic vaccine. Syst Synth Biol 5: 21–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amidi M., Van Helden M., Tabataei N., De Goede A., Schouten M., De Bot V., et al. (2012) Induction of humoral and cellular immune responses by antigen-expressing immunostimulatory liposomes. J Control Release 164: 323–330 [DOI] [PubMed] [Google Scholar]
- Anderson P., Meyers P., Kleinerman E., Venkatakrishnan K., Hughes D., Herzog C., et al. (2014) Mifamurtide in metastatic and recurrent osteosarcoma: a patient access study with pharmacokinetic, pharmacodynamic, and safety assessments. Pediatr Blood Cancer 61: 238–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews C., Huh M., Patton K., Higgins D., Van Nest G., Ott G., et al. (2012) Encapsulating immunostimulatory CpG oligonucleotides in listeriolysin O-liposomes promotes a Th1-type response and CTL activity. Mol Pharm 9: 1118–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari M., Zubair S., Tufail S., Ahmad E., Khan M., Quadri Z., et al. (2012) Ether lipid vesicle-based antigens impart protection against experimental listeriosis. Int J Nanomedicine 7: 2433–2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiee A., Khamesipour A., Samiei A., Soroush D., Shargh V., Kheiri M., et al. (2012) The role of liposome size on the type of immune response induced in BALB/C mice against leishmaniasis: rgp63 as a model antigen. Exp Parasitol 132: 403–409 [DOI] [PubMed] [Google Scholar]
- Bal S., Hortensius S., Ding Z., Jiskoot W., Bouwstra J. (2011) Co-encapsulation of antigen and Toll-like receptor ligand in cationic liposomes affects the quality of the immune response in mice after intradermal vaccination. Vaccine 29: 1045–1052 [DOI] [PubMed] [Google Scholar]
- Balcarova J., Helenius A., Simons K. (1981) Antibody response to spike protein vaccines prepared from Semliki Forest virus. J Gen Virol 53: 85–92 [DOI] [PubMed] [Google Scholar]
- Barnier Quer C., Elsharkawy A., Romeijn S., Kros A., Jiskoot W. (2012) Cationic liposomes as adjuvants for influenza hemagglutinin: more than charge alone. Eur J Pharm Biopharm 81: 294–302 [DOI] [PubMed] [Google Scholar]
- Barnier-Quer C., Elsharkawy A., Romeijn S., Kros A., Jiskoot W. (2013) Adjuvant effect of cationic liposomes for subunit influenza vaccine: influence of antigen loading method, cholesterol and immune modulators. Pharmaceutics 5: 392–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benvegnu T., Lemiegre L., Cammas-Marion S. (2009) New generation of liposomes called archaeosomes based on natural or synthetic archaeal lipids as innovative formulations for drug delivery. Recent Pat Drug Deliv Formul 3: 206–220 [DOI] [PubMed] [Google Scholar]
- Bernstein D., Earwood J., Bravo F., Cohen G., Eisenberg R., Clark J., et al. (2011) Effects of herpes simplex virus type 2 glycoprotein vaccines and CLDC adjuvant on genital herpes infection in the guinea pig. Vaccine 29: 2071–2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein D., Farley N., Bravo F., Earwood J., McNeal M., Fairman J., et al. (2010) The adjuvant CLDC increases protection of a herpes simplex type 2 glycoprotein D vaccine in guinea pigs. Vaccine 28: 3748–3753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrero R., Garcia Mde L., Canet L., Zayas C., Reyes F., Prieto J., et al. (2013) Evaluation of the humoral immune response and cross reactivity against mycobacterium tuberculosis of mice immunized with liposomes containing glycolipids of Mycobacterium smegmatis. BMC Immunol 14 Suppl. 1: S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovier P., Bock J., Ebengo T., Frosner G., Glaus J., Herzog C., et al. (2010) Predicted 30-year protection after vaccination with an aluminum-free virosomal hepatitis a vaccine. J Med Virol 82: 1629–1634 [DOI] [PubMed] [Google Scholar]
- Brignole C., Marimpietri D., Di Paolo D., Perri P., Morandi F., Pastorino F., et al. (2010) Therapeutic targeting of TLR9 inhibits cell growth and induces apoptosis in neuroblastoma. Cancer Res 70: 9816–9826 [DOI] [PubMed] [Google Scholar]
- Brito L., Malyala P., O’Hagan D. (2013) Vaccine adjuvant formulations: a pharmaceutical perspective. Semin Immunol 25: 130–145 [DOI] [PubMed] [Google Scholar]
- Butts C., Maksymiuk A., Goss G., Soulieres D., Marshall E., Cormier Y., et al. (2011) Updated survival analysis in patients with stage IIIB or IV non-small-cell lung cancer receiving BLP25 liposome vaccine (L-BLP25): phase IIB randomized, multicenter, open-label trial. J Cancer Res Clin Oncol 137: 1337–1342 [DOI] [PubMed] [Google Scholar]
- Butts C., Murray R., Smith C., Ellis P., Jasas K., Maksymiuk A., et al. (2010) A multicenter open-label study to assess the safety of a new formulation of BLP25 liposome vaccine in patients with unresectable stage III non-small-cell lung cancer. Clin Lung Cancer 11: 391–395 [DOI] [PubMed] [Google Scholar]
- Carroll T., Matzinger S., Barry P., McChesney M., Fairman J., Miller C. (2014) Efficacy of influenza vaccination of elderly rhesus macaques is dramatically improved by addition of a cationic lipid/DNA adjuvant. J Infect Dis 209: 24–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstens M., Camps M., Henriksen-Lacey M., Franken K., Ottenhoff T., Perrie Y., et al. (2011) Effect of vesicle size on tissue localization and immunogenicity of liposomal DNA vaccines. Vaccine 29: 4761–4770 [DOI] [PubMed] [Google Scholar]
