Abstract
BRAF alterations, namely BRAF fusion and BRAF V600E mutation, have been recently reported in low-grade epilepsy-associated tumors. Twenty low-grade epilepsy-associated tumors were retrieved to evaluate the BRAF mutational status. BRAF mutations were present in 10 tumors and concomitantly in associated dysplastic tissue of three patients. We here show for the first time that BRAF mutations are present not only in low-grade epilepsy-associated tumors but, in some cases, also in the associated focal cortical dysplasia.
Introduction
Low-grade epilepsy-associated tumors (LEAT) represent a common pathologic substrate in the setting of medically intractable chronic epilepsy.1 These tumors most commonly arise in the temporal lobe, mainly in the temporo-anterior-basal-mesial site.2 A significant part of these neoplasms, particularly ganglioglioma (GG) and dysembryoplastic neuroepithelial tumor (DNT), is associated with malformations of cortical development, in particular focal cortical dysplasia (FCD) (30–80% of cases).3–5 Moreover, a case of GG associated with FCD type IIa has been recently reported, where findings suggest the dynamic-evolutive-oncogenic potential of cells comprising FCD type IIa.6 A possible common origin of GG and FCD, from a precursor that undergoes abnormal glioneuronal development has been proposed.7
LEAT share the expression of CD348: CD34-immunoreactive cells could represent dysplastic or undifferentiated neural precursors.7
Furthermore, BRAF alterations, namely BRAF fusion9 and BRAF V600E mutation,8,10 have been recorded in LEAT. Interestingly, it has been recently observed that mutant BRAF protein in GG is predominantly expressed by neuronal tumor cells.11
As a member of the RAF family of serine/threonine kinases, BRAF is a key mediator of the mitogen-activated protein kinase (MAPK) signaling pathway (also known as the RAF-MEK-ERK pathway). BRAF is involved in a wide variety of cellular functions, including cell proliferation, cell cycle arrest, terminal differentiation, and apoptosis.12 The BRAF gene is activated by oncogenic mutations. Most BRAF mutations are missense mutations at amino acid position 600, resulting in an exchange of valine for glutamate (referred to as BRAFV600E). A small-molecule inhibitor with antineoplastic activity (vemurafenib) that targets BRAF V600E mutant has been recently developed.13
We here show for the first time that BRAF mutations are present not only in LEAT but, in a significant proportion of cases, also in the FCD tissue associated with LEAT.
Methods
Twenty LEATs referred to the Bellaria Hospital, Bologna, Italy, were retrieved (Male/Female ratio 8/12) to evaluate the BRAF mutational status. Epilepsy onset mean age was 12 years (range 1 months to 51 years), mean age at surgery was 22.5 years (range 3–51 years), and mean duration of epilepsy was 5 years (range 6 months to 38 years). Mean follow-up was 6.5 years (range 1–11 years). All patients were submitted to a pre-surgical neurophysiological assessment by means of noninvasive long-term video-EEG monitoring aimed to define the epileptogenic area. Seizure semiology, obtained by clinical history and ictal video-EEG monitoring were consistent with focal temporal lobe seizures in all patients. In all cases magnetic resonance imaging was performed. All the patients were submitted to a tailored surgery, consisting in removing the tumor, the temporal pole, the anterior neocortical lateral cortex, the uncus–entorhinal area, and the hippocampus and parahippocampal gyrus.
LEATs were histologically classified according to the WHO classification of tumors of the central nervous system.14 Namely the study included eight GG (40%), two gangliocytomas (GC) (10%), four DNT (20%), four pleomorphic xanthoastrocytomas (PXA) (20%), one papillary glioneuronal tumor (5%), and one grade II diffuse astrocytoma (5%).
Furthermore, all the specimens were also histologically classified according to the published criteria for mesial temporal sclerosis (MTS) diagnosis15 and the most updated classification of FCD,16 which has been shown to considerably improve the reproducibility of FCD diagnoses.17
In 10 cases (50%), the tumors were associated with FCD (associated cases), while in 10 cases (50%) FCD was not present (isolated tumoral cases). The areas identified as dysplastic were carefully evaluated with CD34, MAP2, p53, Ki67, and IDH1 antisera, in order to rule out the possibility of tumor infiltration misdiagnosed as dysplastic tissue. Specifically, the group of cases with FCD showed an architectural cortical dysplasia, without dysmorphic neurons or ballon cells (FCD type IIIb), in 4/10 cases (40%). The remaining 6/10 (60%) patients had FCD type IIa. No patient presented concomitant MTS.
