Abstract
The hallmark of neuronopathic Gaucher disease (GD) is oculomotor abnormalities, but ophthalmological assessment is difficult in uncooperative patients. Chromatic pupillometry is a quantitative method to assess the pupillary light reflex (PLR) with minimal patient cooperation. Thus, we investigated whether chromatic pupillometry could be useful for neurological evaluations in GD. In our neuronopathic GD patients, red light-induced PLR was markedly impaired, whereas blue light-induced PLR was relatively spared. In addition, patients with non-neuronopathic GD showed no abnormalities. These novel findings show that chromatic pupillometry is a convenient method to detect neurological signs and monitor the course of disease in neuronopathic GD.
Introduction
Gaucher disease (GD) is the lysosomal storage disorder, caused by a deficiency of the lysosomal enzyme glucocerebrosidase (GBA), which catalyzes the degradation of glucosylceramide. GD phenotypes are clinically divided into three types: type 1 (GD1), the non-neuronopathic form; type 2 (GD2), infantile onset and rapid relentless neurological progression leading to death, usually by 2 years of age; type 3 (GD3), subacute neuronopathic, characterized by slower and more variable neurological progression.1
GD often involves the visual system and oculomotor deficits are the earliest symptoms identified in neuronopathic GD patients.2 The most common manifestation is saccadic initiation failure, which has been variously labeled as “ocular motor apraxia” or “horizontal supranuclear gaze palsy.”3,4 These findings are pathognomonic and play an important role in diagnosis, whereas it is difficult to record saccades objectively in uncooperative patients and in children.
In addition to these ocular manifestations, we noticed that some patients with neuronopathic GD have dilated pupils and reacted sluggishly to a broad-spectrum (white) light. Pupillary light reflex (PLR) can be measured noninvasively using a pupillometer, which uses an infrared camera to measure the pupil's reaction to light. Certain commercially available models allow for short testing times and minimal patient cooperation. Such devices are currently used in the clinic and in clinical research to measure retina, optic nerve, oculomotor, and brainstem functions.5,6
In this study, we evaluated pupil responses using chromatic pupillometry in GD patients to assess the incidence of PLR impairment and establish whether PLR is useful in the detection of neurological symptoms.
Subjects and Methods
Subjects
Data were acquired from 10 GD patients (one GD1 patient, five GD2 patients, and four GD3 patients) and 32 healthy controls (Control 1: n = 30, median age 23, range 22–37 years of age; Control 2: 4-year-old female; Control 3: 6-year-old female). The diagnosis of GD was confirmed by a deficiency of GBA activity in leukocytes or cultured fibroblasts and mutation analysis of the GBA gene. All GD patients underwent enzyme replacement therapy (imiglucerase, 60 IU/kg every 2 weeks) during the study. Moreover, they underwent general ophthalmological assessments and electrophysiologic studies (electroretinogram: ERG and visual evoked potential: VEP) to exclude other causes of visual impairment before PLR was assessed. All participants gave written informed consent to participation in the study, which had secured ethical approval from the institutional review board (Tottori University School of Medicine Ethics Committee Approval 2012).
Chromatic pupillometry
A binocular infrared pupillometer, Iriscoder Dual C10641 (Hamamatsu Photonics, Hamamatsu, Japan) was used for all experiments. This device is the same as that described by Ishikawa et al.,7 and capable of recording PLR under blue (470 nm) and red (635 nm) LED light stimuli. The stimulation luminance is selectable from 10, 100, and 270 cd/m2. For this experiment, we selected 1-sec blue and red stimuli of 100 cd/m2. This protocol was based on the assumption that a high-intensity blue stimulus (100 cd/m2, <480 nm) sensitizes intrinsically photosensitive retinal ganglion cells (ipRGCs), whereas a high-intensity red stimulus (100 cd/m2, >620 nm) sensitizes L/M cones.8,9
Before recording, the subjects were asked to wear the goggles for 10 min for dark adaptation. In each series, the red stimulus was presented first, followed by the blue stimulus. PLR was measured in the same eye that received the light stimulation (closed-loop paradigm). One set of pupillary response tests consisted of three parts: 1 sec before stimulation, 1 sec during stimulation, and 3 sec after stimulation (a total of 5 sec). Figure1 shows a representative normal pupil response.
