Abstract
Background
Endoscopic ultrasound (EUS)-guided fine-needle aspiration (FNA) is a technique frequently used to diagnose solid and cystic lesions of the pancreas. Antibiotic prophylaxis has been recommended for EUS-FNA of pancreatic cystic lesions but is not universally observed. The most effective antibiotic and the most efficacious route and regimen of administration are also unknown.
Objective
This cohort study was undertaken to evaluate whether single-dose piperacillin/tazobactam or ciprofloxacin given at the time of the procedure effectively prevents major adverse events and to audit the adherence to this protocol in the setting of EUS-FNA of pancreatic cystic lesions.
Design
Consecutive EUS-FNA procedures of pancreatic cystic lesions were performed at Concord Hospital and significant variables regarding the procedure and adverse events were recorded. Patients were also contacted by telephone to follow-up any subacute adverse events they may have experienced.
Patients
Over a 30 month period (January 2010–July 2012), a total of 85 EUS-FNAs of pancreatic cysts were performed on 80 different patients. The mean age was 63.2 years (range 17–89 years; 58% females).
Interventions
Single-dose piperacillin/tazobactam IVs was administered to 87% of patients, while 12% of patients received ciprofloxacin IVs.
Results
No patients developed cyst infection, fever, or sepsis (0%) and one patient (1.2%) was hospitalised for self-limited nausea without adverse sequelae.
Conclusions
Single-dose piperacillin/tazobactam at the time of EUS-FNA of pancreatic cysts is an effective prophylaxis of cyst infection or sepsis and can be conveniently given as a single-dose peri-procedurally without further oral antibiotics.
Keywords: EUS, FNA, pancreatic cyst, antibiotic, prophylaxis
Background
Endoscopic ultrasound (EUS) is a safe method of accurately diagnosing and staging various cysts, lesions, and tumours of the gastrointestinal (GI) tract, pancreas, and mediastinal lymph nodes. EUS-guided fine-needle aspiration (FNA) is utilised to assist diagnosis of submucosal lesions, pancreatic disease, or to detect metastatic cancer in mediastinal lymph nodes.1,2 In EUS-guided FNA, the insertion of a needle through the gastrointestinal wall to the target lesion creates a site of potential entry of microorganisms through the mucosa to enter into the blood stream and/or stagnant fluid collections.3
Bacteraemia with and without septicaemia has been reported to occur in up to 6% of endoscopic procedures, but mostly resolves without sequelae.4 Bacteraemia most often occurs within 30 minutes of the end of the procedure.5, 6 Most adverse events as a result of EUS are related to FNA; however, the risk of infection due to EUS-guided FNA is very low, up to 0.3%.7,8 Cystic lesions are known to represent an increased risk of infection compared with solid lesions.7–9 Guidelines from the European Society of Gastrointestinal Endoscopy (ESGE) advocate for antibiotic prophylaxis with EUS-FNA of pancreatic cystic lesions, while the most recent (June 2013) guidelines from the American Society for Gastrointestinal Endoscopy (ASGE) state that additional prospective data are needed to clarify the risks and benefits of prophylactic antibiotics for EUS-FNA of pancreatic cysts. ASGE previously recommended a 3–5 day course of fluoroquinolone, while ESGE makes no specific choice of antibiotics.9 A prolonged course of antibiotics adds to the inconvenience of the procedure, cost, and risk of antibiotic-associated adverse events including Clostridium difficile-associated diarrhoea. Whether single shot peri-procedural antibiotic may effectively prevent sepsis without generating adverse events remains unknown.