- Chen C., Lin Y., Liu Y., He P., Lin C., Chiu Y., et al. (2012) Liposome-based polymer complex as a novel adjuvant: enhancement of specific antibody production and isotype switch. Int J Nanomedicine 7: 607–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Flies D. (2013) Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol 13: 227–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G., Zhao X., Yan W., Wang W., Zuo X., Huang K., et al. (2007) Alpha interferon is a powerful adjuvant for a recombinant protein vaccine against foot-and-mouth disease virus in swine, and an effective stimulus of in vivo immune response. Vaccine 25: 5199–5208 [DOI] [PubMed] [Google Scholar]
- Christensen D., Henriksen-Lacey M., Kamath A., Lindenstrom T., Korsholm K., Christensen J., et al. (2012) A cationic vaccine adjuvant based on a saturated quaternary ammonium lipid have different in vivo distribution kinetics and display a distinct CD4 T cell-inducing capacity compared to its unsaturated analog. J Control Release 160: 468–476 [DOI] [PubMed] [Google Scholar]
- Christensen D., Korsholm K., Andersen P., Agger E. (2011) Cationic liposomes as vaccine adjuvants. Expert Rev Vaccines 10: 513–521 [DOI] [PubMed] [Google Scholar]
- Cote P., Butler S., George A., Fairman J., Gerin J., Tennant B., et al. (2009) Rapid immunity to vaccination with woodchuck hepatitis virus surface antigen using cationic liposome-DNA complexes as adjuvant. J Med Virol 81: 1760–1772 [DOI] [PubMed] [Google Scholar]
- Cruz L., Rueda F., Simon L., Cordobilla B., Albericio F., Domingo J. (2014) Liposomes containing NY-ESO-1/tetanus toxoid and adjuvant peptides targeted to human dendritic cells via the Fc receptor for cancer vaccines. Nanomedicine (Lond) 9: 435–449 [DOI] [PubMed] [Google Scholar]
- De Bernardis F., Amacker M., Arancia S., Sandini S., Gremion C., Zurbriggen R., et al. (2012) A virosomal vaccine against candidal vaginitis: immunogenicity, efficacy and safety profile in animal models. Vaccine 30: 4490–4498 [DOI] [PubMed] [Google Scholar]
- Desel C., Werninghaus K., Ritter M., Jozefowski K., Wenzel J., Russkamp N., et al. (2013) The Mincle-activating adjuvant TDB induces MyD88-dependent Th1 and Th17 responses through IL-1R signaling. PLoS One 8: e53531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich G., Hess J., Gentschev I., Knapp B., Kaufmann S., Goebel W. (2001) From evil to good: a cytolysin in vaccine development. Trends Microbiol 9: 23–28 [DOI] [PubMed] [Google Scholar]
- Ding Q., Chen J., Wei X., Sun W., Mai J., Yang Y., et al. (2013) Raftsomes containing epitope-MHC-II complexes mediated CD4+ T cell activation and antigen-specific immune responses. Pharm Res 30: 60–69 [DOI] [PubMed] [Google Scholar]
- Dissanayake D., Wijewardana T., Gunawardena G., Poxton I. (2010) Potential use of a liposome-encapsulated mixture of lipopolysaccharide core types (R1, R2, R3 and R4) of Escherichia coli in controlling colisepticaemia in chickens. J Med Microbiol 59: 100–107 [DOI] [PubMed] [Google Scholar]
- Dong L., Liu F., Fairman J., Hong D., Lewis D., Monath T., et al. (2012) Cationic liposome–DNA complexes (CLDC) adjuvant enhances the immunogenicity and cross-protective efficacy of a pre-pandemic influenza a H5N1 vaccine in mice. Vaccine 30: 254–264 [DOI] [PubMed] [Google Scholar]
- Engler O., Schwendener R., Dai W., Wolk B., Pichler W., Moradpour D., et al. (2004) A liposomal peptide vaccine inducing CD8+ T cells in HLA-A2.1 transgenic mice, which recognise human cells encoding hepatitis C virus (HCV) proteins. Vaccine 23: 58–68 [DOI] [PubMed] [Google Scholar]
- Erikci E., Gursel M., Gursel I. (2011) Differential immune activation following encapsulation of immunostimulatory CPG oligodeoxynucleotide in nanoliposomes. Biomaterials 32: 1715–1723 [DOI] [PubMed] [Google Scholar]
- Even-Or O., Joseph A., Itskovitz-Cooper N., Samira S., Rochlin E., Eliyahu H., et al. (2011) A new intranasal influenza vaccine based on a novel polycationic lipid-ceramide carbamoyl-spermine (CCS). II. Studies in mice and ferrets and mechanism of adjuvanticity. Vaccine 29: 2474–2486 [DOI] [PubMed] [Google Scholar]
- Fairman J., Moore J., Lemieux M., Van Rompay K., Geng Y., Warner J., et al. (2009) Enhanced in vivo immunogenicity of SIV vaccine candidates with cationic liposome–DNA complexes in a rhesus macaque pilot study. Hum Vaccin 5: 141–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faisal S., Chen J., McDonough S., Chang C., Teng C., Chang Y. (2011) Immunostimulatory and antigen delivery properties of liposomes made up of total polar lipids from non-pathogenic bacteria leads to efficient induction of both innate and adaptive immune responses. Vaccine 29: 2381–2391 [DOI] [PubMed] [Google Scholar]
- Faisal S., Yan W., McDonough S., Chang C., Pan M., Chang Y. (2009) Leptosome-entrapped leptospiral antigens conferred significant higher levels of protection than those entrapped with PC-liposomes in a hamster model. Vaccine 27: 6537–6545 [DOI] [PubMed] [Google Scholar]
- Fan Y., Wang D., Hu Y., Liu J., Han G., Zhao X., et al. (2012) Liposome and epimedium polysaccharide-propolis flavone can synergistically enhance immune effect of vaccine. Int J Biol Macromol 50: 125–130 [DOI] [PubMed] [Google Scholar]