Figure 1.
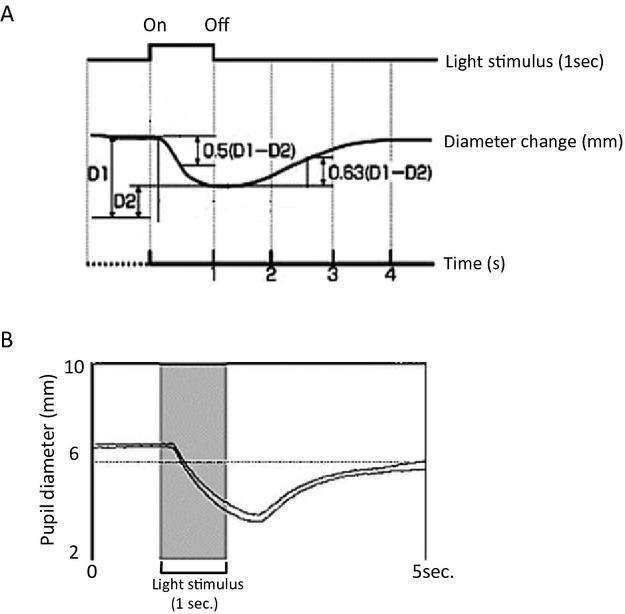
Example of a normal pupillary light reflex (PLR) profile. (A) PLR parameters. D1 = Initial pupil diameter (mm), D2 = Minimum pupil diameter (mm) after a pupillary reaction to light, CR = Initial constriction rate (%) = (D1 − D2)/D1 × 100 (B) Actual PLR recordings for both pupils.
Pupil recording and analysis
We evaluated the initial constriction rate (CR). CR was calculated using the following formula: CR (%) = (initial pupil diameter: D1 − minimum pupil diameter: D2)/D1 × 100. D1 was derived from the median size during the 1 sec just before the onset of each light stimulus, after dark adaptation. Negative constriction was defined as responses <5% to distinguish evoked pupil responses from random noise. Each procedure was tested twice after a minimum interval of 1 min without light stimulation. For each pupillometric variable, we used the average of all measurements for both eyes.
Results
The clinical characteristics and findings are provided in Figure2 and Table1. The graphic representation of the pupillary response to monochromatic light stimulation showed that red light-induced CRs (R-CRs) were markedly attenuated or absent in neuronopathic GD patients (Patients 2–10). Although R-CR was measured quantitatively in four patients (Patients 2, 3, 4, and 7), visibly clear waveforms were confirmed in only one patient (Patient 2); the remaining three patients showed significantly low R-CRs. In contrast, blue light-induced CRs (B-CRs) were relatively spared. All neuronopathic patients already exhibited horizontal saccadic initiation failure at initial PLR examination. Patient 1 (GD1) had no abnormalities in all assessments.
Figure 2.
