Abstract
Objective
To study the safety of antiepileptic drug (AED) withdrawal after temporal lobe epilepsy (TLE) surgery.
Methods
We reviewed patients who underwent TLE surgery from 1995 to 2011, collecting data on doses, dates of AED initiation, reduction, and discontinuation. Predictors of seizure outcome were defined using Cox-proportional hazard modeling and adjusted for, while comparing longitudinal seizure-freedom in patients for whom AEDs were unchanged after resection as opposed to reduced or stopped.
Results
A total of 609 patients (86% adults) were analyzed. Follow-up ranged from 0.5 to 16.7 years. Most (64%) had hippocampal sclerosis. Overall, 229 patients had remained on their same baseline AEDs, while 380 patients stopped (127 cases) or reduced (253 cases) their AEDs. Mean timing of the earliest AED change was shorter in patients with recurrent seizures (1.04 years) compared to those seizure-free at last follow-up (1.44 years; P-value 0.03). Whether AEDs were withdrawn 12 or 24 months after surgery, there was a 10–25% higher risk of breakthrough seizures within the subsequent 2 years. However, 70% of patients with seizure recurrence after AED discontinuation reachieved remission, as opposed to 50% of those whose seizures recurred while reducing AEDs (P = 0.0001). Long-term remission rates were similar in both AED discontinuation and “unchanged” groups (82% remission for AEDs withdrawn after 1 year and 90% for AEDs withdrawn after 2 years), while only 65% of patients whose recurrences started during AED reduction achieved a 2-year remission by last follow-up.
Interpretation
AED withdrawal increases the short-term risk of breakthrough seizures after TLE surgery, and may alter the long-term disease course in some patients.
Introduction
The debate is ageless on how to manage antiepileptic drugs (AED)s after epilepsy surgery. Several factors underlie a need to reduce medications, including concerns about toxicity of polytherapy,1,2 a perpetuation of the “sick role” with seizure-free patients continuing a complicated medication regimen years after surgery,3,4 and ballooning AED costs.5,6 Conversely, many epileptologists fear the unknown risk of provoking a breakthrough seizure or recurrence of refractory epilepsy, and most would not consider stopping AEDs before at least 2 years of postoperative seizure-freedom.7,8 Seizures do recur in 10–30% of all patients by one postoperative year. Seizure-free rates continue to decline by 2–4% per year beyond three to five postoperative years, despite ongoing AED polytherapy.9–18 It is unknown whether these rates of seizure recurrence would be any higher if patients were on no or less AEDs instead.
There is little information guiding decision-making on postoperative AED management. Multiple retrospective series,19–25 and two observational prospective studies are available.25,26 All added valuable information, but some were restricted to children,19,22 most did not include a control group where AEDs were continued,24,25,27–29 none distinguished patients with AED reduction from those with AED discontinuation, and none were adequately powered to answer this basic question: expecting that seizures will recur over time in some patients after surgery, do they recur more often solely because AEDs are tapered? Furthermore, even if breakthrough seizures occur with AED withdrawal, do they herald the recurrence of intractable epilepsy30,31?
In this manuscript, we evaluate the immediate and long-term implications of AED reduction, AED discontinuation, or continuation of preoperative AED regimen in patients who underwent temporal lobe epilepsy (TLE) surgery.
Methods
Patient selection
With approval from our Institutional Review Board, patients of all ages who had TLE surgery between September 1995 and June 2011 were reviewed. Prior to surgery, patients had failed at least two AEDs, completed a presurgical workup and were selected for surgery after a multidisciplinary patient management conference. Routine postsurgical follow-up involved outpatient visits at 6 weeks, 3 months, 6 months, 1 year, and then yearly thereafter for seizure-free patients, with more frequent visits in patients with recurrent seizures. Patients who were not seen in clinic were contacted yearly by phone to update their clinical and medication status. The decision to withdraw AEDs was generally made under physician guidance, and if made by the patient was reported to the physician.
Data collection
We collected data on clinical, imaging, and electrophysiological patient characteristics, focusing on variables previously associated with seizure outcome after TLE surgery.
The doses of AEDs used at the time of surgery, at seizure recurrence, and at last available follow-up were recorded. Dates of initiation, reduction, and termination of AEDs were collected. When the precise date of medication change between two clinic encounters was not found, it was approximated to a midway point between these two visits. This approximation was made in 18% of the cases. The “AED status” for every patient was then classified into one of three categories: “unchanged” if the AED regimen (medication type and dose) was the same from surgery through the last follow-up; “reduced” if the number of AEDs or their doses were reduced at some point after surgery; or “stopped” if all AEDs were discontinued at some point.
Outcome definition
The primary outcome is complete seizure-freedom after surgery. In patients with acute postoperative seizures, the timing of the first seizure occurring beyond the acute postoperative period (>1 week) was considered as the timing of recurrence. We also performed a separate analysis defining a favorable seizure outcome as an “Engel class I” to allow comparisons regarding the probability of achieving an eventual 2-year remission.
