Abstract
Understanding the function of T cells at the maternal-fetal interface remains one of the most difficult problems in reproductive immunology. A great deal of work over the last two decades has led to the view that the T cells that populate the decidua have important roles in both normal and pathological pregnancies, but the exact nature of these roles has remained unclear. Indeed, the old assumption that decidual T cells are uniformly threatening to fetal survival because the placenta is fundamentally an ‘allograft’ has given way to the idea that different T cells subsets contribute in different ways to pregnancy success or failure. Accordingly, some T cells are thought to protect the placenta from immune rejection and facilitate embryo implantation, while others are thought to contribute to pregnancy pathologies such as preeclampsia and spontaneous abortion. Here, we review the current state of information on the behavior of decidual T cells with a focus on both mouse and human studies, and with an emphasis on the many unresolved areas within this overall emerging framework.
Introduction
Many different kinds of maternal leukocytes populate the maternal-fetal interface (i.e. the decidua), each with diverse functions in implantation, placental development, parturition and infectious disease control (for review, see (Bulmer et al., 2010, Erlebacher, 2013a, Trundley and Moffett, 2004)). For example, natural killer cells (NK cells) are well known as critical regulators of spiral artery remodeling in both mice and humans. Here we will discuss the role of decidual αβ T cells (henceforth referred to as simply T cells), whose functions remain particularly poorly understood. The difficulty on working on these cells in part comes from the existence of multiple T cell subsets possessing diverse sets of functions. In addition, each T cell possesses a distinct antigen specificity as determined by its uniquely expressed T cell receptor (TCR) generated during T cell development in the thymus as the result of TCR α and TCR β gene rearrangements. Nonetheless, subset differentiation and antigen specificity will ultimately be central to understanding the function and significance of decidual T cells. Due to the complexity of these issues, we begin with a broad introduction into T cell biology, along with a brief historical perspective on the role of T cell subsets in pregnancy. We then move on to the specifics of T cell biology at the maternal-fetal interface, with an emphasis on the many unresolved issues concerning these cells. Several of the pathways we cover are diagrammed in Figure 1. An introduction to natural killer T (NKT) cells, CD4− CD8− T cells, and γδ T cells, which are relatively rare in the pregnant uterus, can be found elsewhere (Tilburgs et al., 2010a); other reviews of decidual αβ T cells, with additional perspectives, can also be found elsewhere (Ernerudh et al., 2011, Saito et al., 2010, Teles et al., 2013b, Tilburgs and Strominger, 2013).
Figure 1. T cell behavior at the maternal-fetal interface.
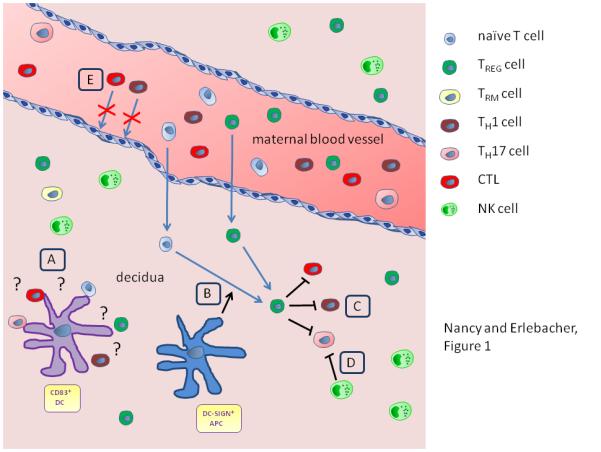
A schematized representation of the pregnant uterus, illustrating several of the regulatory pathways discussed in this review. (A) CD83+ DCs form clusters with decidual T cells, presumably as a consequence of ongoing antigen presentation. The significance of this observation is not currently known, nor are the T cell subsets involved (naïve, regulatory, effector, memory). (B) The potential role of decidual CD14+ DC-SIGN+ APCs in converting naïve decidual T cells into TReg cells. (C, D) The potential role of NK cells and TReg cells in repressing decidual CTLs, TH1 and TH17 cells. (E) Impeded TH1 cell and CTL recruitment to the decidua from the blood as a result of Cxcl9, Cxcl10, Cxcl11, and Ccl5 chemokine gene silencing in decidual stromal cells. Naïve T cells and TReg cells are shown entering the decidua from maternal blood, however there is currently no direct evidence that this happens to a major extent. The idea that naïve T cells enter into the decidua from the blood is particularly unlikely.