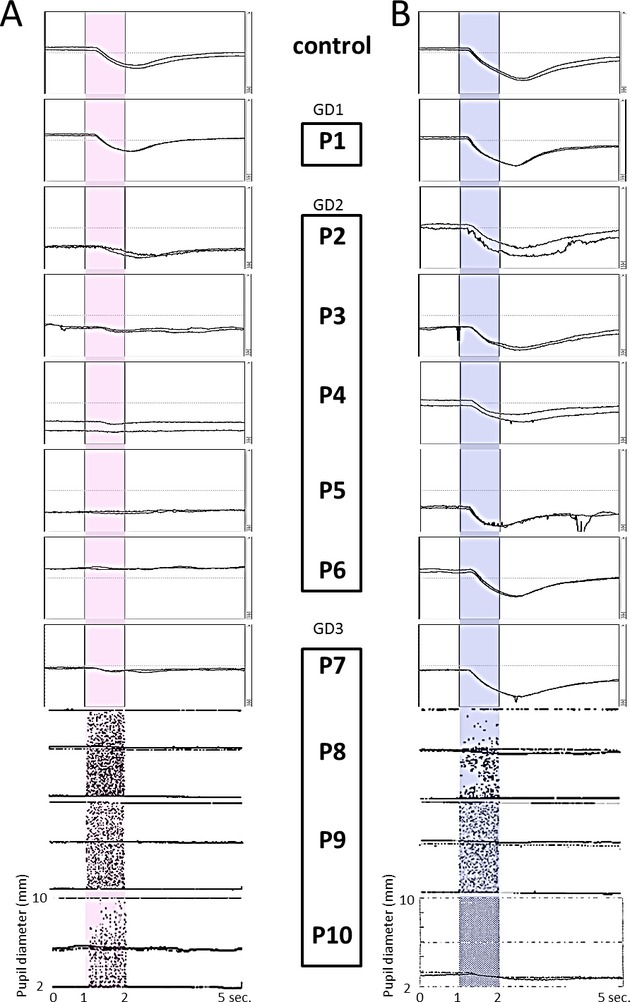
The pupillary light reflex (PLR) to monochromatic light stimulation, as measured in 10 GD patients and controls. Each trace was elicited by a red (A) or blue (B) single flash stimulus, with duration of 1 sec and intensity of 100 cd/m2. (A) Red light-induced PLRs were normal in P1 (GD1: non-neuronopathic type) but markedly attenuated or absent in neuronopathic GD patients (P2–6: type 2, P7–10: type 3) except for P2. (B) Blue light-induced PLRs were normal in P1 and relatively spared in GD2 patients but absent in GD3 patients (except for P7). The mean measurements of initial constriction rate (CR) are shown in Table1.
Table 1.
Genotypes, phenotypes, and clinical findings of GD patients.
| Results of pupillometry |
Horizontal |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Sex | Age at onset of HSIF | Age at PLR exam | Phenotype | Genotype | ADL | Communication | R-CR (%) | B-CR (%) | Visual acuity | SIF | PF | Vertical gaze palsy | VOR | VEP | ERG |
| 1 | M | (−) | 24 years | 1 | L444P/D409H | N | N | 28.8 | 46.8 | N | (−) | (−) | (−) | (+) | N | N |
| 2 | M | 7 months | 7 months | 2 | F213I/R120W | N | N | 22.7 | 55.0 | N | (+) | (+) | (−) | (+) | N | N |
| 3 | F | 6 months | 7 months | 2 | V230G/R296X | B/Tra/Tu | I | 15.0 | 44.5 | NA | (+) | (+) | (+) | NA | NA | N |
| 4 | F | 3 months | 9 months | 2 | L444P/R120W | B/Tra/Tu/V | I | 5.5 | 40.0 | NA | (+) | (+) | (+) | (−) | Giant VEP | OPs↓ |
| 5 | F | ? | 2 years | 2 | RecNciI/? | B/Tra/Tu/V | I | (−) | 44.7 | NA | (+) | (+) | (+) | (−) | N | OPs↓ |
| 6 | F | 2 months | 3 years | 2 | F213I/RecNciI | B/Tra/Tu/V | I | (−) | 38.8 | NA | (+) | (+) | (+) | (−) | Giant VEP | OPs↓ |
| 7 | F | 8 months | 9 years | 3 | L444P/L444P | N | N | 7.0 | 51.0 | N | (+) | (+) | (−) | (−) | N | N |
| 8 | F | 16 years | 16 years | 3 | N188S/? | TA | N | (−) | (−) | N | (+) | (−) | (−) | Fast phase (−) | N | N |
| 9 | F | 20 years | 20 years | 3 | N188S/? | TA | N | (−) | (−) | N | (+) | (−) | (−) | Fast phase (−) | Giant VEP | N |
| 10 | F | 14 years | 29 years | 3 | N188S/G193W | B/Tra/Tu | I | (−) | (−) | NA | (+) | (+) | (+) | (−) | Giant VEP | N |
| Control 1 (n = 30) | Median age: 23 years (range: 22 – 37 years, M:F = 9:13) | 35.1 ± 7.0 | 48.1 ± 5.6 | |||||||||||||
| Control 2 | 4 years (F) | 45.0 | 56.0 | |||||||||||||
| Control 3 | 6 years (F) | 38.0 | 53.5 | |||||||||||||
F, female; M, male; HSIF, horizontal saccadic initiation failure; PLR, pupillary light reflex; ADL, activity of daily living; N, normal or age-appropriate; B, bedridden; Tra, tracheotomy; V, ventilation; Tu, tube feeding; TA, total assistance; I, impaired; R-CR, red light-induced initial constriction rate; B-CR, blue light-induced initial constriction rate; (−), negative construction (CR < 5%); SIF, saccadic initiation failure; PF, pursuit failure; VOR, vestibulo-ocular reflex; ERG, electroretinogram; VEP, visual evoked potential; NA, not available; OPs, oscillatory potentials; ↓, attenuated.