Statistical methods and variable definition
Descriptive statistics were used for each variable and data were analyzed using Wilcoxon rank sum, Chi square and Fisher's exact tests to compare between seizure-free and seizure recurrent patients to provide potential prognostic factors. Variables with a significance level of 5% on univariate analysis were then tested in a multivariate Cox-proportional hazards regression model (again at 5% significance level). Multicolinearity was addressed and controlled for when appropriate. Predictors of AED outcome (reduced vs. stopped vs. unchanged) were similarly defined using a multivariate regression model. These two analyses (predictors of seizure outcome and predictors of AED management) identified the confounding variables to be considered with the subsequent analysis of the seizure outcomes in the various AED management groups.
Kaplan–Meier survival analysis was then done to calculate the probability of favorable seizure outcomes in patients who were seizure-free at 1 or 2 years and had their AEDs subsequently either reduced, stopped, or unchanged. These time-points were chosen as they clinically represent the various cutoffs discussed in the literature for “safe” AED withdrawal. The survival analysis included the subgroup with unchanged AEDs so that outcomes in the AED withdrawal categories can be put in perspective with a control group in place. Statistical significance was tested using the log-rank test and comparison of 95% confidence intervals.
Results
A total of 609 patients (including 85 children) fulfilled study criteria and were analyzed. The cohort's clinical, imaging, and surgical characteristics are summarized in Table1.
Table 1.
Overall baseline cohort characteristics.
| Baseline cohort characteristics | |
| Clinical characteristics | |
| Female (%) | 52 |
| Left-sided surgery (%) | 54 |
| Mean preoperative seizure-frequency/month (SD, range) | 25.4 (74.3, 0.5–900) |
| Mean age at seizure onset, years (SD, range) | 15.5 (13.8, 0.5–16.7) |
| Mean age at surgery, years (SD, range) | 34.7 (14.8, 1–74.3) |
| Mean follow-up duration, years (SD, range) | 4.62 (3.48, 0.5–16.7) |
| MRI findings (N = 609) | |
| Normal, number (%) | 81 (13) |
| Unilateral temporal lobe abnormality, number (%) | 453 (75) |
| Extratemporal abnormality, number (%) | 75 (12) |
| PET findings (N = 608) | |
| Normal, number (%) | 17 (3) |
| Abnormal, number (%) | 492 (81) |
| Not done, number (%) | 99 (16) |
| Type of surgery (N = 601) | |
| Standard temporal lobectomy, number (%) | 571 (95) |
| Selective amygdalohippocampectomy, number (%) | 17 (2.8) |
| Tailored cortical resection, number (%) | 13 (2.2) |
| Etiology (N = 608) | |
| Hippocampal sclerosis, number (%) | 389 (64) |
| Malformations of cortical development, number (%) | 103 (17) |
| Tumors, number (%) | 78 (13) |
| Vascular malformations, number (%) | 21 (4) |
| Others, number (%) | 17 (3) |
N = total number of patients with available data on variable of interest.
AED management
By last follow-up, there was no change in baseline preoperative AEDs in 229 patients (38%). AEDs were stopped completely at some point in 127 (21%) patients, and were reduced in 253 (42%) patients. The number of AEDs at the time of surgery ranged from one to five (mean 1.95, SD 0.7; median 2), and from zero to five AEDs at last follow-up (mean 1.42; SD 0.92; median 1). Tables2, 3 detail the dosing of the most commonly used AEDs.
Table 2.
Anti-epileptic drug (AED) usage at the time of surgery.
| Drug | #Patients (%) | Min dose (mg/day) | Max dose (mg/day) | Mean (mg/day) | Median (mg/day) | SD |
|---|---|---|---|---|---|---|
| LEV | 253 (42) | 500 | 6000 | 2406 | 2500 | 1047 |
| LTG | 217 (36) | 12 | 900 | 447 | 400 | 195 |
| CBZ | 169 (28) | 200 | 2400 | 1144 | 1200 | 439 |
| TOP | 113 (19) | 45 | 1800 | 385 | 300 | 295 |
| PHT | 110 (18) | 50 | 1000 | 403 | 400 | 152 |
| OXC | 93 (15) | 300 | 3000 | 1496 | 1500 | 664 |
LEV, levetiracetam; LTG, lamotrigine; CBZ, carbamazepine; TOP, topiramate; PHT, phenytoin; OXC, oxcarbazepine.
Table 3.
Antiepileptic drugs patients were taking at the point of last follow-up.
| Drug | #Patients (%) | Min dose (mg/day) | Max dose (mg/day) | Mean (mg/day) | Median (mg/day) | SD |
|---|---|---|---|---|---|---|
| LEV | 186 (31) | 250 | 5500 | 2290 | 2000 | 1086 |
| LTG | 197 (32) | 50 | 2250 | 487 | 425 | 260 |
| CBZ | 109 (18) | 200 | 2400 | 982 | 900 | 420 |
| TOP | 67 (11) | 50 | 800 | 330 | 300 | 183 |
| PHT | 52 (9) | 100 | 1200 | 370 | 342 | 157 |
| OXC | 71 (12) | 375 | 2400 | 1425 | 1500 | 503 |
LEV, levetiracetam; LTG, lamotrigine; CBZ, carbamazepine; TOP, topiramate; PHT, phenytoin; OXC, oxcarbazepine.