Piperacillin is a penicillin of the ureidopenicillin class with broad-spectrum coverage against both Gram-positive and Gram-negative bacteria.10 Beta-lactamase inhibitors, such as tazobactam, have been shown to bind secondary penicillin-binding proteins, which augments the bactericidal effect of β-lactam drugs, even in penicillin- and ampicillin-sensitive strains.11 Piperacillin in combination with tazobactam has been shown to be stable to beta-lactamases produced by staphylococci, Enterobacteriaceae, Pseudomonas species, and anaerobes.10 Current literature supports the efficacy of the combination of piperacillin/tazobactam in the treatment of moderate to severe polymicrobial nosocomial infections and against susceptible strains of extended-spectrum beta-lactamase-producing bacteria such as Escherichia coli and Klebsiella spp.11,12 Thus intravenous piperacillin/tazobactam has ideal cyst penetration and antibiotic coverage characteristics but has not been previously recommended as a prophylactic antibiotic. The objective of this cohort study was, therefore, to evaluate the efficacy of a protocol-driven strategy using a single-dose peri-procedural piperacillin/tazobactam, or ciprofloxacin if there was a documented penicillin allergy, in the prophylaxis of EUS-FNA-associated infectious adverse events.
Methods
This was a single-centre pilot study to evaluate the safety and efficacy of single-dose intravenous antibiotics at the time of EUS-FNA. Patients who underwent ambulatory EUS-FNA of pancreatic cystic lesions at Concord Hospital over a 30 month period (December 2009–July 2012) were recruited. The number of patients recruited was limited due to the nature of the study as a pilot study of a new prophylactic regimen that has not been previously tested for efficacy or safety. Concord Hospital is a EUS-referral hospital of the Sydney Local Health District that services a population of 800,000 residents. EUS cases had been performed since 1991 by three trained consultant gastroenterologists or their advanced therapeutic endoscopy fellows under direct supervision. An antibiotic protocol was developed in conjunction with the infectious disease department to allow the use of piperacillin/tazobactam for all EUS-FNA cases. The primary indications for EUS-FNA of the cysts were for assessment of the relevant biomarkers and enzymes, such as carcinoembryonic antigen (CEA) levels and amylase, and for obtaining cytology specimens for diagnosis. This was often at the request of the referring pancreatic surgeon. The study was designed to validate the efficacy of single-dose antibiotics in preventing infectious adverse events, and it was deemed unethical to incorporate a control group into the study receiving only a placebo. The use of this antibiotic was otherwise limited in Australia; hence, it was unlikely that patients had prior exposure to this drug. Cases were identified through the ProVation MD (version 5, ProVation Medical Inc., USA) or GI Scribe (Health Communication Network, Australia) computer-based endoscopy reporting database programs. The endoscopy reports and medical records were entered prospectively for analysis including the procedural variables of lesion characteristic (location, size, and solid or cystic), FNA needle gauge (19G, 22G, or 25G; Echotip Ultra Ultrasound Needle, Boston Scientific Corporation, Massachusetts, USA), the number of passes required to obtain an adequate sample, the operating endoscopist, the quality of material obtained (including clarity, colour, and viscosity), final diagnosis, and type of peri-procedural antibiotics administered. Our EUS-FNA results are comparable with established benchmarks as previously published.13
Procedures were performed under conscious sedation using midazolam and fentanyl. All patients were given piperacillin/tazobactam IV (Wyeth Pharmaceuticals, USA; Tazocin: piperacillin 4 g and tazobactam 0.5 g) over 30 minutes unless they had a known penicillin allergy, in which case, ciprofloxacin IV (Bayer, Germany; Ciproxin) 200 mg over 1 hour was substituted. Antibiotics were administered at the time of the EUS once the cyst was identified without further ambulatory dosing. Patients were monitored for 1–2 hours following the procedure prior to discharge. All peri-procedural and post-procedural adverse events were documented with prospective follow-up data of any undocumented adverse events obtained through structured telephone calls at least a week following discharge from the hospital. The ProVation database was used to prospectively enter clinical data and to review and audit antibiotic usage. All patients provided signed informed consent for the procedure and follow-up, and the audit was approved by the Concord Hospital Human Research Ethics Committee (code EC00118). Patients could be contacted twice, once within 30 days and the second contact was planned for after six months. Patients who could not be contacted at the initial time point were contacted at the second time point. As such, data regarding peri-operative and early post-operative sequelae were monitored and recorded prospectively for each procedure, and any potential later sequelae were identified through the telephone follow-up of each patient.