Discussion
In this study, we successfully used pupillometry to identify PLR impairment in neuronopathic GD patients. We noted that the qualitative trend for reduced R-CRs was associated with the severity of the neurological symptoms, as indicated by activity of daily living (ADL) deficits. Our findings suggest that neuronopathic GD progression induces severe attenuation of R-CR, with relative sparing of B-CR. To our knowledge, the abnormal PLR in neuronopathic GD is a novel physiological finding.
The PLR pathway begins with the axons of photosensitive retinal ganglion cells (RGCs) that convey information to the optic nerve. The optic nerve subsequently connects to several targets in the midbrain, including the olivary pretectal nucleus (OPN) and Edinger–Westphal nucleus (EWN), and the oculomotor nerve. Neuronopathic GD is known to involve the midline of the dorsal brainstem, EWN, and oculomotor nucleus.10–12 Thus, it is possible that PLR abnormalities may reflect dysfunctional parasympathetic innervation between the dorsal midline of the midbrain and the iris. However, the physiology that underlies the difference between R-CR and B-CR remains unclear. These findings led us to propose two hypotheses: (1) relative sparing of B-CR may be derived from the function of ipRGCs; (2) functional changes of the inner retina may lead to loss of R-CR.
For decades, rods and cones were considered the only photoreceptors, and both provide excitatory input to conventional RGCs via bipolar cells. However, the discovery of ipRGCs and the presence of the characteristic photopigment melanopsin in them have led to changes in this classical view.13,14 Melanopsin-expressing ipRGCs comprise 0.2% RGCs and respond to light stimulation in the absence of any synaptic rod/cone input.15 The ipRGCs combine their direct photoresponses with synaptic rod/cone input and project to several brain nuclei that regulate circadian rhythms (the suprachiasmatic nucleus), PLR (OPN) and the imaging-forming system (the lateral geniculate nucleus). However, little is known about the differences in the pathways of conventional RGCs and ipRGCs in the optic nerve and brainstem.
Recently, increasing evidence has indicated that ipRGCs are resistant to neurodegeneration. Histopathological studies in patients with mitochondrial optic neuropathy have shown relative sparing of ipRGCs compared with conventional RGCs,16 with pupillometry analysis showing a slight reduction in CR in the affected eye.17 In addition, only ˜17% ipRGCs need to be activated to drive full pupillary constriction in ipRGC-knockout mice.18 Thus, we speculate that ipRGCs likely have a high cellular resistance to metabolic derangement, leading to relative preservation of B-CR.
Next, we investigated why neuronopathic GD patients show R-CR impairment even though their rods and cones remain functional. Several prior studies have suggested the utility of chromatic pupillometry to monitor each photoreceptor separately using stimuli of different wavelengths and intensities.8,9,19 M/L cones can be uniquely stimulated at wavelengths beyond 620 nm (red light, λmax = 543, 566 nm), whereas other photoreceptors are thought to be insensitive. Rods respond to blue light (λmax = 507 nm) at low luminance levels (normal threshold at −3 to −5 log cd/m2) and ipRGCs are sensitized to blue light (λmax = 482 nm) at higher luminance levels (100 cd/m2).