Patient characteristics in various AED management groups
Patients within the “reduction,” “discontinuation,” and “unchanged” cohorts had similar baseline seizure burden, epilepsy duration and imaging (MRI and positron emission tomography [PET]) findings, but differed in relation to: (1) etiology, (2) the number of AEDs being used at the time of surgery, and (3) presence of spikes on routine electroencephalography (EEG) done 6 months after surgery (Table4).
Table 4.
Relationship between baseline patient characteristics and AED management decisions.
| AED reduced (N = 253) | AED stopped (N = 127) | AED unchanged (N = 228) | P-value | |
|---|---|---|---|---|
| Etiology N (%) | ||||
| MTS | 173 (68%) | 66 (51%) | 150 (66%) | <0.0001 |
| MCD | 47 (19%) | 19 (15%) | 37 (16%) | |
| Tumor | 17 (7%) | 35 (28%) | 26 (12%) | |
| Vascular | 10 (4%) | 5 (4%) | 6 (2%) | |
| Other | 6 (2%) | 2 (2%) | 9 (4%) | |
| Baseline # of AEDs | ||||
| Mean ±SD error | 2.13 ± 0.04 | 1.69 ± 0.06 | 1.89 ± 0.05 | <0.0001 |
| Ipsilateral spikes on postoperative EEG | ||||
| Present | 29 (11%) | 19 (15%) | 42 (18%) | 0.02 |
| Absent | 212 (84%) | 96 (75%) | 152 (67%) | |
| Type of surgery | ||||
| ATL | 238 (94%) | 116 (92%) | 217 (95%) | 0.88 |
| SAH | 7 (3%) | 5 (4%) | 5 (2%) | |
| Neocortical | 6 (3%) | 2 (2%) | 5 (2%) | |
| Age group | ||||
| Adults | 226 (89%) | 102 (80%) | 189 (84%) | 0.06 |
| Children | 25 (10%) | 21 (17%) | 38 (17%) | |
| Side of surgery | ||||
| Left | 137 (54%) | 68 (53%) | 116 (51%) | 0.67 |
| Right | 114 (45%) | 55 (43%) | 111 (49%) | |
| MRI findings | ||||
| Unilateral temporal abnormality | 191 (75%) | 98 (77%) | 160 (70%) | 0.34 |
| Normal | 34 (13%) | 13 (10%) | 32 (14%) | |
| Temporal + extratemporal abnormalities | 26 (10%) | 12 (9%) | 34 (15%) | |
| PET scan | ||||
| Abnormal | 210 (83%) | 94 (74%) | 183 (80%) | 0.16 |
| Normal | 5 (2%) | 3 (2%) | 9 (4%) | |
| History of generalized tonic clonic seizures | ||||
| Present | 193 (76%) | 83 (65%) | 177 (77%) | 0.11 |
| Absent | 57 (23%) | 38 (30%) | 48 (21%) | |
| Baseline seizure-frequency mean (±SD) | 23.0 (± 4.7) | 17.4 (± 6.7) | 31.5 (± 4.9) | 0.20 |
| N patients (%) with any seizure recurrence | 143 (57%) | 59 (48%) | 130 (58%) | 0.17 |
MTS, mesial temporal sclerosis; MCD, malformation of cortical development; AED, antiepileptic drug; ATL, anterior temporal lobectomy; SAH, selective amygdalohippocampectomy.
The “AED reduction” category included 128 patients who had the number of their AEDs reduced, 78 whose number of AEDs was unchanged, but daily doses were decreased, and 47 who had both number and dose of AEDs reduced. The AED reduction involved a conversion into monotherapy from two to four baseline AEDs in 123 patients, a conversion into two AEDs in 23 patients, and a reduction from four to three AEDs in three patients.
Time frame of AED change
The exact dates of AED changes were available in 311 patients (82%) who had any AEDs withdrawn (253 reduced and 127 stopped).
In these 311 patients, the mean interval from surgery to starting AED withdrawal was 1.34 years (range 0–13.0 years, SD 1.70, median 0.67 years). This interval was longer in patients with >20 preoperative seizures/month (1.43 years vs. 0.95 years; P = 0.04), and it tended to be shorter in children (mean 0.88 years vs. 1.40 years in adults; P = 0.08).
The mean interval from surgery to the latest AED change was 1.64 years (range 0–12.2 years, SD 1.87 years, median 1.07 years). This interval correlated with age group (shorter in children 0.95 years vs. 1.76 years in adults; P = 0.002), suggesting a higher sense of “urgency” to stop AEDs in children compared to adults. We were unable to reliably determine from reviewing the records whether medications were being reduced with the eventual intent of complete discontinuation or solely to reduce side effects. On average though, the latest AED change occurred earlier in patients within the “AED reduction” category (1.57 ± 0.14 years after surgery) compared to 1.94 (±0.19) years in those with the discontinuation category (P = 0.11) suggesting that many patients were in the process of being tapered off medications when this was halted due to seizure recurrence.