T cell activation and subsets – a primer
Following their development in the thymus, T cells are considered to be naïve until they first encounter cognate peptide antigen. This encounter takes place in the secondary lymphoid organs – i.e. the spleen for blood-borne antigens and the regional lymph nodes for blood-borne antigens and antigens that drain via lymphatic vessels from peripheral tissues – and entails interactions with dendritic cells (DCs) that present the cognate antigen on their cell surface in association with major histocompatibility complex (MHC) molecules. For CD4 T cells, TCR signaling is stimulated upon engagement of peptide antigen:MHC class II complexes; for CD8 T cells, TCR signaling is stimulated upon engagement of peptide antigen:MHC class I complexes. If the DC also supplies sufficient costimulation, which includes critical signals generated from the surface molecules CD80 and CD86, the responding T cell proliferates (i.e. undergoes clonal expansion) and then differentiates into one of several T cells subsets as specified by the set of cytokines encountered during antigen exposure. As discussed below, these subsets are TH1, TH2, TH17 effector CD4 T cells, regulatory CD4 T (TReg) cells, and cytotoxic T lymphocytes (CTLs), which are effector CD8 T cells. Once activated, an effector T cell exits the spleen or lymph node and then homes via the blood to the peripheral tissues. Although a relatively open area of research, T cell behavior within peripheral tissues is also thought to be actively regulated through pathways that include local antigen presentation and cytokine production by DCs and macrophages, as well as cross-inhibition of the different TH cell subsets via their own production of cytokines.
Effector CD4 T cells
TH1, TH2, and TH17 cells are the three main effector CD4 T cell subsets currently known (for review, see (Zhu et al., 2010)). They are defined by the set of transcription factors they respectively express in order to maintain their differentiated state, as well as by the set of cytokines they express that mediate their effector functions. In addition, each subset expresses a characteristic set of chemokine receptors that directs recruitment to sites of inflammation by promoting the extravasation of the cells across the endothelium. Consequently, the set of chemokine ligands expressed by an inflamed tissue will determine to a large extent the effector T cell subsets that can gain access to this tissue.
TH1 cells primarily function within peripheral tissues to promote the eradication of virus-infected cells and intracellular pathogens. The cells express the transcription factors T-bet and STAT4, and secrete interferon-γ (IFNγ) as their signature cytokine. They also express the chemokine receptors CXCR3, which is the receptor for CXCL9 (MIG), CXCL10 (IP-10), CXCL11 (I-TAC), and CCR5, which is a receptor for CCL5 (RANTES). IFNγ produced by TH1 cells promotes macrophage activation as well as stromal and endothelial cell expression of the CXCL9, CXCL10, and CXCL11, which means that activated TH1 cells promote both their own recruitment as well as the recruitment of CTLs, which also express CXCR3 (Nakanishi et al., 2009). TH1 cells also produce tumor necrosis factor-α (TNFα), which serves to promote inflammation in a variety of ways. Importantly, TH1 cells are the primary CD4 T cells that drive surgical allograft rejection and therefore have long been considered major threats to fetal survival and potential contributors to pregnancy pathologies.
TH2 cells primarily function in allergic reactions through their regulation of antibody isotype switching, and in the eradication of helminths. The cells express the transcription factors GATA3 and STAT6, secrete the cytokines IL-4, IL-5 and IL-10, and preferentially express the chemokine receptor CCR4, which is required for migration to sites of allergic inflammation such as the airways. Historically, interest in TH2 cells in pregnancy has come from the fact that these cells provided an alternative, less potentially embryotoxic differentiation state for CD4 T cells in comparison to TH1 cells, as well as the ability of TH2 cytokines to repress TH1 cell differentiation and function in trans. Thus, it had been thought that pregnancy would involve a general Th2-skewing of the T cell response in order to minimize the generation of TH1 cells. A great deal of data, however, has rendered this idea overly simplistic (discussed further in (Saito et al., 2010)).
TH17 cells augment acute inflammatory responses and mediate host immunity against extracellular bacteria and fungi. The cells express the transcription factors RORγt, STAT3 and IRF4, and secrete members of the IL-17 family of pro-inflammatory cytokines, most notably IL-17A. IL-17A acts on epithelial cells to induce neutrophil chemoattractants such as CXCL1 and neutrophil survival factors such as G-CSF. The predominant chemokine receptor of TH17 cells is CCR6, which attracts them to epithelial surfaces expressing their ligand CCL20. As the most recently described TH cell subset, their role in reproduction is only now beginning to be elucidated.