In this study, we selected high-intensity blue and red stimuli to sensitize ipRGCs and L/M cones. Although all patients exhibited normal a-wave amplitudes with flash-ERG, which reflects cone activity, R-CR was severely impaired. On the other hand, decreased ERG OPs were found in our patients and decreased ERG b-wave amplitudes have been reported in a visually asymptomatic GD patient.20 Lowering OPs and b-wave amplitudes are typically attributed to changes in the inner nuclear layer (amacrine cells and Müller cells) of the retina.
Therefore, we assume that the primary deficit (storage of substrate) caused secondary functional changes to the inner retina, and the synaptic rod/cone inputs to both RGCs via bipolar cells may be blocked, resulting eventually in the progression of R-CR (derived from cone/rod activation via conventional RGCs and ipRGCs) to loss. On the other hand, B-CR may be relatively spared because of their intrinsic response of ipRGCs. While further studies are warranted, chromatic pupillometry can be used to facilitate future investigation of GD pathophysiology.
In conclusion, neuronopathic GD patients have PLR impairments and chromatic pupillometry appears to be a useful method to evaluate such patients, regardless of age or neurocognitive status. Further studies are required to investigate the utility of this method to monitor prognosis and as a predictor of disease progression in larger patient samples.
Acknowledgments
This study was supported by grants from the Ministry of Health, Labor and Welfare of Japan (H23-25 Nanji-Ippan-002).
Author Contribution
Kentarou Shirai, M.D., Koyo Ohno, M.D., Yoko Nishimura, M.D., and Akiko Tamasaki, M.D. were involved in examining the patients and collecting the resulting data. Norika Kubota, Ph.D., M.D., Rumiko Takayama, M.D., Yukitoshi Takahashi, Ph.D., M.D., Takanori Onuki, M.D., Chikahiko Numakura, Ph.D., M.D., Mitsuhiro Kato, Ph.D., M.D., Yusuke Hamada, M.D., Norio Sakai, Ph.D., M.D., Atsuko Ohno, M.D., Maya Asami, M.D., Shoko Matsushita, Ph.D., M.D., Anri Hayashi, M.D., Tomohiro Kumada, Ph.D., M.D., Tatsuya Fujii, Ph.D., M.D., Asako Horino, M.D., and Takeshi Inoue, M.D., Ichiro Kuki, M.D. were responsible for introducing the patients to our hospital and actively involved in the methods employed for the purpose of this study. Ken Asakawa, Ph.D., Hitoshi Ishikawa, Ph.D., M.D., Yoshihiro Maegaki, Ph.D., M.D., and Kousaku Ohno, Ph.D., M.D. supervised the entire study, providing value inputs for the methods and discussion aspects of the manuscript.
Conflict of Interest
None declared.
Funding Information
This study was supported by grants from the Ministry of Health, Labor and Welfare of Japan (H23-25 Nanji-Ippan-002).
