Abstract
Background:
In May 2012, universal vaccination with the 13-valent pneumococcal conjugate vaccine (PCV-13) was introduced for all children in the Tijuana region of Mexico, with a coverage of 80%.
Method:
Between October 2005 and September 2013 active surveillance was undertaken for all invasive pneumococcal diseases (IPDs) in children admitted to the Tijuana General Hospital.
Results:
Following PCV-13 implementation, there was a 75% reduction in overall IPD, and no cases of serotype 19A, pneumococcal meningitis, and pneumococcal-associated deaths.
Conclusions:
These results are the first to show the effectiveness of PCV-13 in Mexico.
Keywords: pneumococcal diesase, pneumococcal vaccine, streptococcus pneumoniae, vaccine effectiveness, mexico
Introduction
Invasive pneumococcal disease (IPD) is a serious and life-threatening condition. Introduction of the 7-valent pneumococcal conjugate vaccine (PCV-7) in young children in the USA and many other countries was associated with a reduction in IPD, especially on PCV-7-associated serotypes. Furthermore, a decrease in IPD by herd effect on other age groups was also seen [Centers for Disease Control and Prevention 2005; Pittet and Posfay-Barbe, 2012]. However, replacement by other pneumococcal serotypes appeared (e.g. 19-A, 7F, 3, among others), and the use of a vaccine with a wider serotype coverage was needed [McIntosh and Reinert, 2011; Rozenbaum et al. 2011]. Accordingly, the 13-valent pneumococcal conjugate vaccine (PCV-13) was soon implemented in the USA, UK, and other developed and developing countries, with clear evidence of its effectiveness on IPD by most serotypes included in some countries where PCV-13 was introduced [Kaplan et al. 2013; van Hoek et al. 2014].
In Latin America, based on results from a multinational passive surveillance system known as the System Network Monitoring Responsible Agents of Bacterial Meningitis and Pneumonia (SIREVA II), serotype 19A has emerged as the second leading cause of IPD in children, especially in countries where PCV-7 was implemented [Pan American Health Organization, 2014].
In Mexico, PCV-7 was introduced in 2006 as part of the universal immunization program in children, and the emergence of serotype 19A has been reported by SIREVA II in up to 41.8% of all pneumococcal isolates in children younger than 5 years of age during 2012 [Pan American Health Organization, 2014].
The Tijuana, Baja-California, Mexico and San Diego, California is the world’s most transited frontier, with up to 50,000 people daily crossing the border. We have previously published and presented the replacement of a pneumococcal serotype following the introduction of PCV-7, especially by serotypes 19-A, 7F, 3, and 6A/C [Chacon-Cruz et al. 2012]. In May 2012, universal vaccination with PCV-13 was introduced for all children in the region, with coverage of 80%. This study analyzes the effectiveness of PCV-13 16 months after vaccine implementation in the Tijuana region.
Methods
Between October 2005 and September 2013 (8 years), an active hospital-based surveillance was undertaken for all IPDs in children under 16 years of age admitted to the Tijuana General Hospital (TGH). The TGH covers approximately 40% of Tijuana’s population. The active surveillance consisted of actively looking at all children admitted to the emergency room with suspected sepsis, suspected meningitis, pneumonia with effusion, suspected bacteremic pneumonia, and/or mastoiditis. After a patient was detected clinically, blood/cerebrospinal fluid (CSF)/ pleural or mastoid cultures were immediately taken and incubated at 37°C and 5% carbon dioxide. Only culture-confirmed cases were included.
Following pneumococcal identification, serotyping was performed using the Quellung reaction (Statens Serum Institute®, Copenhagen, Denmark). Once a culture was positive for Streptococcus pneumoniae the patient was followed during his/her hospitalization. All cases were prospectively captured and followed, and further descriptive analysis (e.g. clinical, demographic, microbiological data) was performed using Excel®.
Results
A total of 48 cases of confirmed IPD were found. Clinical diagnosis was pleural empyema (48%), sepsis with/without other conditions (27%), meningitis (25%), otomastoiditis (18.75%), and bacteremic pneumonia (4.15%).