- Figueiredo L., Cadete A., Goncalves L., Corvo M., Almeida A. (2012) Intranasal immunisation of mice against Streptococcus equi using positively charged nanoparticulate carrier systems. Vaccine 30: 6551–6558 [DOI] [PubMed] [Google Scholar]
- Garcon N., Van Mechelen M. (2011) Recent clinical experience with vaccines using MPL- and QS-21-containing adjuvant systems. Expert Rev Vaccines 10: 471–486 [DOI] [PubMed] [Google Scholar]
- Gluck R. (1992) Immunopotentiating reconstituted influenza virosomes (IRIVs) and other adjuvants for improved presentation of small antigens. Vaccine 10: 915–919 [DOI] [PubMed] [Google Scholar]
- Gluck R., Burri K., Metcalfe I. (2005) Adjuvant and antigen delivery properties of virosomes. Curr Drug Deliv 2: 395–400 [DOI] [PubMed] [Google Scholar]
- Gluck R., Walti E. (2000) Biophysical validation of Epaxal Berna, a hepatitis A vaccine adjuvanted with immunopotentiating reconstituted influenza virosomes (IRIV). Dev Biol (Basel) 103: 189–197 [PubMed] [Google Scholar]
- Gowen B., Fairman J., Dow S., Troyer R., Wong M., Jung K., et al. (2009) Prophylaxis with cationic liposome–DNA complexes protects hamsters from phleboviral disease: importance of liposomal delivery and CpG motifs. Antiviral Res 81: 37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory A., Titball R., Williamson D. (2013) Vaccine delivery using nanoparticles. Front Cell Infect Microbiol 3: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P., Vyas S. (2011) Investigation of lectinized liposomes as M-cell targeted carrier-adjuvant for mucosal immunization. Colloids Surf B Biointerfaces 82: 118–125 [DOI] [PubMed] [Google Scholar]
- Hansen J., Lindenstrom T., Lindberg-Levin J., Aagaard C., Andersen P., Agger E. (2012) CAF05: cationic liposomes that incorporate synthetic cord factor and poly(I:C) induce CTL immunity and reduce tumor burden in mice. Cancer Immunol Immunother 61: 893–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebishima T., Yuba E., Kono K., Takeshima S., Ito Y., Aida Y. (2012) The pH-sensitive fusogenic 3-methyl-glutarylated hyperbranched poly(glycidol)-conjugated liposome induces antigen-specific cellular and humoral immunity. Clin Vaccine Immunol 19: 1492–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heegaard P., Dedieu L., Johnson N., Le Potier M., Mockey M., Mutinelli F., et al. (2011) Adjuvants and delivery systems in veterinary vaccinology: current state and Future developments. Arch Virol 156: 183–202 [DOI] [PubMed] [Google Scholar]
- Helenius A., Fries E., Kartenbeck J. (1977) Reconstitution of Semliki Forest virus membrane. J Cell Biol 75: 866–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen-Lacey M., Bramwell V., Christensen D., Agger E., Andersen P., Perrie Y. (2010) Liposomes based on dimethyldioctadecylammonium promote a depot effect and enhance immunogenicity of soluble antigen. J Control Release 142: 180–186 [DOI] [PubMed] [Google Scholar]
- Henriksen-Lacey M., Christensen D., Bramwell V., Lindenstrom T., Agger E., Andersen P., et al. (2011) Comparison of the depot effect and immunogenicity of liposomes based on dimethyldioctadecylammonium (DDA), 3β-[N-(N’,N’-dimethylaminoethane)carbomyl] cholesterol (DC-CHOL), and 1,2-dioleoyl-3-trimethylammonium propane (DOTAP): prolonged liposome retention mediates stronger Th1 responses. Mol Pharm 8: 153–161 [DOI] [PubMed] [Google Scholar]
- Herzog C., Hartmann K., Kunzi V., Kursteiner O., Mischler R., Lazar H., et al. (2009) Eleven years of inflexal V-A virosomal adjuvanted influenza vaccine. Vaccine 27: 4381–4387 [DOI] [PubMed] [Google Scholar]
- Hiszczynska-Sawicka E., Li H., Boyu Xu J., Akhtar M., Holec-Gasior L., Kur J., et al. (2012) Induction of immune responses in sheep by vaccination with liposome-entrapped DNA complexes encoding Toxoplasma gondii MIC3 gene. Pol J Vet Sci 15: 3–9 [DOI] [PubMed] [Google Scholar]
- Hong D., Chang S., Botham C., Giffon T., Fairman J., Lewis D. (2010) Cationic lipid/DNA complex-adjuvanted influenza a virus vaccination induces robust cross-protective immunity. J Virol 84: 12691–12702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihashi T., Satoh T., Sugimoto C., Kajino K. (2013) Emulsified phosphatidylserine, simple and effective peptide carrier for induction of potent epitope-specific T cell responses. PLoS One 8: e60068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingvarsson P., Schmidt S., Christensen D., Larsen N., Hinrichs W., Andersen P., et al. (2013) Designing CAF-adjuvanted dry powder vaccines: spray drying preserves the adjuvant activity of CAF01. J Control Release 167: 256–264 [DOI] [PubMed] [Google Scholar]
- Ingvarsson P., Yang M., Nielsen H., Rantanen J., Foged C. (2011) Stabilization of liposomes during drying. Expert Opin Drug Deliv 8: 375–388 [DOI] [PubMed] [Google Scholar]
- Irache J., Salman H., Gamazo C., Espuelas S. (2008) Mannose-targeted systems for the delivery of therapeutics. Expert Opin Drug Deliv 5: 703–724 [DOI] [PubMed] [Google Scholar]
- Jain V., Vyas S., Kohli D. (2009) Well-defined and potent liposomal hepatitis B vaccines adjuvanted with lipophilic MDP derivatives. Nanomedicine 5: 334–344 [DOI] [PubMed] [Google Scholar]