The primary outcome was peri-procedural local or systemic FNA-associated sepsis and the secondary outcome measure related to any EUS-FNA or drug-induced severe adverse events. Sepsis was defined as an infective adverse outcome consistent with the introduction of microbial organisms either locally into a previously-sterile locus of fluid or systemically. This was diagnosed through clinical features, imaging, aspiration of septic focus, and response to anti-microbial treatment. Sepsis data were further sub-classified as FNA-related, endoscope-associated trauma, and aspiration pneumonia. A severe adverse event was defined as one that required hospitalisation, was life-threatening, or resulted in death or disability. The observed proportion was expressed as percentages and 95% confidence intervals (CI). Missing data were addressed through the last variable carried forward method.
Results
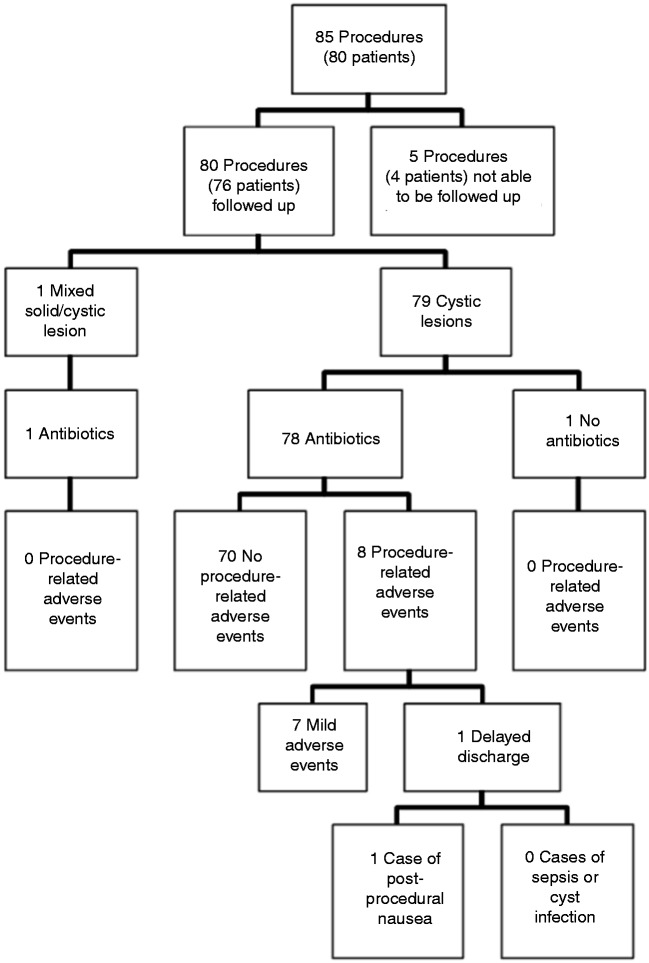
A total of 85 EUS-FNAs of pancreatic cysts were performed on 80 individual subjects (Figure 1, Table 1). The mean age was 63.2 years (range 17–89 years; 58% females). Almost all the procedures were performed in an ambulatory outpatient setting (96%). Three different gauged needles were utilised as follows: 19G (9%), 22G (46%), and 25G (44%). The mean number of passes was 1.4 per patient. A transgastric approach was utilised in 71% and a transduodenal one in 29%. A pancreatic cyst was the primary target of almost all procedures (96%), but targeting of extra-pancreatic tissue was performed if considered relevant to the case (two peripancreatic lymph nodes and one from the left adrenal gland). Most of the lesions (98%) were cystic, with the remaining two lesions classified as mixed cystic and solid. The location of the cysts was found in various sites in the pancreas (head: 26%, head and neck: 1%, neck: 19%, neck and body: 2%, body: 16%, body and tail: 7%, tail: 9%, uncinate process: 8%). Analysis of cytology from EUS-FNA of pancreatic cysts was often non-diagnostic (28.2% of cases), but yielded diagnoses for the majority of the cases (Table 2). The five most common diagnoses were intraductal papillary mucinous neoplasm (24.7%), pseudocyst (14.1%), mucinous cystadenoma (10.6%), pancreatitis-associated cyst (4.7%), and serous cystadenoma (3.5%).