References
- 1.Scriver CR. The metabolic and molecular bases of inherited disease. 7th ed. New York: McGraw-Hill, Health Professions Division; 1995. [Google Scholar]
- 2.Tylki-Szymanska A, Vellodi A, El-Beshlawy A, et al. Neuronopathic Gaucher disease: demographic and clinical features of 131 patients enrolled in the International Collaborative Gaucher Group Neurological Outcomes Subregistry. J Inherit Metab Dis. 2010;33:339–346. doi: 10.1007/s10545-009-9009-6. [DOI] [PubMed] [Google Scholar]
- 3.Patterson MC, Horowitz M, Abel RB, et al. Isolated horizontal supranuclear gaze palsy as a marker of severe systemic involvement in Gaucher's disease. Neurology. 1993;43:1993–1997. doi: 10.1212/wnl.43.10.1993. [DOI] [PubMed] [Google Scholar]
- 4.Harris CM, Taylor DS, Vellodi A. Ocular motor abnormalities in Gaucher disease. Neuropediatrics. 1999;30:289–293. doi: 10.1055/s-2007-973507. [DOI] [PubMed] [Google Scholar]
- 5.Kardon R, Anderson SC, Damarjian TG, et al. Chromatic pupillometry in patients with retinitis pigmentosa. Ophthalmology. 2011;118:376–381. doi: 10.1016/j.ophtha.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 6.Kawasaki A, Crippa SV, Kardon R, et al. Characterization of pupil responses to blue and red light stimuli in autosomal dominant retinitis pigmentosa due to NR2E3 mutation. Invest Ophthalmol Vis Sci. 2012;53:5562–5569. doi: 10.1167/iovs.12-10230. [DOI] [PubMed] [Google Scholar]
- 7.Ishikawa H, Onodera A, Asakawa K, et al. Effects of selective-wavelength block filters on pupillary light reflex under red and blue light stimuli. Jpn J Ophthalmol. 2012;56:181–186. doi: 10.1007/s10384-011-0116-1. [DOI] [PubMed] [Google Scholar]
- 8.Kardon R, Anderson SC, Damarjian TG, et al. Chromatic pupil responses: preferential activation of the melanopsin-mediated versus outer photoreceptor-mediated pupil light reflex. Ophthalmology. 2009;116:1564–1573. doi: 10.1016/j.ophtha.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Park JC, Moura AL, Raza AS, et al. Toward a clinical protocol for assessing rod, cone, and melanopsin contributions to the human pupil response. Invest Ophthalmol Vis Sci. 2011;52:6624–6635. doi: 10.1167/iovs.11-7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buttner-Ennever JA, Uemura T, Arai Y, et al. Horizontal saccadic palsy associated with gliosis of the brainstem midline. Prog Brain Res. 2008;171:597–603. doi: 10.1016/S0079-6123(08)00687-0. [DOI] [PubMed] [Google Scholar]
- 11.Kaga K, Ono M, Yakumaru K, et al. Brainstem pathology of infantile Gaucher's disease with only wave I and II of auditory brainstem response. J Laryngol Otol. 1998;112:1069–1073. doi: 10.1017/s0022215100142483. [DOI] [PubMed] [Google Scholar]
- 12.Grafe M, Thomas C, Schneider J, et al. Infantile Gaucher's disease: a case with neuronal storage. Ann Neurol. 1988;23:300–303. doi: 10.1002/ana.410230315. [DOI] [PubMed] [Google Scholar]
- 13.Qiu X, Kumbalasiri T, Carlson SM, et al. Induction of photosensitivity by heterologous expression of melanopsin. Nature. 2005;433:745–749. doi: 10.1038/nature03345. [DOI] [PubMed] [Google Scholar]
- 14.Hattar S, Kumar M, Park A, et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dacey DM, Liao HW, Peterson BB, et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- 16.La Morgia C, Ross-Cisneros FN, Sadun AA, et al. Melanopsin retinal ganglion cells are resistant to neurodegeneration in mitochondrial optic neuropathies. Brain. 2010;133:2426–2438. doi: 10.1093/brain/awq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawasaki A, Herbst K, Sander B, et al. Selective wavelength pupillometry in Leber hereditary optic neuropathy. Clin Experiment Ophthalmol. 2010;38:322–324. doi: 10.1111/j.1442-9071.2010.02212.x. [DOI] [PubMed] [Google Scholar]
- 18.Guler AD, Ecker JL, Lall GS, et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Markwell EL, Feigl B, Zele AJ. Intrinsically photosensitive melanopsin retinal ganglion cell contributions to the pupillary light reflex and circadian rhythm. Clin Exp Optom. 2010;93:137–149. doi: 10.1111/j.1444-0938.2010.00479.x. [DOI] [PubMed] [Google Scholar]
- 20.Seidova SF, Kotliar K, Foerger F, et al. Functional retinal changes in Gaucher disease. Doc Ophthalmol. 2009;118:151–154. doi: 10.1007/s10633-008-9142-9. [DOI] [PubMed] [Google Scholar]