- Jamali A., Holtrop M., De Haan A., Hashemi H., Shenagari M., Memarnejadian A., et al. (2012) Cationic influenza virosomes as an adjuvanted delivery system for CTL induction by DNA vaccination. Immunol Lett 148: 77–82 [DOI] [PubMed] [Google Scholar]
- Jones A., Bosio C., Duffy A., Goodyear A., Schriefer M., Dow S. (2010) Protection against pneumonic plague following oral immunization with a non-replicating vaccine. Vaccine 28: 5924–5929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi M., Unger W., Storm G., Van Kooyk Y., Mastrobattista E. (2012) Targeting tumor antigens to dendritic cells using particulate carriers. J Control Release 161: 25–37 [DOI] [PubMed] [Google Scholar]
- Kamath A., Mastelic B., Christensen D., Rochat A., Agger E., Pinschewer D., et al. (2012) Synchronization of dendritic cell activation and antigen exposure is required for the induction of Th1/Th17 responses. J Immunol 188: 4828–4837 [DOI] [PubMed] [Google Scholar]
- Kamphuis T., Shafique M., Meijerhof T., Stegmann T., Wilschut J., De Haan A. (2013) Efficacy and safety of an intranasal virosomal respiratory syncytial virus vaccine adjuvanted with monophosphoryl lipid a in mice and cotton rats. Vaccine 31: 2169–2176 [DOI] [PubMed] [Google Scholar]
- Kamphuis T., Stegmann T., Meijerhof T., Wilschut J., De Haan A. (2013) A virosomal respiratory syncytial virus vaccine adjuvanted with monophosphoryl lipid a provides protection against viral challenge without priming for enhanced disease in cotton rats. Influenza Other Respir Viruses 7: 1227–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson I., Brandt L., Vinner L., Kromann I., Andreasen L., Andersen P., et al. (2013) Adjuvanted HLA-supertype restricted subdominant peptides induce new T-cell immunity during untreated HIV-1-Infection. Clin Immunol 146: 120–130 [DOI] [PubMed] [Google Scholar]
- Kaur R., Bramwell V., Kirby D., Perrie Y. (2012) Pegylation of DDA:TDB liposomal adjuvants reduces the vaccine depot effect and alters the Th1/Th2 immune responses. J Control Release 158: 72–77 [DOI] [PubMed] [Google Scholar]
- Kawai T., Akira S. (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11: 373–384 [DOI] [PubMed] [Google Scholar]
- Kheiri M., Jamali A., Shenagari M., Hashemi H., Sabahi F., Atyabi F., et al. (2012) Influenza virosome/DNA vaccine complex as a new formulation to induce intra-subtypic protection against influenza virus challenge. Antiviral Res 95: 229–236 [DOI] [PubMed] [Google Scholar]
- Korsholm K., Andersen P., Christensen D. (2012) Cationic liposomal vaccine adjuvants in animal challenge models: overview and current clinical status. Expert Rev Vaccines 11: 561–577 [DOI] [PubMed] [Google Scholar]
- Kozako T., Arima N., Yoshimitsu M., Honda S., Soeda S. (2012) Liposomes and nanotechnology in drug development: focus on oncotargets. Int J Nanomedicine 7: 4943–4951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamachari Y., Salem A. (2009) Innovative strategies for co-delivering antigens and CPG oligonucleotides. Adv Drug Deliv Rev 61: 205–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan L., Dicaire C., Patel G., Sprott G. (2000) Archaeosome vaccine adjuvants induce strong humoral, cell-mediated, and memory responses: comparison to conventional liposomes and alum. Infect Immun 68: 54–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan L., Sprott G. (2008) Archaeosome adjuvants: immunological capabilities and mechanism(s) of action. Vaccine 26: 2043–2055 [DOI] [PubMed] [Google Scholar]
- Lakshminarayanan V., Thompson P., Wolfert M., Buskas T., Bradley J., Pathangey L., et al. (2012) Immune recognition of tumor-associated mucin MUC1 is achieved by a fully synthetic aberrantly glycosylated MUC1 tripartite vaccine. Proc Natl Acad Sci U S A 109: 261–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lay M., Callejo B., Chang S., Hong D., Lewis D., Carroll T., et al. (2009) Cationic lipid/DNA complexes (JVRS-100) combined with influenza vaccine (Fluzone) increases antibody response, cellular immunity, and antigenically drifted protection. Vaccine 27: 3811–3820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux-Roels G. (2010) Unmet needs in modern vaccinology: adjuvants to improve the immune response. Vaccine 28 Suppl. 3: C25–36 [DOI] [PubMed] [Google Scholar]
- Leroux-Roels G., Maes C., Clement F., Van Engelenburg F., Van Den Dobbelsteen M., Adler M., et al. (2013) Randomized phase I: safety, immunogenicity and mucosal antiviral activity in young healthy women vaccinated with HIV-1 GP41 P1 peptide on virosomes. PLoS One 8: e55438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Chen S., Jiang Y., Jiang J., Zhang Z., Sun X. (2013) Dendritic cell targeted liposomes–protamine–DNA complexes mediated by synthetic mannosylated cholesterol as a potential carrier for DNA vaccine. Nanotechnology 24: 295101. [DOI] [PubMed] [Google Scholar]
- Li Y., Hu Y., Jin Y., Zhang G., Wong J., Sun L., et al. (2011) Prophylactic, therapeutic and immune enhancement effect of liposome-encapsulated polcyclic on highly pathogenic H5N1 influenza infection. J Gene Med 13: 60–72 [DOI] [PubMed] [Google Scholar]
- Lin Y., Deng M., Tseng L., Jiang P., Jan T., Hsieh F., et al. (2011) Adjuvant effect of liposome in chicken result from induction of nitric oxide. Biomed Mater 6: 015011. [DOI] [PubMed] [Google Scholar]