CTLs
CTLs are the CD8 T cell counterparts of effector TH1 cells, and like TH1 cells play a major role in virus and intracellular pathogen clearance. They express the same set of transcription factors (T-bet and STAT4) and cytokines (IFNγ and TNFα) as TH1 cells, and their recruitment to peripheral tissues is similarly governed by localized expression of CXCR3 and CCR5 ligands. In contrast to TH1 cells, however, CTLs have the capacity to directly kill target cells by virtue of their expression of the cytolytic molecules perforin and granzyme. These molecules are released upon TCR-mediated interactions with target cells expressing cognate peptide:MHC class I complexes. As with TH1 cells, CTLs play a major role in graft rejection and so too have been considered direct threats to fetal survival. This view has been tempered, however, by the realization that invasive trophoblasts, in both mice an humans, express a limited set of classical MHC class I molecules (King et al., 2000, Madeja et al., 2011), which means that there is less opportunity for CTLs to directly attack the placenta as they might a surgical organ transplant. On the other hand, the cytokines IL-12 and IL-18 produced by DCs and macrophages can induce CD8 T cells to produce IFNγ in a TCR-independent manner (Freeman et al., 2012). Thus, the mere presence of these cells at the maternal-fetal interface has, in principle, the potential to augment decidual inflammation to the detriment of pregnancy success.
Regulatory T cells
TReg cells suppress the activity of other immune cell types and are involved in down regulating immune responses after the elimination of invading organisms, in preventing autoimmunity, and in minimizing pathogenic responses to commensals (for a review, see (Campbell and Koch, 2011)). The cells are defined by their expression of the FOXP3 transcription factor, and are identified as such using intracellular staining protocols in conjunction with the cells’ CD4+ CD25hi surface phenotype. The cells express a wide variety of different chemokines receptors, and so have a rather promiscuous homing capacity. TReg cells are thought to primarily mediate their immunosuppressive effects though secretion of the cytokines IL-10 and TGF-β, as well as by acting as a sink for IL-2, which is a T cell mitogen and the ligand for CD25.
TReg cells are further classified in two groups based upon their origin. Natural TReg (nTReg) cells are generated in the thymus directly from T cell precursors and have reactivity towards self antigen. As a result, these cells are primarily thought to be involved in preventing the development of autoimmune reactions. In contrast, induced TReg (iTReg) cells are generated in secondary lymphoid organs from naïve CD4 T cells following their simultaneous exposure to innocuous foreign antigen and TGF-β. iTReg cells are therefore thought to play a specialized role in mitigating pathogenic responses to commensal flora. Recently, nTReg cells and iTReg cells, including those within the human decidua, have been distinguished by virtue of their differential expression of the Helios transcription factor, but the use of this marker has been challenged (Akimova et al., 2011, Gottschalk et al., 2012, Verhagen and Wraith, 2010).
Interest in the role of TReg cells in pregnancy, which is currently very intense, was initially stimulated by the dual findings that the cells increased in frequency in blood during both human and mouse pregnancy (Aluvihare et al., 2004, Somerset et al., 2004), and were required to prevent pregnancy failure in mice in allogeneic mating combinations (Aluvihare et al., 2004) (of note, the finding of TReg cell expansion during human pregnancy has since become controversial (Ernerudh et al., 2011)). More recently, the CNS1 enhancer element of the FOXP3 gene, which is specifically required for iTReg cell generation, was found to exist only in placental mammals, being absent from even monotremes and marsupials (Samstein et al., 2012). This result suggested a highly specific role for iTReg cells in pregnancy, potentially both systemically and at the maternal-fetal interface. We discuss the possible role of TReg cells within the decidua further below (for an additional review, see (Ernerudh et al., 2011)); their potential systemic function during pregnancy has been discussed elsewhere (Erlebacher, 2013b).