Figure 1.
Flow chart of follow-up results of subjects in the study.
Table 1.
Summary of data characterising patients and findings
| Age, mean (SD), y | 63.2 (±13) |
| Sex, male/female, no. (%) | 34 (42), 46 (58) |
| Needle Gauge, no. (%) | |
| 19G | 8 (9) |
| 22G | 39 (46) |
| 25G | 38 (44) |
| Number of Passes, no. (%) | |
| 1 | 64 (75) |
| 2 | 14 (16) |
| 3 | 4 (5) |
| 4 | 2 (2) |
| 5 | 2 (2) |
| Mean Number of Passes | 1.4 |
| Cyst Location, no. (%) | |
| Head | 22 (26) |
| Head and Neck | 1 (1) |
| Neck | 16 (19) |
| Neck and Body | 2 (2) |
| Body | 14 (16) |
| Body and Tail | 6 (7) |
| Tail | 9 (9) |
| Uncinate | 7 (8) |
| Antibiotics administered, no. (%) | |
| Piperacillin/tazobactam | 74 (87) |
| Ciprofloxacin | 10 (12) |
Table 2.
Most common five diagnoses based on cytology
| Diagnosis | Number of cases, n (%) |
|---|---|
| Non-diagnostic/benign | 24 (28.2) |
| IPMN | 21 (24.7) |
| Pseudocyst | 12 (14.1) |
| Mucinous cystadenoma | 9 (10.6) |
| Pancreatitis-associated cyst | 4 (4.7) |
| Serous cystadenoma | 3 (3.5) |
IPMN: intraductal papillary mucinous neoplasm.
During the study period, there was 1% non-adherence to the antibiotic protocol. Antibiotic prophylaxis was administered in all but one patient comprising of piperacillin/tazobactam in 87% and ciprofloxacin in 12% (for known or suspected penicillin allergy in 78% and unavailability of piperacillin/tazobactam in 22%). Additional post-procedure oral 875 mg amoxicillin/125 mg clavulanic acid (Augmentin duo forte, GlaxoSmithKline, United Kingdom) was given to two patients (3%) due to incomplete cyst drainage that contained blood and pancreatic pseudocysts that could not be excluded.
Regarding acute adverse events, one patient was admitted for post-procedure observation for opioid-related nausea. The case was conducted in the late afternoon as the endoscopy observation day ward was closing. He had no peritonism or sepsis and a radiological examination was deemed unnecessary. Transient neutrophilia (white cell count (WCC) 18 × 109/L) was observed and managed with empirical IV antibiotics. His symptoms had resolved by the following day. One other patient represented with acute-on-chronic pancreatitis without sepsis. Two additional patients experienced mild abdominal pain with one presenting to a general practitioner. Both improved without sequelae and did not meet the sepsis definition. Two patients experienced sore throats; one of which was mild, while the other experienced bleeding, but neither of these patients had abdominal pain or infective symptoms. One patient experienced a single episode of opioid-associated vomiting that resolved without specific treatment after his procedure. None of these patients had sepsis-associated adverse events according to the a priori definition.
A structured telephone follow-up was conducted after a median of 256 days to capture late adverse events and outcomes of lesions that may have included definitive resectional surgery. Only four patients could not be contacted or refused participation. One patient had unstable angina a month following the EUS-FNA which was deemed unrelated to the procedure. He was subsequently diagnosed with triple vessel disease necessitating coronary artery bypass surgery. Another patient presented a month later with epigastric pain. He was afebrile with a C-reactive protein (CRP) level of 20, but with normal lipase and liver function tests. The pain was consistent with acute-on-chronic pancreatitis and he was treated with simple analgesics.
None of these patients had acute or late sepsis attributable to the EUS-FNA procedure that included FNA-introduced intra-abdominal abscess, endoscope-trauma, or systemic adverse events (0%, 95% CI: 0% to 0%). All adverse events were self-limited, mild, or resulted from premorbid conditions unrelated to the procedure. None of the patients developed adverse antibiotic reactions or diarrhoea (0%, 95% CI: 0% to 0%). One case with nausea required hospitalisation (1.2%, 95% CI: −1.1% to 3.1%) but without sepsis or long-term sequelae.