- Lin Y., Slight S., Khader S. (2010) Th17 cytokines and vaccine-induced immunity. Semin Immunopathol 32: 79–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenstrom T., Woodworth J., Dietrich J., Aagaard C., Andersen P., Agger E. (2012) Vaccine-induced Th17 cells are maintained long-term postvaccination as a distinct and phenotypically stable memory subset. Infect Immun 80: 3533–3544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., De Vries-Idema J., Ter Veer W., Wilschut J., Huckriede A. (2013) Influenza virosomes supplemented with GPI-0100 adjuvant: a potent vaccine formulation for antigen Dose sparing. Med Microbiol Immunol 203: 47–55 [DOI] [PubMed] [Google Scholar]
- Liu J., Wu J., Wang B., Zeng S., Qi F., Lu C., et al. (2014) Oral vaccination with a liposome-encapsulated influenza DNA vaccine protects mice against respiratory challenge infection. J Med Virol 86: 886–894 [DOI] [PubMed] [Google Scholar]
- Logue C., Phillips A., Mossel E., Ledermann J., Welte T., Dow S., et al. (2010) Treatment with cationic liposome–DNA complexes (CLDCs) protects mice from lethal western equine encephalitis virus (WEEV) challenge. Antiviral Res 87: 195–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonez C., Vandenbranden M., Ruysschaert J. (2012) Cationic lipids activate intracellular signaling pathways. Adv Drug Deliv Rev 64: 1749–1758 [DOI] [PubMed] [Google Scholar]
- Ludewig B., Barchiesi F., Pericin M., Zinkernagel R., Hengartner H., Schwendener R. (2000) In vivo antigen loading and activation of dendritic cells via a liposomal peptide vaccine mediates protective antiviral and anti-tumour immunity. Vaccine 19: 23–32 [DOI] [PubMed] [Google Scholar]
- Ma Y., Zhuang Y., Xie X., Wang C., Wang F., Zhou D., et al. (2011) The role of surface charge density in cationic liposome-promoted dendritic cell maturation and vaccine-induced immune responses. Nanoscale 3: 2307–2314 [DOI] [PubMed] [Google Scholar]
- Mandal M., Kawamura K., Wherry E., Ahmed R., Lee K. (2004) Cytosolic delivery of viral nucleoprotein by listeriolysin O-liposome induces enhanced specific cytotoxic T lymphocyte response and protective immunity. Mol Pharm 1: 2–8 [DOI] [PubMed] [Google Scholar]
- Mandal M., Lee K. (2002) Listeriolysin O-liposome-mediated cytosolic delivery of macromolecule antigen in vivo: enhancement of antigen-specific cytotoxic T lymphocyte frequency, activity, and tumor protection. Biochim Biophys Acta 1563: 7-17 [DOI] [PubMed] [Google Scholar]
- Martel C., Agger E., Poulsen J., Hammer Jensen T., Andresen L., Christensen D., et al. (2011) Caf01 potentiates immune responses and efficacy of an inactivated influenza vaccine in ferrets. PLoS One 6: e22891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masek J., Bartheldyova E., Turanek-Knotigova P., Skrabalova M., Korvasova Z., Plockova J., et al. (2011) Metallochelating liposomes with associated lipophilised norAbuMDP as biocompatible platform for construction of vaccines with recombinant his-tagged antigens: preparation, structural study and immune response towards rHsp90. J Control Release 151: 193–201 [DOI] [PubMed] [Google Scholar]
- McAleer J., Vella A. (2010) Educating CD4 T cells with vaccine adjuvants: lessons from lipopolysaccharide. Trends Immunol 31: 429–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milicic A., Kaur R., Reyes-Sandoval A., Tang C., Honeycutt J., Perrie Y., et al. (2012) Small cationic DDA:TDB liposomes as protein vaccine adjuvants obviate the need for TLR agonists in inducing cellular and humoral responses. PLoS One 7: e34255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki J., Nishiyama H., Yano I., Nakaya A., Kohama H., Kawai K., et al. (2011) The therapeutic effects of R8-liposome-BCG-CWS on BBN-induced rat urinary bladder carcinoma. Anticancer Res 31: 2065–2071 [PubMed] [Google Scholar]
- Mizuuchi M., Hirohashi Y., Torigoe T., Kuroda T., Yasuda K., Shimizu Y., et al. (2012) Novel oligomannose liposome-DNA complex DNA vaccination efficiently Evokes anti-HPV E6 and E7 CTL responses. Exp Mol Pathol 92: 185–190 [DOI] [PubMed] [Google Scholar]
- Moon J., Suh H., Bershteyn A., Stephan M., Liu H., Huang B., et al. (2011) Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat Mater 10: 243–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrey J., Motter N., Chang S., Fairman J. (2011) Breaking B and T cell tolerance using cationic lipid–DNA complexes (CLDC) as a vaccine adjuvant with hepatitis B virus (HBV) surface antigen in transgenic mice expressing HBV. Antiviral Res 90: 227–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser C., Muller M., Kaeser M., Weydemann U., Amacker M. (2013) Influenza virosomes as vaccine adjuvant and carrier system. Expert Rev Vaccines 12: 779–791 [DOI] [PubMed] [Google Scholar]
- Moser M., Leo O. (2010) Key concepts in immunology. Vaccine 28 Suppl. 3: C2–C13 [DOI] [PubMed] [Google Scholar]
- Nagill R., Kaur S. (2010) Enhanced efficacy and immunogenicity of 78 kDa antigen formulated in various adjuvants against murine visceral leishmaniasis. Vaccine 28: 4002–4012 [DOI] [PubMed] [Google Scholar]
- Nakamura T., Yamazaki D., Yamauchi J., Harashima H. (2013) The nanoparticulation by octaarginine-modified liposome improves alpha-galactosylceramide-mediated antitumor therapy via systemic administration. J Control Release 171: 216–224 [DOI] [PubMed] [Google Scholar]