Memory and tissue resident memory T cells
Virtually all effector T cells generated in response to an immunogenic antigen undergo apoptosis once antigen clearance is complete. However, a few of these antigen-experienced cells survive and differentiate into memory T cells, which are capable of mounting rapid responses after a second contact with antigen. Classically, memory cells have been divided into two subsets based upon their homing patterns (for review, see (Mueller et al., 2013)). Central memory T cells express CD62L (L-selectin) and the chemokine receptor CCR7 and recirculate throughout the blood and secondary lymphoid organs like naïve T cells. In contrast, effector memory T cells are CD62L− CCR7− and recirculate throughout peripheral tissues patrolling for antigen. More recently, a third class of memory T cells has been described, termed resident memory T cells (TRM cells) that, as implied by their name, remain stationary within the tissues where they are thought to provide an immediate, yet antigen-specific, defense against a re-infecting pathogen (Mueller et al., 2013). These cells have recently been identified in the mouse reproductive tract (Schenkel et al., 2013), but their role in pregnancy has not yet been investigated.
T cell composition in the decidua
A thorough analysis of the T cell subset composition of the mouse decidua has not been published. Compared to other leukocyte subtypes such as NK cells and monocytes, T cells are quite rare, and comprise only about 3% of total decidual leukocytes on embryonic day (E) 8.5 ((Croy et al., 2012) and Nancy and Erlebacher, unpublished data). The cells are split about 50:50 between CD4 and CD8 T cells (Croy et al., 2012), and approximately 15% of the CD4 T cells are FOXP3+ TReg cells on E13.5-14.5 (Samstein et al., 2012). The proportion of these TReg cells that are nTReg versus iTReg cells is unknown. In contrast, a great deal more work has been performed on the human decidua, where T cells comprise a much greater proportion of total leukocytes (10-20%; for review see (Erlebacher, 2013a)). About ~30-45% of the cells are CD4 T cells and 45-75% are CD8 T cells (Bulmer et al., 1991, Marlin et al., 2011, Mjosberg et al., 2010, Tilburgs et al., 2009b, Vassiliadou and Bulmer, 1996), with the large majority of both subsets being antigen-experienced (CD45RA− or CD45RO+) (Saito et al., 1994, Slukvin et al., 1996, Tilburgs et al., 2010b). Based upon their chemokine expression profile, putative TH2 and TH17 cells comprise only ~5% and 2% of first trimester decidual CD4 T cells, respectively, while TH1 (CCR4− CXCR3+ CCR6−) cells surprisingly comprise ~5-30% of the cells (Mjosberg et al., 2010). About 5% of the CD4 T cells are CD25hi FOXP3+ TReg cells (Mjosberg et al., 2010, Tilburgs et al., 2008), with about 55% of these cells at term in turn being putative Helios+ nTReg cells, and 45% being putative Helios− iTReg cells (Hsu et al., 2012). In humans, TReg cell frequencies have also been noted to be higher in the decidua than in the blood (Dimova et al., 2011, Mjosberg et al., 2010, Nagamatsu et al., 2011, Sasaki et al., 2004, Schumacher et al., 2009, Tilburgs et al., 2008, Tilburgs et al., 2006).
These data have an important caveat, however, since they were generated via flow cytometric analyses of disaggregated tissues. Namely, it is currently unknown how many of the T cells visualized in this matter are present within the tissue parenchyma versus the blood that is flowing through the tissue. In mice, this issue has been highlighted by our recent data discussed below demonstrating that TH1 cells and CTLs are actively prevented from extravasating into the decidua (Nancy et al., 2012). Indeed, using a technique we developed for discriminating intravascular from extravascular leukocytes in the decidua (Tagliani et al., 2011), we have found that the majority of the CD4 and CD8 T cells isolated from non-perfused decidua on E8.5 are actually intravascular under steady state conditions (Nancy and Erlebacher, unpublished data). It is unclear whether vascular perfusion is able to remedy this problem, as many leukocytes within the mouse decidua are adherent to the endothelium (Kruse et al., 1999). Judging from work in rats, these leukocytes are likely to remain even after vascular perfusion (Welsh and Enders, 1985).