Median age was 3 years (15 days to 15 years), with 58.34% older than 2 years of age; 58.3% were male, 41.6% female. Median hospitalization days was 14 (1–90), and overall lethality was five cases (10.42%), of which four (80% of all deceased patients) had meningitis.
As seen in Figure 1, following PCV-13 implementation in May 2012, with eight confirmed IPD cases during 2011–12, there were only two cases during 2012–13 (75% reduction in overall IPD cases). Accordingly, after the universal introduction of PCV-13, there was a 100% reduction of IPD cases secondary to serotype 19-A, as well as an initial absence of cases of all pneumococcal meningitis and fatalities during the 2012–13 period. The two cases presented during the last year of surveillance were a pleural empyema caused by serotype 6A, and a bacteremic pneumonia caused by serotype 7B. Both were children older than 2 years, with a 2 + 1 completed schedule of PCV-7, but no PCV-13 immunization.
Figure 1.
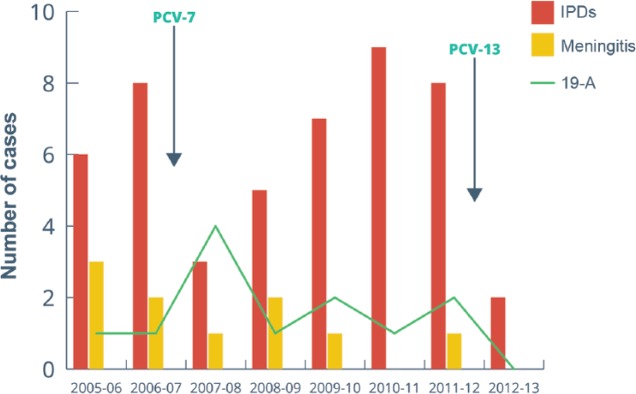
Reduction in overall IPD cases, and disappearance of pneumococcal meningitis and serotype 19-A following PCV-13 vaccination (n = 48). IPD, invasive pneumococcal disease; PCV-7, 7-valent pneumococcal conjugate vaccine PCV-13, 13-valent pneumococcal conjugate vaccine.
Conclusion
Surveillance in Mexico and Latin America of IPD is mostly based on passive surveillance (SIREVA II) [Pan American Health Organization, 2014]. In Mexico, universal immunization with PCV-7 started between 2005 and 2006, and serotype 19A emerged as the most frequent invasive serotype [Chacon-Cruz et al. 2012].
Previous studies carried out in our hospital showed that following PCV-7 introduction in Tijuana, the emergence of serotypes 19A, 7F, 3, and 6A/C occurred, with serotype 19A mostly associated with higher fatalities, meningitis, and hospitalization days, while serotype 3 was associated with pleural empyemas [Chacon-Cruz et al. 2011, 2012].
There is another publication in Mexico, also based on active surveillance in hospitals from four states, in which serotype 19A was the leading cause of IPD in children younger than 5 years, closely followed by serotypes 35B, 6A, and 19F [Bautista-Márquez et al. 2013].
PCV-13 has also been proved to be effective both on IPD and community-acquired pneumonia in children in other Latin American countries (i.e. Uruguay and Nicaragua) [Becker-Dreps et al. 2014; Pirez et al. 2014].
This is the first Mexican study based on active surveillance that shows early findings of the effectiveness of PCV-13 on reduction of overall IPD, decrease in pneumococcal serotype 19-A, and early effects of pneumococcal meningitis and fatalities in children. We are aware that further follow up is needed to confirm these findings, and also that this information comes from just one hospital. However, as mentioned above, the TGH covers approximately 40% of Tijuana’s population, and our study is based on strict identification of patients with suspected IPD, with blood/CSF, pleural and/or mastoid cultures taken immediately after admission, strongly suggesting that the effectiveness of PCV-13 is real, and consistent with data from other countries where PCV-13 has been implemented [Becker-Dreps et al. 2014; Kaplan et al. 2013; van Hoek et al. 2014].
We should continue this surveillance in order to see changes in the epidemiology associated with IPD, including circulation of serotypes and age presentation, among other factors, and also to investigate whether our data are in agreement with further findings from SIREVA II.