- Nishimura M., Kohara J., Kuroda Y., Hiasa J., Tanaka S., Muroi Y., et al. (2013) Oligomannose-coated liposome-entrapped dense granule protein 7 induces protective immune response to Neospora caninum in cattle. Vaccine 31: 3528–3535 [DOI] [PubMed] [Google Scholar]
- Nordly P., Agger E., Andersen P., Nielsen H., Foged C. (2011) Incorporation of the TLR4 agonist monophosphoryl lipid A into the bilayer of DDA/TDP liposomes: physico-chemical characterization and induction of CD8+ T-cell responses in vivo. Pharm Res 28: 553–562 [DOI] [PubMed] [Google Scholar]
- Nordly P., Madsen H., Nielsen H., Foged C. (2009) Status and future prospects of lipid-based particulate delivery systems as vaccine adjuvants and their combination with immunostimulators. Expert Opin Drug Deliv 6: 657–672 [DOI] [PubMed] [Google Scholar]
- North S., Butts C. (2005) Vaccination with BLP25 liposome vaccine to treat non-small cell lung and prostate cancers. Expert Rev Vaccines 4: 249–257 [DOI] [PubMed] [Google Scholar]
- North S., Graham K., Bodnar D., Venner P. (2006) A pilot study of the liposomal MUC1 vaccine BLP25 in prostate specific antigen failures after radical prostatectomy. J Urol 176: 91–95 [DOI] [PubMed] [Google Scholar]
- Ogawa C., Liu Y., Kobayashi K. (2011) Muramyl dipeptide and its derivatives: peptide adjuvant in immunological disorders and cancer therapy. Curr Bioact Compd 7: 180–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyanagi F., Horai T., Sekine I., Yamamoto N., Nakagawa K., Nishio M., et al. (2011) Safety of BLP25 liposome vaccine (L-BLP25) in Japanese patients with unresectable stage III NSCLC after primary chemoradiotherapy: preliminary results from a phase I/II study. Jpn J Clin Oncol 41: 718–722 [DOI] [PubMed] [Google Scholar]
- Onuigbo E., Okore V., Ofokansi K., Okoye J., Nworu C., Esimone C., et al. (2012) Preliminary evaluation of the immunoenhancement potential of Newcastle disease vaccine formulated as a cationic liposome. Avian Pathol 41: 355–360 [DOI] [PubMed] [Google Scholar]
- Oussoren C., Zuidema J., Crommelin D., Storm G. (1997) Lymphatic uptake and biodistribution of liposomes after subcutaneous injection. II. Influence of liposomal size, lipid composition and lipid dose. Biochim Biophys Acta 1328: 261–272 [DOI] [PubMed] [Google Scholar]
- Pang Y., Zhang Y., Wang H., Jin J., Piao J., Piao J., et al. (2013) Reduction of Salmonella enteritidis number after infections by immunization of liposome-associated recombinant SEFA. Avian Dis 57: 627–633 [DOI] [PubMed] [Google Scholar]
- Patel G., Chen W. (2010) Archaeal lipid mucosal vaccine adjuvant and delivery system. Expert Rev Vaccines 9: 431–440 [DOI] [PubMed] [Google Scholar]
- Patel G., Zhou H., Kuolee R., Chen W. (2004) Archaeosomes as adjuvants for combination vaccines. J Liposome Res 14: 191–202 [DOI] [PubMed] [Google Scholar]
- Peduzzi E., Westerfeld N., Zurbriggen R., Pluschke G., Daubenberger C. (2008) Contribution of influenza immunity and virosomal-formulated synthetic peptide to cellular immune responses in a phase I subunit malaria vaccine trial. Clin Immunol 127: 188–197 [DOI] [PubMed] [Google Scholar]
- Perche F., Benvegnu T., Berchel M., Lebegue L., Pichon C., Jaffres P., et al. (2011) Enhancement of dendritic cells transfection in vivo and of vaccination against B16F10 melanoma with mannosylated histidylated lipopolyplexes loaded with tumor antigen messenger RNA. Nanomedicine 7: 445–453 [DOI] [PubMed] [Google Scholar]
- Perez O., Romeu B., Cabrera O., Gonzalez E., Batista-Duharte A., Labrada A., et al. (2013) Adjuvants are key factors for the development of future vaccines: lessons from the Finlay adjuvant platform. Front Immunol 4: 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips A., Schountz T., Toth A., Rico A., Jarvis D., Powers A., et al. (2014) Liposome–antigen–nucleic acid Complexes protect mice from lethal challenge with western and eastern equine encephalitis viruses. J Virol 88: 1771–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichon C., Midoux P. (2013) Mannosylated and histidylated LPR technology for vaccination with tumor antigen mRNA. Methods Mol Biol 969: 247–274 [DOI] [PubMed] [Google Scholar]
- Puangpetch A., Anderson R., Huang Y., Sermswan R., Chaicumpa W., Sirisinha S., et al. (2012) Cationic liposomes extend the immunostimulatory effect of CpG oligodeoxynucleotide against Burkholderia pseudomallei infection in BALB/C mice. Clin Vaccine Immunol 19: 675–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran R., Maji M., Ali N. (2012) Vaccination with liposomal leishmanial antigens adjuvanted with monophosphoryl lipid-trehalose dicorynomycolate (MPL-TDM) confers long-term protection against visceral leishmaniasis through a human administrable route. Mol Pharm 9: 59–70 [DOI] [PubMed] [Google Scholar]
- Reed S., Orr M., Fox C. (2013) Key roles of adjuvants in modern vaccines. Nat Med 19: 1597–1608 [DOI] [PubMed] [Google Scholar]
- Ribeiro A., Souza A., Amaral A., Vasconcelos N., Jeronimo M., Carneiro F., et al. (2013) Nanobiotechnological approaches to delivery of DNA vaccine against fungal infection. J Biomed Nanotechnol 9: 221–230 [DOI] [PubMed] [Google Scholar]
- Rizwan S., McBurney W., Young K., Hanley T., Boyd B., Rades T., et al. (2013) Cubosomes containing the adjuvants imiquimod and monophosphoryl lipid A stimulate robust cellular and humoral immune responses. J Control Release 165: 16–21 [DOI] [PubMed] [Google Scholar]