The mean interval between surgery and seizure recurrence was 1.59 years ± 0.19 in patients with seizure recurrence in context of reduction versus 2.58 years ± 0.26 in the AED discontinuation group (P = 0.004). The mean interval between surgery and recurrence was 1.19 ± 0.19 years in those with no AED change.
Seizure outcome in overall cohort
A total of 338 patients (55%) had breakthrough seizures after surgery. However, 429 (71%) were in remission by last follow-up (Engel I), 81 cases (13%) had an Engel II (rare seizures), 68 (11%) had Engel III, and 30 (5%) had Engel IV. In fact, of the 338 patients who experienced any seizure recurrence, 163 (48%) were seizure-free by last follow-up (Engel I), 78 (23%) were significantly improved (Engel II), 67 (20%) had no worthwhile improvement (Engel III) and 30 (9%) were worse off (Engel IV).
In the subgroup with recurrence in the setting of AED reduction or discontinuation (112 patients), 75 were able to eventually achieve remission (68%), 26 had an Engel score of II at last follow-up, eight had Engel score III, and two had Engel score IV.
After multivariate modeling, two independent seizure outcome predictors were identified: ipsilateral spiking on postoperative EEG and preoperative seizure-frequency (whole model P-value <0.0001). These variables predicted outcome in the group as a whole, and within each subgroup of AED management individually.
Seizure outcome and AED management
The mean timing of the earliest AED change was shorter in patients with recurrent seizures (1.04 years) compared to those who were seizure-free (1.44 years after surgery; P-value=0.03). A similar trend (P = 0.08) was observed correlating the interval to the latest AED change with seizure outcome.
Subsequent analyses focused on patients who were seizure-free at 12 months (Fig.1A and B) and 24 months (Fig.2A and B) before undergoing AED withdrawal. We report seizure outcomes as A-complete seizure-freedom since surgery and B-Engel score I (allowing patients to have some breakthrough seizures) as long as they eventually achieve 2-year remission.
Figure 1.
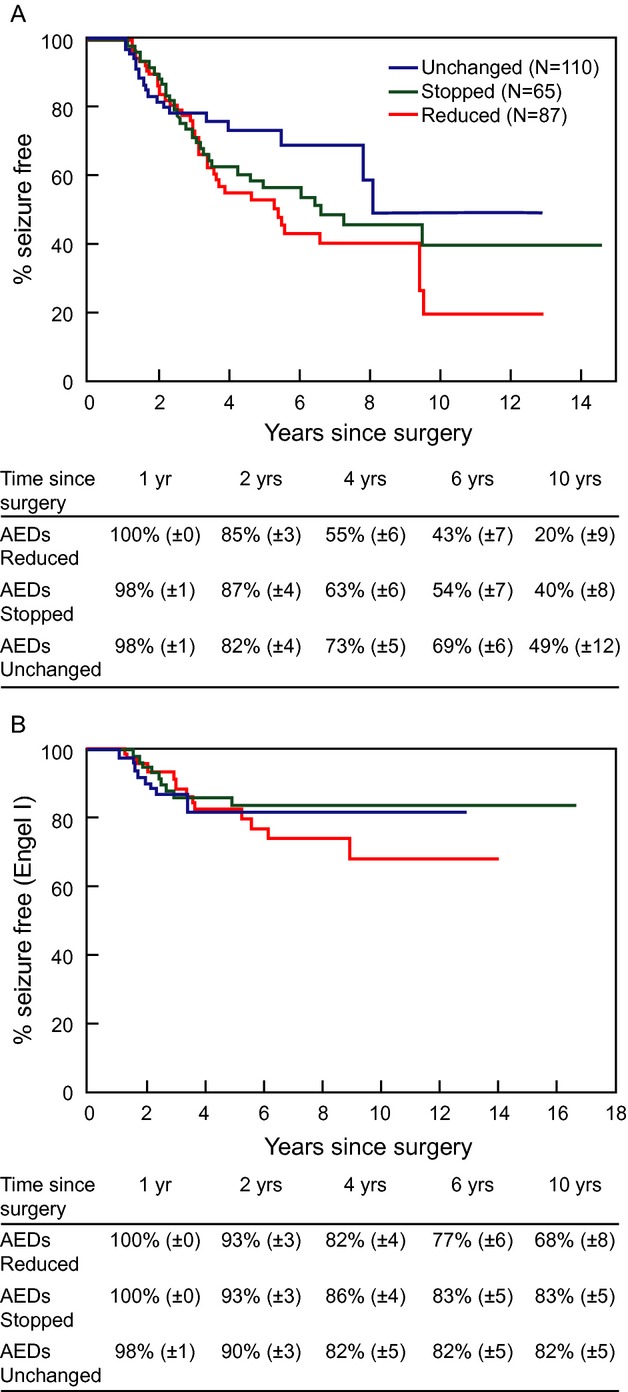
This figure illustrates the rates of a favorable seizure outcome as defined by complete seizure-freedom after surgery (A) and by Engel score of 1 (B) in the various medication management categories for patients who were seizure-free at one postoperative year. Median timing of the actual earliest AED change was 1.90 years.
Figure 2.