Discussion
EUS-FNA is a relatively safe procedure. The overall rate of adverse events is reported to be around 1.5%. Another study found the rates of perforation, bleeding, and infection to be each up to 1.3%, while the risk of pancreatitis was reported to be about 1–2%.7 EUS-FNA of cystic lesions is reported to have a higher risk of adverse events compared with those of solid lesions, in particular representing an increased risk of cyst infection and fever.7,8 This risk is of added concern in the subset of patients requiring EUS-FNA of pancreatic lesions, who are more likely to have underlying diseases such as chronic pancreatitis. Thus, there is a strong theoretical advantage in providing antibiotic prophylaxis to these patients in order to prevent cyst infections, fevers, and sepsis. Several studies have shown that EUS-FNA of pancreatic cystic lesions with peri-procedural antibiotic prophylaxis is safe and the risks of infection are low.4,7,8 However, complex regimens involving parenteral antibiotics hours in advance of the procedure or oral courses after the procedure increases the complexity of the procedure and may result in non-adherence.
This study showed that EUS-FNA was safe and that single-dose peri-procedural piperacillin/tazobactam or ciprofloxacin if there was an allergy to penicillin effectively prevents cyst infection and local and systemic sepsis. The telephone call performed prospectively as part of an ethics committee-approved audit ensured collection of both acute and long-term data. The late adverse events collected were deemed unrelated to the index EUS-FNA procedure and were almost invariably from comorbidity. As such, this study sufficiently supports the safety of EUS-FNA of pancreatic cystic lesions with single-dose antibiotic prophylaxis with either piperacillin/tazobactam or ciprofloxacin for those allergic to penicillin and supports the use of antibiotics timed to the actual procedure.
Expert opinions indicate that the use of antibiotic prophylaxis for EUS-FNA to be controversial as there is insufficient evidence that it significantly reduces the risk of adverse events. Conversely some studies suggest that the rate of bacteraemia following EUS is low both without and with FNA,14 and that the benefit of antibiotic prophylaxis in EUS-FNA is thus likely to be low.15 It should be noted that while there was no significant difference in the rates of adverse events between groups that were given and withheld antibiotic prophylaxis,15 the only cases of cyst infection and fever were seen in the group that was withheld the prophylaxis. Thus, while the conclusion is drawn that there is little to no benefit seen from antibiotic prophylaxis in this study, it is arguable that the nature of the adverse events in the group that was withheld the prophylaxis was potentially more serious and life-threatening, particularly when considering patients who are more likely to suffer from chronic diseases such as pancreatitis. Furthermore, additional adverse events caused by antibiotic prophylaxis have been recorded in other studies; in particular, allergic reactions and anaphylaxis and secondary infections (such as Clostridium difficile associated diarrhoea).15 Due to this uncertainty regarding the true benefit and potential harms of antibiotic prophylaxis, it has been also proposed that antibiotic prophylaxis be specifically administered only to select patients at a high risk of infective endocarditis.13 However, the rates of adverse events related to antibiotics are similarly very low, and no such adverse events were seen in this study. As such, this study confirms that the rate of adverse events caused by antibiotic prophylaxis is low, and that the potential benefits of preventing cyst infection, fever, and sepsis in compromised patients outweighs these concerns, and supports the protocol of antibiotic prophylaxis during EUS-FNA of pancreatic cystic lesions.
This study assesses the utility of single-dose antibiotics given at the time of the procedure and it was shown to be convenient as it did not require pre-arranged antibiotic administration and recruited only cases where FNA was actually performed. Although this was a single-centre study, the practice and results were consistent among the three gastroenterologists and was likely to be applicable to other centres. The study design allowed for complete collection of data of all consecutive cases and for the prospective telephone follow-up to ensure late events were not missed. The approach of single-dose antibiotics at the time of the procedure and the use of piperacillin/tazobactam was novel. This was not designed to be a randomised study to evaluate the need for prophylactic antibiotics in EUS-FNA which would require recruitment of large numbers due to the rarity of septic events (0%) and was beyond the scope of this study.