- Rodriguez A., Zamorano P., Wilkowsky S., Torra F., Ferreri L., Dominguez M., et al. (2013) Delivery of recombinant vaccines against bovine herpesvirus type 1 gD and Babesia bovis MSA-2c to mice using liposomes derived from egg yolk lipids. Vet J 196: 550–551 [DOI] [PubMed] [Google Scholar]
- Roman V., Jensen K., Jensen S., Leo-Hansen C., Jespersen S., Da Silva Te D., et al. (2013) Therapeutic vaccination using cationic liposome-adjuvanted HIV type 1 peptides representing HLA-supertype-restricted subdominant T cell epitopes: safety, immunogenicity, and Feasibility in Guinea-Bissau. AIDS Res Hum Retroviruses 29: 1504–1512 [DOI] [PubMed] [Google Scholar]
- Rosenkrands I., Vingsbo-Lundberg C., Bundgaard T., Lindenstrom T., Enouf V., Van Der Werf S., et al. (2011) Enhanced humoral and cell-mediated immune responses after immunization with trivalent influenza vaccine adjuvanted with cationic liposomes. Vaccine 29: 6283–6291 [DOI] [PubMed] [Google Scholar]
- Roulois D., Gregoire M., Fonteneau J. (2013) MUC1-specific cytotoxic T lymphocytes in cancer therapy: induction and challenge. Biomed Res Int 2013: 871936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksawad R., Likitnukul S., Warachit B., Hanvivatvong O., Poovorawan Y., Puripokai P. (2011) Immunogenicity and safety of a pediatric dose virosomal hepatitis a vaccine in Thai HIV-infected children. Vaccine 29: 4735–4738 [DOI] [PubMed] [Google Scholar]
- Sarkar S., Salyer A., Wall K., Sucheck S. (2013) Synthesis and immunological evaluation of a MUC1 glycopeptide incorporated into l-rhamnose displaying liposomes. Bioconjug Chem 24: 363–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwendener R., Ludewig B., Cerny A., Engler O. (2010) Liposome-based vaccines. Methods Mol Biol 605: 163–175 [DOI] [PubMed] [Google Scholar]
- Schweneker K., Gorka O., Schweneker M., Poeck H., Tschopp J., Peschel C., et al. (2013) The mycobacterial cord factor adjuvant analogue trehalose-6,6’-dibehenate (TDB) activates the NLRP3 inflammasome. Immunobiology 218: 664–673 [DOI] [PubMed] [Google Scholar]
- Senchi K., Matsunaga S., Hasegawa H., Kimura H., Ryo A. (2013) Development of oligomannose-coated liposome-based nasal vaccine against human parainfluenza virus type 3. Front Microbiol 4: 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shargh V., Jaafari M., Khamesipour A., Jaafari I., Jalali S., Abbasi A., et al. (2012) Liposomal SLA co-incorporated with PO CpG ODNs or PS CpG ODNs induce the same protection against the murine model of leishmaniasis. Vaccine 30: 3957–3964 [DOI] [PubMed] [Google Scholar]
- Shirota H., Klinman D. (2014) Recent progress concerning CpG DNA and its use as a vaccine adjuvant. Expert Rev Vaccines 13: 299–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singha H., Mallick A., Jana C., Fatima N., Owais M., Chaudhuri P. (2011) Co-immunization with interlukin-18 enhances the protective efficacy of liposomes encapsulated recombinant Cu-Zn superoxide dismutase protein against Brucella abortus. Vaccine 29: 4720–4727 [DOI] [PubMed] [Google Scholar]
- Smith D., Simon J., Baker J., Jr (2013) Applications of nanotechnology for immunology. Nat Rev Immunol 13: 592–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spang A., Martijn J., Saw J., Lind A., Guy L., Ettema T. (2013) Close encounters of the third domain: the emerging genomic view of archaeal diversity and evolution. Archaea 2013: 202358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprott G., Yeung A., Dicaire C., Yu S., Whitfield D. (2012) Synthetic archaeosome vaccines containing triglycosylarchaeols can provide additive and long-lasting immune responses that are enhanced by archaetidylserine. Archaea 2012: 513231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Provoda C., Lee K. (2010) Enhanced in vivo gene expression mediated by listeriolysin O incorporated anionic LPDII: its utility in cytotoxic T lymphocyte-inducing DNA vaccine. J Control Release 148: 219–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi A., Kobayashi N., Taneichi M., Uchida T., Akatsuka T. (2013) Coupling to the surface of liposomes alters the immunogenicity of hepatitis C virus-derived peptides and confers sterile immunity. Biochem Biophys Res Commun 430: 183–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamborrini M., Stoffel S., Westerfeld N., Amacker M., Theisen M., Zurbriggen R., et al. (2011) Immunogenicity of a virosomally-formulated plasmodium falciparum GLURP-MSP3 chimeric protein-based malaria vaccine candidate in comparison to adjuvanted formulations. Malar J 10: 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneichi M., Tanaka Y., Kakiuchi T., Uchida T. (2010) Liposome-coupled peptides induce long-lived memory CD8 T cells without CD4 T cells. PLoS One 5: e15091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur A., Aagaard C., Stockmarr A., Andersen P., Jungersen G. (2013) Cell-mediated and humoral immune responses after immunization of calves with a recombinant multiantigenic Mycobacterium avium subsp. paratuberculosis subunit vaccine at different ages. Clin Vaccine Immunol 20: 551–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomann J., Heurtault B., Weidner S., Braye M., Beyrath J., Fournel S., et al. (2011) Antitumor activity of liposomal ERBB2/HER2 epitope peptide-based vaccine constructs incorporating TLR agonists and mannose receptor targeting. Biomaterials 32: 4574–4583 [DOI] [PubMed] [Google Scholar]