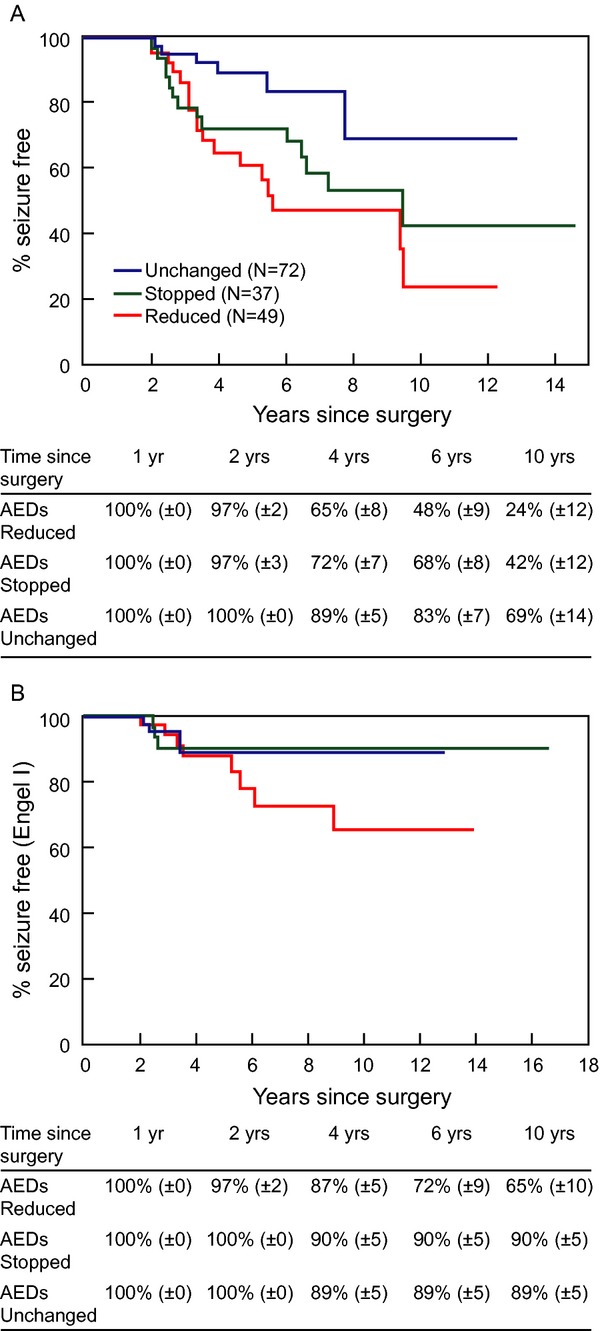
This figure illustrates the rates of a favorable seizure outcome as defined by complete seizure-freedom after surgery (A) and by Engel score of 1 (B) in the various medication management categories for patients who were seizure-free at two postoperative years.
Subgroup analyses in tumors versus other pathologies, and in patients with or without spikes on postoperative EEG showed the same findings as those illustrated in Figures1, 2.
Discussion
While recognizing that no definite guidelines on postoperative AED management can be derived from this retrospective observational cohort study, the work presented here provides both confirmatory and novel knowledge about this difficult subject.
AED management decisions
An adequate assessment of the reasons underlying patient and physician decision-making about AED withdrawal could not be reliably derived from our record review. Our findings suggest though that postoperative AED management in this TLE cohort may reflect a compromise between needing to relieve patients from significant medication-related side effects and confidence about surgical success. Patients with brain tumors (and thus an easily ascertainable complete removal of the epileptic focus) were at least twice as likely as those with any other epilepsy pathology to have their AEDs completely discontinued after surgery, while those with spikes on postoperative EEG (and thus an easily ascertainable presence of an epileptic focus) were almost twice as likely to continue their AEDs unchanged (Table4). The baseline number of AEDs (and potentially the degree of medication side effects) was, however, higher in this group with unchanged AEDs compared to that where AEDs were stopped, suggesting that the need to reduce side effects may have been mitigated by concerns over recurrence risk with higher baseline seizure severity. This finding aligns well with earlier studies suggesting that AEDs are often continued in patients felt to be at “high risk” of postoperative seizure recurrence.19,22,25 A thorough understanding of this issue requires a prospective study evaluating both the patient and physician perspectives as the decisions to continue or stop AEDs are being made.