BRAF mutational status was separately analyzed in all 20 LEATS, in the 10 tumor-associated FCD specimens and in the 10 histologically normal cortical tissue samples adjacent to the LEATS that were not associated with FCD. For comparison 10 samples of “isolated FCD” (five of FCD type I, four of FCD type IIa and one of FCD type IIb) have also been tested for BRAF mutational status.
In order to assess the BRAF molecular status DNA was extracted from formalin-fixed paraffin-embedded (FFPE) material scraped under microscope guidance from five 10-μm thick sections, using the MasterPure DNA FFPE extraction kit (Roche Diagnostic, Manheim, Germany).
About 10 ng of DNA were used for amplification with primers specific for BRAF exon 15 (Fw 5′ – TGCTTGCTCTGATAGGAAAATGA – 3′; Rv 5′ – TGGATCCAGACAACTGTTCAAA – 3′) modified with universal tail sequences and multiple identifier (MID) nucleotides. Sequencing was performed using the 454 GS-Junior next generation sequencer (Roche Diagnostic, Mannheim, Germany) according to established protocols (http://www.454.com/). Results were analyzed using Amplicon Variant Analyzer (AVA) software (Roche Diagnostic, Mannheim, Germany). Next generation sequencing of target DNA sequences (reads) not only allows to identify a mutation but also gives the percentage of mutated amplicons in the sample analyzed.
Results
Histopathological and molecular results of LEATs and of associated FCD are summarized in Table1. Findings observed in isolated LEATs (without FCD) and in adjacent histologically normal cortex are summarized in Table2. At least 519 consensual reads (analyzed sequences) were obtained with parallel sequencing (median number 922 reads, range 519–1352 reads). The BRAF V600E mutation was detected in eight neoplastic specimens. One GG sample had the BRAF V600K mutation. One DNT sample had a mutation resulting in the insertion of a threonine residue between codons 599 and 600 (BRAF T599_V600 InsT). Altogether BRAF mutations were found in three out of four PXA (75%), in four out of eight GG (50%) (including the case with the BRAF V600K mutation), in two out of four DNT (50%) (including the BRAF T599_V600insT mutation), and in the single case of grade II diffuse astrocytoma. The remaining 10 LEATs were not mutated for BRAF. Three dysplastic specimens, constituted by FCD type IIa, had the BRAF V600E mutation: all these three cases were associated with a BRAF V600E mutated tumor (one GG and two PXA) (see Fig.1A and B). On the other hand, seven dysplastic cases (four FCD type IIIb and three FCD type IIa), the 10 tumor-associated brain samples without cortical dysplasia and the 10 cases of “isolated FCD” were not mutated for BRAF.
Table 1.
Histological and molecular results of LEATs with associated FCD.
| Case | Histological diagnosis | BRAF status | % of mutated reads | Total number of reads |
|---|---|---|---|---|
| 1 | GG | V600E | 2.5 | 571 |
| FCD type IIIb | WT | |||
| 2 | GG | V600E | 12 | 1091 |
| FCD type IIa | V600E | 5 | 559 | |
| 3 | DNT | T599_V600InsT | 2 | 1047 |
| FCD type IIIb | WT | |||
| 4 | GG | V600E | 3.8 | 1109 |
| FCD type IIa | WT | |||
| 5 | GG | WT | ||
| FCD type IIa | WT | |||
| 6 | DNT | WT | ||
| FCD type IIIb | WT | |||
| 7 | GG | WT | ||
| FCD type IIa | WT | |||
| 8 | PXA | WT | ||
| FCD type IIIb | WT | |||
| 9 | PXA | V600E | 33 | 940 |
| FCD type IIa | V600E | 5 | 903 | |
| 10 | PXA | V600E | 25 | 967 |
| FCD type IIa | V600E | 3.5 | 820 |
DNT, dysembryoplastic neuroepithelial tumor; FCD, focal cortical dysplasia; GG, ganglioglioma; PXA, pleomorphic xanthoastrocytoma; WT, wild type.