- Torchilin V.P. (2005) Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov 4: 145–160 [DOI] [PubMed] [Google Scholar]
- Traub S., Von Aulock S., Hartung T., Hermann C. (2006) MDP and other muropeptides – direct and synergistic effects on the immune system. J Endotoxin Res 12: 69–85 [DOI] [PubMed] [Google Scholar]
- Tseng L., Liang H., Deng M., Lee K., Pan R., Yang J., et al. (2010) The influence of liposomal adjuvant on intranasal vaccination of chickens against Newcastle disease. Vet J 185: 204–210 [DOI] [PubMed] [Google Scholar]
- Underwood C., Van Eps A. (2012) Nanomedicine and veterinary science: the reality and the practicality. Vet J 193: 12–23 [DOI] [PubMed] [Google Scholar]
- Van Den Berg J., Oosterhuis K., Hennink W., Storm G., Van Der Aa L., Engbersen J., et al. (2010) Shielding the cationic charge of nanoparticle-formulated dermal DNA vaccines is essential for antigen expression and immunogenicity. J Control Release 141: 234–240 [DOI] [PubMed] [Google Scholar]
- Walczak M., De Mare A., Riezebos-Brilman A., Regts J., Hoogeboom B., Visser J., et al. (2011) Heterologous prime-boost immunizations with a virosomal and an alphavirus replicon vaccine. Mol Pharm 8: 65–77 [DOI] [PubMed] [Google Scholar]
- Wang C., Zhuang Y., Zhang Y., Luo Z., Gao N., Li P., et al. (2012) Toll-like receptor 3 agonist complexed with cationic liposome augments vaccine-elicited antitumor immunity by enhancing TLR3-IRF3 signaling and type I interferons in dendritic cells. Vaccine 30: 4790–4799 [DOI] [PubMed] [Google Scholar]
- Watson D., Endsley A., Huang L. (2012) Design considerations for liposomal vaccines: influence of formulation parameters on antibody and cell-mediated immune responses to liposome associated antigens. Vaccine 30: 2256–2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D., Platt V., Cao L., Venditto V., Szoka F., Jr (2011) Antibody response to polyhistidine-tagged peptide and protein antigens attached to liposomes via lipid-linked nitrilotriacetic acid in mice. Clin Vaccine Immunol 18: 289–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werninghaus K., Babiak A., Gross O., Holscher C., Dietrich H., Agger E., et al. (2009) Adjuvanticity of a synthetic cord factor analogue for subunit mycobacterium tuberculosis vaccination requires FcRgamma-Syk-Card9-dependent innate immune activation. J Exp Med 206: 89–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C., Fox G. (1977) Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc Natl Acad Sci U S A 74: 5088–5090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Park K., Soo R., Sun Y., Tyroller K., Wages D., et al. (2011) Inspire: a phase III study of the BLP25 liposome vaccine (L-BLP25) in Asian patients with unresectable stage III non-small cell lung cancer. BMC Cancer 11: 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaguchi K., Ohgitani T., Noro T., Kaneshige T., Shimizu Y. (2009) Vaccination of chickens with liposomal inactivated avian pathogenic Escherichia coli (APEC) vaccine by eye drop or coarse spray administration. Avian Dis 53: 245–249 [DOI] [PubMed] [Google Scholar]
- Yanasarn N., Sloat B., Cui Z. (2011) Negatively charged liposomes show potent adjuvant activity when simply admixed with protein antigens. Mol Pharm 8: 1174–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B., Mao Y., Bai L., Herman S., Wang X., Ramanunni A., et al. (2013) Targeted nanoparticle delivery overcomes off-target immunostimulatory effects of oligonucleotides and improves therapeutic efficacy in chronic lymphocytic leukemia. Blood 121: 136–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Karunakaran K., Jiang X., Shen C., Andersen P., Brunham R. (2012) Chlamydia muridarum T cell antigens and adjuvants that induce protective immunity in mice. Infect Immun 80: 1510–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Wang D., Abula S., Hu Y., Zhao X., Huang Y., et al. (2013) The immunological adjuvant activity of gypenosides liposome against Newcastle disease vaccine. Int J Biol Macromol 60: 116–121 [DOI] [PubMed] [Google Scholar]
- Zaman M., Good M., Toth I. (2013) Nanovaccines and their mode of action. Methods 60: 226–231 [DOI] [PubMed] [Google Scholar]
- Zepp F. (2010) Principles of vaccine design – lessons from nature. Vaccine 28 Suppl. 3: C14–C24 [DOI] [PubMed] [Google Scholar]
- Zhao X., Fan Y., Wang D., Hu Y., Guo L., Ruan S., et al. (2011) Immunological adjuvant efficacy of glycyrrhetinic acid liposome against Newcastle disease vaccine. Vaccine 29: 9611–9617 [DOI] [PubMed] [Google Scholar]
- Zhong Z., Wei X., Qi B., Xiao W., Yang L., Wei Y., et al. (2010) A novel liposomal vaccine improves humoral immunity and prevents tumor pulmonary metastasis in mice. Int J Pharm 399: 156–162 [DOI] [PubMed] [Google Scholar]
- Zhuang Y., Ma Y., Wang C., Hai L., Yan C., Zhang Y., et al. (2012) Pegylated cationic liposomes robustly augment vaccine-induced immune responses: role of lymphatic trafficking and biodistribution. J Control Release 159: 135–142 [DOI] [PubMed] [Google Scholar]