A related interpretative issue concerns the comparison made frequently between the lymphocyte subset composition of peripheral blood of a pregnant woman and the composition of the woman’s decidua. This comparison, in which the over-representation of a given subset is sometimes taken to imply the existence of local mechanisms that directly enhance that subset’s recruitment or survival have not sufficiently considered the fact that naïve T cells, which lack tissue-homing chemokine receptors (e.g. CXCR3, CCR4, CCR5, CCR6), enter non-lymphoid peripheral tissues relatively poorly. For example, in a recent study on pregnant women at term, naïve (CD45RA+) CD8 T cells were shown to comprise ~50% of all CD8 T cells in the blood, but only ~5% of CD8 T cells in the decidua, with these latter cells including ones that might actually be intravascular (Tilburgs et al., 2010b). Similar results have been obtained for both CD4 and CD8 T cells in studies of the first trimester (Saito et al., 1994, Slukvin et al., 1996). These data imply that that all differentiated T cell subsets will, without any specific recruitment mechanism, appear enriched within the decidua compared to the blood when cell numbers are expressed as proportions of respective total CD4 or CD8 T cells. Despite some evidence pointing to the involvement of specific chemoattractants (Schumacher et al., 2009), this issue is particularly relevant to suggestions that TReg cells are actively recruited to the human maternal-fetal interface (Schumacher et al., 2009, Tilburgs et al., 2008, Tilburgs et al., 2006).
Antigen specificity and locations of antigen presentation
Two of the most persistent and difficult questions regarding the biology of decidual T cells are whether the cells have specificity for placental antigens, and if so, where they encounter these antigens. The former question is far from trivial given the existence of resident memory cells and the well-known property of effector memory T cells to continuously traffic through peripheral tissues even in the absence of local inflammation or infection. TReg cells also have promiscuous homing properties and are thought to populate all tissues to some extent at steady state (Campbell and Koch, 2011). Thus, it is entirely conceivable that a large fraction of decidual T cells do not have placental specificity but rather populate the decidua either because they just happen to be migrating through this tissue in an antigen non-specific fashion, or because they are the same cells that were present in the endometrium at the time of implantation. These possibilities are also supported by the lack of data showing that maternal T cells are robustly activated to placental antigens, as discussed further below, as well as data indicating that certain T cell subsets are actively prevented from extravasating into the decidua, also discussed below.
The question of where T cells encounter placental antigens, while understood to a much greater extent, still has unresolved aspects. In mice, the uterine draining lymph nodes (LN) are sites of placental antigen presentation, but this is surprisingly not the result of DC migration from the decidua. Rather, decidual DC become trapped within the tissue, and placental antigens instead are disseminated in cell-free form both via the regional lymphatics as well as through the blood, which gives them access to the spleen and all LN throughout the body (Collins et al., 2009, Erlebacher et al., 2007, Moldenhauer et al., 2009, Rowe et al., 2012). Ensuing antigen presentation by the antigen presenting cells (APCs) that reside within these secondary lymphoid organs is ultimately non-immunogenic, however, as effector T cells are not generated from naïve precursors. Instead, the responding T cells are largely deleted as they simultaneously undergo multiple rounds of cell division. This work has been based upon the use of transgenic mice that express model antigens as surrogate fetal/placental antigens in order to clearly detect antigen-specific T cell responses, but it is assumed that findings also apply to endogenous placental antigens (for review, see (Moldenhauer et al., 2010)). In the case of at least one model antigen, it has recently been shown that the systemic CD4 T cell response can also involve conversion into iTReg cells, but the number of such cells generated in this matter is at maximum on the order of 1000 cells per mouse (Rowe et al., 2012).
The upshot of this work is that it might be incredibly difficult to detect maternal T cells specifically responding to placental antigens in humans, especially if blood alone is analyzed. In one recent attempt, CD8 T cells specific for male HY minor histocompatibility antigen were indeed detected in the blood of pregnant women bearing male concepti, but the cells were only detectable in half of the relevant pregnancies and their percentage (of total CD8 T cells) was on average only 0.043% after 10 days of in vitro peptide stimulation (Lissauer et al., 2012). Interestingly, the cells that grew out had an effector memory phenotype. In another study, a mother/child mismatch in human leukocyte antigen- (HLA-) C, which is one of the classical MHC class I molecules in humans, was found to enrich the frequency of CD25dim (i.e. activated or memory) decidual CD4 T cells by about 10%, an observation that was attributed to the presentation of paternal HLA-C-derived peptides by maternal APCs (Tilburgs et al., 2009a). HLA-C-specific CD4 T cells were not directly identified, however, and no change was observed in the frequency of decidual CD28− (effector or effector/memory) CD8 T cells.