Table 2.
Histological and molecular results of isolated LEATs (without FCD) and of adjacent histologically normal cortex.
| Case | Histological diagnosis | BRAF status | % of mutated reads | Total number of reads |
|---|---|---|---|---|
| 11 | DNT | V600E | 16.6 | 883 |
| NO FCD | WT | |||
| 12 | DNT | WT | ||
| NO FCD | WT | |||
| 13 | PGNT | WT | ||
| NO FCD | WT | |||
| 14 | Gangliocytoma | WT | ||
| NO FCD | WT | |||
| 15 | PXA | V600E | 17.5 | 519 |
| NO FCD | WT | |||
| 16 | Grade II diffuse astrocytoma | V600E | 31 | 1352 |
| NO FCD | WT | |||
| 17 | GG | WT | ||
| NO FCD | WT | |||
| 18 | GG | V600K | 26 | 630 |
| NO FCD | WT | |||
| 19 | Gangliocytoma | WT | ||
| NO FCD | WT | |||
| 20 | GG | WT | ||
| NO FCD | WT |
DNT, dysembryoplastic neuroepithelial tumor; FCD, focal cortical dysplasia; GG, ganglioglioma; PGNT, papillary glioneuronal tumor; PXA, pleomorphic xanthoastrocytoma; WT, wild type.
Figure 1.
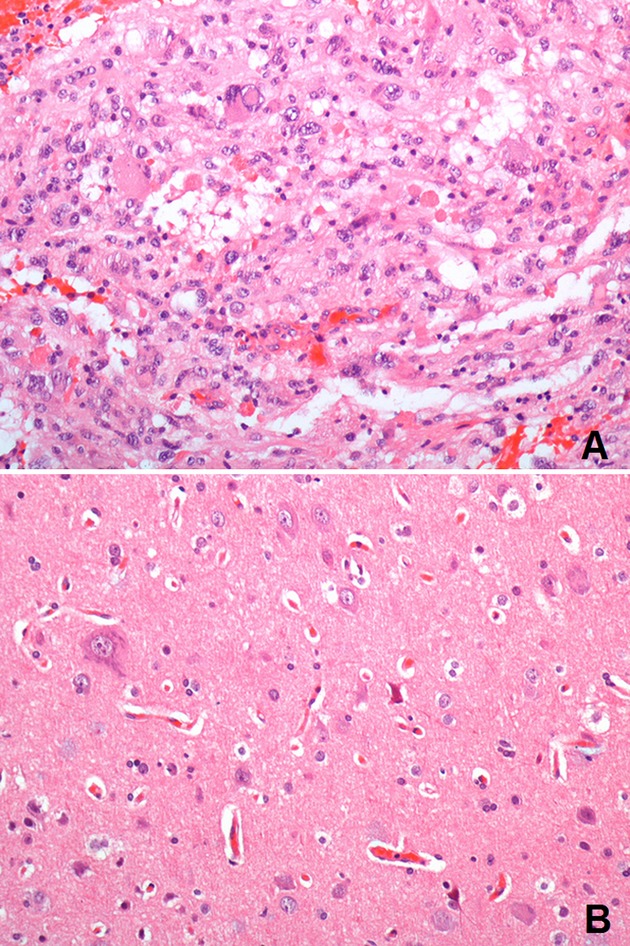
Case no. 9. (A) Representative BRAFV600E mutated LEAT: the tumor is a grade II pleomorphic xanthoastrocytoma composed of giant neoplastic cells showing nuclear pleomorphism and xanthomatous changes; nuclear inclusions and numerous eosinophilic granular bodies are present; in spite of marked cytological pleomorphism the tumor lacks microvascular proliferation and necrosis; mitotic figures are not common (H&E, 200× magnification); (B) corresponding BRAFV600E mutated associated FCD: dysmorphic neurons in focal cortical displasia type IIa (H&E, 200× magnification).
Discussion
We here show for the first time that BRAF mutations are present not only in LEATs, as recently shown8,10 but also in the FCD that accompanies LEAT. These data are very intriguing, as a possible common origin of LEATs and FCD from a precursor that undergoes abnormal glioneuronal development, has been postulated7 but not yet proven.