Short-term implications of AED withdrawal
Whether AEDs were withdrawn after 1 or 2 years following successful surgery, patients whose AEDs were withdrawn were less likely to remain completely seizure-free within the subsequent 1–2 years compared to those whose AEDs were unchanged (Figs.1A, 2A). When patients within the 1-year withdrawal category were evaluated 2 years later, 72% (±5%) within the AED reduction group and 69% (±6%) within the AED discontinuation group had remained completely seizure-free as opposed to 79% (±4%) when AEDs were unchanged. Similarly, when patients whose AEDs were withdrawn at least 2 years after surgery were evaluated at four postoperative years, 55% (±6%) were seizure-free within the AED reduction group and 63% (±6%) within the AED discontinuation group as opposed to 73% (±5%) within the “unchanged AED” group. These observations are consistent with previous work, including a randomized clinical trial32 that evaluated AED withdrawal in the larger population of medically treated seizure-free patients, and found that medication withdrawal may slightly increase the risk of breakthrough seizures within the subsequent 12–24 months. They also concur with a large prospective observational cohort study comparing patients who reduced their medications either from two to one AED or from one to no AED after successful epilepsy surgery and found similarly increased relapse rates in both groups.25
Long-term implications of AED withdrawal
Published data suggest that AED withdrawal (lumping AED reduction and AED discontinuation) after surgery may not alter long-term rates of remission. One prospective observational study reporting the rates of seizure recurrence after AED taper initiated 3 months or 1 year after surgery found an eventual rate of seizure recurrence similar to a small group of historical controls.26 In our cohort, a “withdrawal group” lumping reduction and discontinuation had comparable late outcomes to that with continued AEDs (data not shown). However, examination of the Engel scores at 6 and 10 years shows that the AED discontinuation group typically regained seizure-freedom and achieved long-term remission rates (Engel I) that were similar to the “unchanged AED” group, while patients whose seizures recurred in the context of AED reduction persistently had lower chances of long-term remission (Figs.1B, 2B). This again occurred whether AEDs were withdrawn after 1 or 2 years. In addition, 70% patients with seizure recurrence after AED discontinuation were able to reachieve remission, as opposed to 50% of those whose seizures recurred while reducing AEDs and 37% of the recurrences whose AEDs had remained unchanged since surgery (P = 0.0001). These findings reproduce an earlier study where postoperative seizures in the context of AED reduction were more likely to become intractable than those starting after AED discontinuation.33 Of note, the poor prognostic implications of recurrence during AED reduction occurred regardless of the underlying pathology. Put together, these findings suggest an inherently different pathophysiology and implications of seizures recurring while AEDs are being reduced versus after discontinuation, a pathophysiology that most likely transcends individual medications or traditional epilepsy characteristics and is rather “substrate”-dependent. A high seizure-frequency is associated with poor drug response in both humans and animal models of new-onset epilepsy.34 Variations in the timing of initial exposure to some AEDs following the induction of kindling alters the subsequent effectiveness of these drugs.35 In fact, potential genetic mechanisms of variation in epilepsy severity have been identified, including subclinical mutations in ion channels that increase or reduce epilepsy severity in mice, regardless of underlying pathology.36 Within this framework, our results suggest that similar potential genetic mechanisms may underlie the “rekindling” of intractable epilepsy after breakthrough seizures in some patients, but not others.
On the upside, our results allow the clinician to reassure patients whose seizures recur after completely stopping AEDs about their long-term prognosis. On the downside, we could not identify specific clinical predictors that can adequately distinguish a priori patients who will successfully complete AED withdrawal. This highlights our limitations in understanding the mechanisms of postoperative seizure outcomes and emphasizes the need for specific and reliable biomarkers of disease and treatment response.
Early versus late AED withdrawal
Earlier AED withdrawal in our group was associated with a higher rate of initial seizure recurrence: (1) the mean timing of the earliest AED change was shorter in patients with recurrent seizures compared to those who remained seizure-free, and (2) patients in the 1-year withdrawal category had about 10% lower chance of being seizure-free at 4 years when compared to the 2-year withdrawal category. Specifically, at the 4-year time point (Figs.1A, 2A), 55% were seizure-free in the AED reduction group and 63% in the AED discontinuation group in the 1-year withdrawal cohort, as opposed to 65% and 72%, respectively, in the 2-year withdrawal group. This is consistent with earlier literature,7,28 and intuitively expected. However, the long-term rates of remission were the same within each AED management category, whether the AEDs were withdrawn at 1 or 2 years. For example, evaluating the “AED reduction” category, the 6-year seizure-freedom rate was 77% when AEDs were reduced at 1 year (Fig.1B) and 72% when AEDs were reduced at 2 years (Fig.2B). Similarly, the 10-year seizure rates were also comparable (65–68%) whether AEDs were reduced at 1 or 2 years. These results are consistent with an observational study in children in which earlier time to AED reduction or discontinuation increased the risk of breakthrough seizures but did not affect remission by last follow-up.19 The practical implication becomes that we cannot necessarily mitigate the risk of epilepsy recurrence after AED withdrawal simply by waiting longer after surgery.
Limitations
This is a retrospective cohort study with several limitations and biases. The actual reasons and motivation underlying individual patient and physician decisions to change AEDs cannot be ascertained in this design, and the cohorts evaluated differed with regard to their etiology, baseline number of AEDs, and occurrence of postoperative EEG spiking. We attempted to mitigate for these problems by: (1) studying a large, well-characterized sample; and (2) performing a detailed comparison of the three study groups (AEDs reduced vs. stopped vs. unchanged) and adjusting in subsequent analysis for the baseline differences identified in etiology and EEG findings. We cannot, however, rule out that some unmeasured underlying differences between the different study groups may have accounted for some of the observed outcome differences.
Conclusions
Regardless of its timing, AED withdrawal (reduction or discontinuation) increases the short-term risk of breakthrough seizures after TLE surgery. Seizures occurring after AED discontinuation are usually controlled with reinstitution of medications and long-term prognosis is not altered. Seizures starting while AEDs are being reduced are more difficult to control. These findings and their significance need to be validated in a prospective randomized controlled study.