Footnotes
Funding: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Enrigue Chacon-Cruz, Pediatric Infectious Diseases Service, Hospital General de Tijuana, Tijuana Baja-California, Paseo Centenario S/N, Zona Rio, Tijuana, Baja-California, ZC 22010, Mexico.
R.M. Rivas-Landeros, Microbiology Laboratory, Hospital General de Tijuana, Tijuana Baja-California, Mexico
M.L. Volker-Soberanes, Microbiology Laboratory, Hospital General de Tijuana, Tijuana Baja-California, Mexico
References
- Bautista-Márquez A., Richardson V., Ortiz-Orozco O., Luna-Cruz M., Carnalla-Barajas M., Echaniz-Avilés G., et al. (2013) Prevalence of pneumococcal disease, serotype distribution, and antimicrobial susceptibility in Mexican children younger than 5 years of age. Arch Med Res 44: 142–150 [DOI] [PubMed] [Google Scholar]
- Becker-Dreps S., Amaya E., Liu L., Moreno G., Rocha J., Briceño R., et al. (2014) Changes in childhood pneumonia and infant mortality rates following introduction of the 13-valent pneumococcal conjugate vaccine in Nicaragua. Pediatr Infect Dis J 33: 637–642 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2005) Direct and indirect effect for routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence on invasive pneumococcal disease – United States. 1998–2003. MMWR Morb Mortal Wkly Rep 54: 893–897 [PubMed] [Google Scholar]
- Chacon-Cruz E., Rivas-Landeros R., Volker M. (2011) Pneumococcal pleural empyema and otomastoiditis: emergence of serotypes 19A, 7F and 3 in northern Mexico. Presented at the 29th Annual Meeting of the European Society for Paediatric Infectious Diseases, The Hague, Netherlands, abstract number 54. [Google Scholar]
- Chacon-Cruz E., Velazco-Mendez Y., Navarro-Alvarez S., Rivas-Landeros R., Volker M., Lopez-Espinosa G. (2012) Pneumococcal disease: emergence of serotypes 19A and 7F following pneumococcal conjugate vaccination in a Mexican hospital. J Infect Dev Ctries 6: 516–520 [DOI] [PubMed] [Google Scholar]
- Kaplan S., Barson W., Lin P., Romero J., Bradley J., Tan T., et al. (2013) Early trends for invasive pneumococcal infections in children after the introduction of the 13-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J 32: 203–207 [DOI] [PubMed] [Google Scholar]
- McIntosh E., Reinert R. (2011) Global prevailing and emerging pediatric pneumococcal serotypes. Expert Rev Vaccines 10: 109–129 [DOI] [PubMed] [Google Scholar]
- Pan American Health Organization (2014) SIREVA II. [http://www.paho.org/hq/index.php?option=com_content&view=category&layout=blog&id=3609&Itemid=3953]
- Pirez M., Algorta G., Chamorro F., Romero C., Varela A., Cedres A., et al. (2014) Changes in hospitalizations for pneumonia after universal vaccination with pneumococcal conjugate vaccines 7/13 valent and Haemophilus influenzae type b conjugate vaccine in a pediatric referral hospital in Uruguay. Pediatr Infect Dis J 33: 753–759 [DOI] [PubMed] [Google Scholar]
- Pittet L., Posfay-Barbe K. (2012) Pneumococcal vaccines for children: a global public health priority. Clin Microbiol Infect 18(Suppl. 5): 25–36 [DOI] [PubMed] [Google Scholar]
- Rozenbaum M., Boersma C., Postma M., Hak E. (2011) Observed differences in invasive pneumococcal disease epidemiology after routine infant vaccination. Expert Rev Vaccines 10: 187–199 [DOI] [PubMed] [Google Scholar]
- van Hoek A., Sheppard C., Andrews N., Waight P., Slack M., Harrison T., et al. (2014) Pneumococcal carriage in children and adults two years after introduction of the thirteen valent pneumococcal conjugate vaccine in England. Vaccine 32: 4349–4355 [DOI] [PubMed] [Google Scholar]