Lesional cells may represent a low proportion of FCD tissue requiring a highly sensitive method to identify genetic alterations. Next generation sequencing targeted to BRAF exon 15 was utilized for mutation detection.
In the FCD type IIa cases where we identified BRAF mutations, the diagnostic clue was represented by the presence of dysmorphic neurons. This observation is in line with the recent finding that the mutant BRAFV600E protein in GG is predominantly expressed by neuronal tumor cells.11
We considered the possibility that identification of BRAFV600E mutated alleles in FCD samples simply reflected the presence of rare neoplastic cells interspersed with the dysplastic elements. However, this possibility has been excluded by the careful histologic and immunohistochemical screening (with CD34, MAP2, p53, Ki67 and IDH1 antisera) of all FCD cases prior to molecular analysis. Importantly, the percentage of mutated target sequences (reads) in the BRAFV600E mutated FCD samples was 5% in two cases and 3.5% in the remaining case. Assuming that the BRAFV600E is heterozygous the amount of mutated cells in the sample analyzed was 7–10%, a proportion of possible neoplastic cells well above the threshold for histopathological diagnosis.
We did not find BRAF mutations in the cases of “isolated FCD,” unlike “associated FCD.” This difference could reflect a not common biological pathway. It is well known for example that isolated FCD type I represents a different disease, with different outcome, in comparison to FCD type I associated with another lesion (i.e., hippocampal sclerosis, tumor, vascular malformation), so that today it is diagnosed as FCD type III. Finally, the recent finding that expression of BRAF V600E is associated with a worse postoperative seizure outcome in glioneuronal tumors18 could be explained by the presence, in some cases, of a not removed FCD in the adjacent cortex.
Thus, our results support the hypothesis that – in a significant proportion of cases – FCD and LEATs can show an evolutive oncogenic progression, similar to what has already been shown for other tumors.19
Interestingly, we found the BRAF V600E mutation also in a grade II diffuse astrocytoma, a tumor included in LEATs, but not having a glioneuronal nature. This finding, only rarely documented in large systematic studies,10,20 is consistent with a recent report21 in which the BRAF V600E mutation was identified in five (15%) out of 33 consecutive grade 2 diffuse gliomas. The authors observed that four out of these five BRAF-mutant grade 2 diffuse gliomas presented with long-standing, frequent, sometimes refractory seizures and all four tumors were located within the temporal lobe. They also reported two cases of glioblastoma with BRAF V600E, both in patients presenting with focal seizures. All these data indicate that BRAF mutations occur in a setting specifically linked to epileptogenesis.
To the best of our knowledge this is the first demonstration that BRAF mutations are present in the FCD associated with LEAT, suggesting a pathogenetic role. Our findings prompt further investigation into the potential role of BRAF mutations in cyto-architectural dysplasia and in the tumorigenesis of LEATs.
Author Contributions
G. M. designed the study, discussed and interpreted results, wrote the manuscript. D. d. B. performed molecular experiments, analyzed, and interpreted results. M. V. performed molecular experiments. M. G. performed tailored surgery, discussed results. M. M. performed tailored surgery. L. V. performed pre-surgical neurological assessment. P. R. contributed to pre-surgical neurological assessment. G. R. performed pre-surgical neurological assessment. R. M. discussed and interpreted results, supervised the work. G. T. discussed and interpreted results, coordinated, and supervised the work.
Conflict of Interest
None declared.
Funding Information
No funding information provided.