Conflict of Interest
None declared.
Funding Information
No funding information provided.
References
- 1.Cramer JA, Mintzer S, Wheless J, Mattson RH. Adverse effects of antiepileptic drugs: a brief overview of important issues. Expert Rev Neurother. 2010;10:885–891. doi: 10.1586/ern.10.71. [DOI] [PubMed] [Google Scholar]
- 2.Hoppe C, Poepel A, Sassen R, Elger CE. Discontinuation of anticonvulsant medication after epilepsy surgery in children. Epilepsia. 2006;47:580–583. doi: 10.1111/j.1528-1167.2006.00471.x. [DOI] [PubMed] [Google Scholar]
- 3.Cole AJ, Wiebe S. Debate: should antiepileptic drugs be stopped after successful epilepsy surgery? Epilepsia. 2008;49(Suppl. 9):29–34. doi: 10.1111/j.1528-1167.2008.01924.x. [DOI] [PubMed] [Google Scholar]
- 4.Taylor DC, McMacKin D, Staunton H, et al. Patients’ aims for epilepsy surgery: desires beyond seizure freedom. Epilepsia. 2001;42:629–633. doi: 10.1046/j.1528-1157.2001.34400.x. [DOI] [PubMed] [Google Scholar]
- 5.Langfitt JT, Holloway RG, McDermott MP, et al. Health care costs decline after successful epilepsy surgery. Neurology. 2007;68:1290–1298. doi: 10.1212/01.wnl.0000259550.87773.3d. [DOI] [PubMed] [Google Scholar]
- 6.Ivanova JI, Birnbaum HG, Kidolezi Y, et al. Economic burden of epilepsy among the privately insured in the US. Pharmacoeconomics. 2010;28:675–685. doi: 10.2165/11535570-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Berg AT, Langfitt JT, Spencer SS, Vickrey BG. Stopping antiepileptic drugs after epilepsy surgery: a survey of U.S. epilepsy center neurologists. Epilepsy Behav. 2007;10:219–222. doi: 10.1016/j.yebeh.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tellez-Zenteno JF, Ronquillo LH, Jette N, et al. Discontinuation of antiepileptic drugs after successful epilepsy surgery. A Canadian survey. Epilepsy Res. 2012;102:23–33. doi: 10.1016/j.eplepsyres.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 9.Jeha LE, Najm I, Bingaman W, et al. Surgical outcome and prognostic factors of frontal lobe epilepsy surgery. Brain. 2007;130(Pt 2):574–584. doi: 10.1093/brain/awl364. [DOI] [PubMed] [Google Scholar]
- 10.Jeha LE, Najm IM, Bingaman WE, et al. Predictors of outcome after temporal lobectomy for the treatment of intractable epilepsy. Neurology. 2006;66:1938–1940. doi: 10.1212/01.wnl.0000219810.71010.9b. [DOI] [PubMed] [Google Scholar]
- 11.Sarkis RA, Jehi L, Najm IM, et al. Seizure outcomes following multilobar epilepsy surgery. Epilepsia. 2012;53:44–50. doi: 10.1111/j.1528-1167.2011.03274.x. [DOI] [PubMed] [Google Scholar]
- 12.Fong JS, Jehi L, Najm I, et al. Seizure outcome and its predictors after temporal lobe epilepsy surgery in patients with normal MRI. Epilepsia. 2011;52:1393–1401. doi: 10.1111/j.1528-1167.2011.03091.x. [DOI] [PubMed] [Google Scholar]
- 13.Sarkis RA, Jehi LE, Bingaman WE, Najm IM. Surgical outcome following resection of rolandic focal cortical dysplasia. Epilepsy Res. 2010;90:240–247. doi: 10.1016/j.eplepsyres.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Jehi LE, O'Dwyer R, Najm I, et al. A longitudinal study of surgical outcome and its determinants following posterior cortex epilepsy surgery. Epilepsia. 2009;50:2040–2052. doi: 10.1111/j.1528-1167.2009.02070.x. [DOI] [PubMed] [Google Scholar]
- 15.Tellez-Zenteno JF, Wiebe S. Long-term seizure and psychosocial outcomes of epilepsy surgery. Curr Treat Options Neurol. 2008;10:253–259. doi: 10.1007/s11940-008-0028-7. [DOI] [PubMed] [Google Scholar]
- 16.McIntosh AM, Kalnins RM, Mitchell LA, et al. Temporal lobectomy: long-term seizure outcome, late recurrence and risks for seizure recurrence. Brain. 2004;127(Pt 9):2018–2030. doi: 10.1093/brain/awh221. [DOI] [PubMed] [Google Scholar]
- 17.Spencer SS, Berg AT, Vickrey BG, et al. Predicting long-term seizure outcome after resective epilepsy surgery: the multicenter study. Neurology. 2005;65:912–918. doi: 10.1212/01.wnl.0000176055.45774.71. [DOI] [PubMed] [Google Scholar]