Conclusion
EUS-FNA of pancreatic cystic lesions is a safe procedure and major adverse events are uncommon following single-dose piperacillin/tazobactam or ciprofloxacin for penicillin allergy. This study was designed to assess the utility of single-dose antibiotics given at the time of the procedure and it was shown to be convenient, safe, and effective in preventing infection or sepsis. Additional larger-scale studies are required to confirm the statistically low rate of infectious adverse events suggested by this study.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Rosch T (ed). Endoscopic ultrasonography: State of the art – 1995. Part 1. In: Sivak M Jr (ed.) Gastrointest Endosc Clinics N Am 1995; 5: 475–698.
- 2.Shim CS. Role of endoscopic ultrasonography for gastric lesions. Endoscopy 1998; 30: S55–S59 [DOI] [PubMed] [Google Scholar]
- 3.Barawi M, Gottlieb K, Cunha B, et al. A prospective evaluation of the incidence of bacteremia associated with EUS-guided fine-needle aspiration. Gastrointest Endosc 2001; 53(2): 189–192 [DOI] [PubMed] [Google Scholar]
- 4.Botoman VA, Surawics CM. Bacteremia with gastrointestinal endoscopic procedures. Gastrointest Endosc 1986; 32: 342–346 [DOI] [PubMed] [Google Scholar]
- 5.Gress FG, Hawes RH, Savides TJ, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy using linear array and radial scanning endosonography. Gastrointest Endosc 1977; 45: 243–250 [DOI] [PubMed] [Google Scholar]
- 6.Coughlin GP, Butler RN, Alp MH, et al. Colonoscopy and bacteremia. Gut 1977; 18: 678–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Toole D, Palazzo L, Arotcarena R, et al. Assessment of complications of EUS-guided fine-needle aspiration. Gastrointest Endosc 2001; 53: 470–474 [DOI] [PubMed] [Google Scholar]
- 8.Wiersema MJ, Vilmann P, Giovannini M, et al. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology 1997; 112: 1087–1095 [DOI] [PubMed] [Google Scholar]
- 9.Early DS, Acosta RD, et al. ASGE Standards of Practice Committee. ASGE guideline: adverse events associated with EUS and EUS with FNA. Gastrointest Endosc 2013; 77(6): 839–843 [DOI] [PubMed] [Google Scholar]
- 10.Sanders Jr, WE, Sanders CC. Piperacillin/tazobactam: a critical review of the evolving clinical literature. Clin Infect Dis 1996; 22: 107–123 [DOI] [PubMed] [Google Scholar]
- 11.Peterson LR. Antibiotic policy and prescribing strategies for therapy of extended-spectrum beta-lactamase-producing Enterobacteriaceae: the role of piperacillin-tazobactam. Clin Micriobiol Infect 2008; 14: S181–S184 [DOI] [PubMed] [Google Scholar]
- 12.Schmitt DV, Leitner E, Welte T, et al. Piperacillin/tazobactam vs imipenem/cilastatin in the treatment of nosocomial pneumonia — a double blind prospective multicentre study. Infection 2006; 34(3): 127–134 [DOI] [PubMed] [Google Scholar]
- 13.Fanning S, Kwok A, Jones B, et al. EUS aspiration needle size: smaller is better? Gastrointest Endosc 2010; 72(4): 904–905 [DOI] [PubMed] [Google Scholar]
- 14.Janssen J, König K, Knop-Hammad V, et al. Frequency of bacteremia after linear EUS of the upper GI tract with and without FNA. Gastrointest Endosc 2004; 59(3): 339–344 [DOI] [PubMed] [Google Scholar]
- 15.Guarner-Argente C, Shah P, Buchner A, et al. Use of antimicrobials for EUS-guided FNA of pancreatic cysts: a retrospective, comparative analysis. Gastrointest Endosc 2011; 74(1): 81–86 [DOI] [PubMed] [Google Scholar]