References
- 1.Thom M, Blümcke I, Aronica E. Long-term epilepsy-associated tumors. Brain Pathol. 2012;22:350–379. doi: 10.1111/j.1750-3639.2012.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giulioni M, Rubboli G, Marucci G, et al. Seizure outcome of epilepsy surgery in focal epilepsies associated with temporomesial glioneuronal tumors: lesionectomy compared with tailored resection. J Neurosurg. 2009;111:1275–1282. doi: 10.3171/2009.3.JNS081350. [DOI] [PubMed] [Google Scholar]
- 3.Daumas-Duport C. Dysembryoplastic neuroepithelial tumours. Brain Pathol. 1993;3:283–295. doi: 10.1111/j.1750-3639.1993.tb00755.x. [DOI] [PubMed] [Google Scholar]
- 4.Prayson RA, Khajavi K, Comair YG. Cortical architectural abnormalities and MIB-1 immunoreactivity in gangliogliomas: a study of 60 patients with intracranial tumors. J Neuropathol Exp Neurol. 1994;54:513–520. doi: 10.1097/00005072-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi A, Hong S-C, Seo DW, et al. Frequent association of cortical dysplasia in dysembryoplastic neuroepithelial tumor treated by epilepsy surgery. Surg Neurol. 2005;64:419–427. doi: 10.1016/j.surneu.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Ortiz-Gonzalez XR, Venneti S, Biegel JA, et al. Ganglioglioma arising from dysplastic cortex. Epilepsia. 2011;52:e106–e108. doi: 10.1111/j.1528-1167.2011.03124.x. [DOI] [PubMed] [Google Scholar]
- 7.Deb P, Sharma MC, Tripathi M, et al. Expression of CD34 as a novel marker for glioneuronal lesions associated with chronic intractable epilepsy. Neuropathol Appl Neurobiol. 2006;32:461–468. doi: 10.1111/j.1365-2990.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 8.Chappé C, Padovani L, Scavarda D, et al. Dysembryoplastic neuroepithelial tumors share with pleomorphic xanthoastrocytomas and gangliogliomas BRAF(V600E) mutation and expression. Brain Pathol. 2013;23:574–583. doi: 10.1111/bpa.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin A, Rodriguez FJ, Karajannis MA, et al. BRAF alterations in primary glial and glioneuronal neoplasms of the central nervous system with identification of 2 novel KIAA1549: BRAF fusion variants. J Neuropathol Exp Neurol. 2012;71:66–72. doi: 10.1097/NEN.0b013e31823f2cb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schindler G, Capper D, Meyer J, et al. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011;121:397–405. doi: 10.1007/s00401-011-0802-6. [DOI] [PubMed] [Google Scholar]
- 11.Koelsche C, Wöhrer A, Jeibmann A, et al. Mutant BRAF V600E protein in ganglioglioma is predominantly expressed by neuronal tumor cells. Acta Neuropathol. 2013;125:891–900. doi: 10.1007/s00401-013-1100-2. [DOI] [PubMed] [Google Scholar]
- 12.Peyssonnaux C, Eychene A. The Raf/MEK/ERK pathway: new concepts of activation. Biol Cell. 2001;93:53–62. doi: 10.1016/s0248-4900(01)01125-x. [DOI] [PubMed] [Google Scholar]
- 13.Bollag G, Hirth P, Tsai J, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–599. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. World health organization classification of tumours of the central nervous system. Lyon: IARC Press; 2007. [Google Scholar]
- 15.Blümcke I, Pauli E, Clusmann H, et al. A new clinico-pathological classification system for mesial temporal sclerosis. Acta Neuropathol. 2007;113:235–244. doi: 10.1007/s00401-006-0187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blümcke I, Thom M, Aronica E, et al. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia. 2011;52:158–174. doi: 10.1111/j.1528-1167.2010.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coras R, de Boer OJ, Armstrong D, et al. Good interobserver and intraobserver agreement in the evaluation of the new ILAE classification of focal cortical dysplasias. Epilepsia. 2012;53:1341–1348. doi: 10.1111/j.1528-1167.2012.03508.x. [DOI] [PubMed] [Google Scholar]
- 18.Prabowo AS, Iyer AM, Veersema TJ, et al. BRAF V600E mutation is associated with mTOR signaling activation in glioneuronal tumors. Brain Pathol. 2014;24:52–66. doi: 10.1111/bpa.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 20.Basto D, Trovisco V, Lopes JM, et al. Mutation analysis of B-RAF gene in human gliomas. Acta Neuropathol. 2005;109:207–210. doi: 10.1007/s00401-004-0936-x. [DOI] [PubMed] [Google Scholar]
- 21.Chi AS, Batchelor TT, Yang D, et al. BRAF V600E mutation identifies a subset of low-grade diffusely infiltrating gliomas in adults. J Clin Oncol. 2013;31:e233–e236. doi: 10.1200/JCO.2012.46.0220. [DOI] [PubMed] [Google Scholar]