- 18.Tonini C, Beghi E, Berg AT, et al. Predictors of epilepsy surgery outcome: a meta-analysis. Epilepsy Res. 2004;62:75–87. doi: 10.1016/j.eplepsyres.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Boshuisen K, Arzimanoglou A, Cross JH, et al. Timing of antiepileptic drug withdrawal and long-term seizure outcome after paediatric epilepsy surgery (TimeToStop): a retrospective observational study. Lancet Neurol. 2012;11:784–791. doi: 10.1016/S1474-4422(12)70165-5. [DOI] [PubMed] [Google Scholar]
- 20.Sinclair DB, Jurasek L, Wheatley M, et al. Discontinuation of antiepileptic drugs after pediatric epilepsy surgery. Pediatr Neurol. 2007;37:200–202. doi: 10.1016/j.pediatrneurol.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Schiller Y, Cascino GD, So EL, Marsh WR. Discontinuation of antiepileptic drugs after successful epilepsy surgery. Neurology. 2000;54:346–349. doi: 10.1212/wnl.54.2.346. [DOI] [PubMed] [Google Scholar]
- 22.Lachhwani DK, Loddenkemper T, Holland KD, et al. Discontinuation of medications after successful epilepsy surgery in children. Pediatr Neurol. 2008;38:340–344. doi: 10.1016/j.pediatrneurol.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Kim YD, Heo K, Park SC, et al. Antiepileptic drug withdrawal after successful surgery for intractable temporal lobe epilepsy. Epilepsia. 2005;46:251–257. doi: 10.1111/j.0013-9580.2005.28004.x. [DOI] [PubMed] [Google Scholar]
- 24.Camfield P, Camfield C. The frequency of intractable seizures after stopping AEDs in seizure-free children with epilepsy. Neurology. 2005;64:973–975. doi: 10.1212/01.WNL.0000154517.82748.A7. [DOI] [PubMed] [Google Scholar]
- 25.Berg AT, Vickrey BG, Langfitt JT, et al. Reduction of AEDs in postsurgical patients who attain remission. Epilepsia. 2006;47:64–71. doi: 10.1111/j.1528-1167.2006.00371.x. [DOI] [PubMed] [Google Scholar]
- 26.Rathore C, Panda S, Sarma PS, Radhakrishnan K. How safe is it to withdraw antiepileptic drugs following successful surgery for mesial temporal lobe epilepsy? Epilepsia. 2011;52:627–635. doi: 10.1111/j.1528-1167.2010.02890.x. [DOI] [PubMed] [Google Scholar]
- 27.Kerling F, Pauli E, Lorber B, et al. Drug withdrawal after successful epilepsy surgery: how safe is it? Epilepsy Behav. 2009;15:476–480. doi: 10.1016/j.yebeh.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 28.Park KI, Lee SK, Chu K, et al. Withdrawal of antiepileptic drugs after neocortical epilepsy surgery. Ann Neurol. 2010;67:230–238. doi: 10.1002/ana.21884. [DOI] [PubMed] [Google Scholar]
- 29.Ziemba KS, Wellik KE, Hoffman-Snyder C, et al. Timing of antiepileptic drug withdrawal in adult epilepsy patients after neocortical surgical resection: a critically appraised topic. Neurologist. 2011;17:176–178. doi: 10.1097/NRL.0b013e318217368e. [DOI] [PubMed] [Google Scholar]
- 30.Jehi L. Medication management after epilepsy surgery: are we closer to an answer? Neurology. 2012;79:728–729. doi: 10.1212/WNL.0b013e3182644f95. [DOI] [PubMed] [Google Scholar]
- 31.Jehi L. Medication management after epilepsy surgery: opinions versus facts. Epilepsy Curr. 2013;13:166–168. doi: 10.5698/1535-7597-13.4.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lossius MI, Hessen E, Mowinckel P, et al. Consequences of antiepileptic drug withdrawal: a randomized, double-blind study (Akershus Study) Epilepsia. 2008;49:455–463. doi: 10.1111/j.1528-1167.2007.01323.x. [DOI] [PubMed] [Google Scholar]
- 33.Jehi L, Sarkis R, Bingaman W, et al. When is a postoperative seizure equivalent to “epilepsy recurrence” after epilepsy surgery? Epilepsia. 2010;51:994–1003. doi: 10.1111/j.1528-1167.2010.02556.x. [DOI] [PubMed] [Google Scholar]
- 34.Potschka H. Animal and human data: where are our concepts for drug-resistant epilepsy going? Epilepsia. 2013;54(Suppl. 2):29–32. doi: 10.1111/epi.12181. [DOI] [PubMed] [Google Scholar]
- 35.Srivastava AK, Alex AB, Wilcox KS, White HS. Rapid loss of efficacy to the antiseizure drugs lamotrigine and carbamazepine: a novel experimental model of pharmacoresistant epilepsy. Epilepsia. 2013;54:1186–1194. doi: 10.1111/epi.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogawski MA. The intrinsic severity hypothesis of pharmacoresistance to antiepileptic drugs. Epilepsia. 2013;54(Suppl. 2):33–40. doi: 10.1111/epi.12182. [DOI] [PubMed] [Google Scholar]