Indeed, recent work in mice has complicated even the idea that the expanded numbers of TReg cells observed systemically during both mouse and human gestation represent the aggregate response of a large number of CD4 T cell clones synchronously converting to iTReg cells in response to shed placental antigen. Specifically, it was found that treatment of non-pregnant mice with the pregnancy hormone progesterone, given at doses to achieve serum levels similar to what occur at midgestation, induced the same systemic ~2-4-fold expansion of TReg cells typically seen during mouse pregnancy (Mao et al., 2010). Conversely, this study also revealed that TReg cells expand in a progesterone receptor-dependent fashion in mice rendered “pseudopregnant,” which is a temporary hormonal state induced by the act of copulation alone (without actual embryo implantation) and associated with elevated progesterone production by the ovary. Thus, expanded systemic TReg cell numbers during both mouse and human pregnancy might largely reflect an antigen non-specific effect of progesterone rather than specific induction by placental antigens. Of note, however, another study has shown no effect of progesterone on TReg cell numbers in mice (Zhao et al., 2007).
Importantly, however, it remains possible that decidual APCs present placental antigen within the decidua itself. Indeed, CD83+ DCs in the first trimester human decidua form clusters with decidual T cells (Kammerer et al., 2000), while CD14+ DC-SIGN+ cells, which are thought to be a specialized macrophage subset with antigen presenting capacity (Erlebacher, 2013a, Nagamatsu and Schust, 2010), have been found in close association with decidual TReg cells (Hsu et al., 2012). If these spatial proximities reflect ongoing antigen presentation, however, it remains unclear the extent to which the responding T cells are naïve given the exclusion of naïve T cells from peripheral tissues and the low proportion of naïve T cells in the human decidua (Saito et al., 1994, Slukvin et al., 1996, Tilburgs et al., 2010b). Indeed, the current set of data in mice strongly argues against the intra-decidual presentation of placental antigens to at least naïve CD8 T cells, since such cells can be seen responding to a surrogate placental antigen in the spleen and uterine draining LN without any concurrent response within the decidua itself (Erlebacher et al., 2007). Thus, it is important to consider the alternative possibility that the intra-decidual presentation of placental Ag serves to reinforce the activation of placenta-specific T cells that were first exposed to antigen elsewhere. This possibility is consistent with the demonstrated systemic generation of iTReg cells specific for shed placental antigens (Rowe et al., 2012) followed by the homing of these cells to the decidua. Given recent data that the maternal-fetal interface is normally colonized at low levels with a variety of microbes (Stout et al., 2013), it is also possible that decidual APCs present microbe-derived peptides, perhaps as part of a mechanism to combat decidual infection.
Recruitment
Another dimension to the issue of the composition and function of decidual T cells comes from recent work from our laboratory showing that activated TH1 cells and CTLs are actively excluded from the mouse decidua (Nancy et al., 2012). This exclusion prevented antigen-specific fetal loss but itself was not antigen-specific. Rather, it was linked to an epigenetic program activated in differentiating decidual stromal cells (DSCs) that silenced expression of the Cxcl9, Cxcl10, Cxcl11 and Ccl5 genes that encode the key TH1 cell- and CTL-attracting chemokines CXCL9, CXCL10, CXLC11, and CCL5 discussed above.
In addition to suggesting a major reason why the fetus is not rejected by the maternal immune system, these results demonstrated that epigenetic pathways active in the stromal compartment of the mouse decidua play a major role in controlling T cell subset population dynamics at the maternal-fetal interface. Two pressing questions, therefore, are whether similar pathways are active in humans, and, if so, the extent to which they influence T cell composition of the human decidua in various physiological and pathophysiological settings. In particular, the existence of chemokine gene silencing pathways raises the possibility that the apparent relative proportions of T cell subsets in the decidua in normal pregnancy might not be the consequence of the active, specific recruitment of certain T cell subsets over others, or the selective proliferation, survival or retention of these subsets, but rather because T cell subsets strictly dependent upon CXCL9, CXCL10, CXCL11 and CCL5 for peripheral tissue recruitment are excluded. Indeed, demonstration that CXCL9, CXCL10, CXCL11 and CCL5 are silenced in human decidual stromal cells would suggest that the TH1 cells that populate the human decidua at relatively high numbers are present from the time of implantation and may in fact represent resident memory cells that lack specificity for placental antigen.
Regulation
The decidua-specific factors and pathways that regulate decidual T cell behavior are very poorly understood, and several promising ideas have not panned out in a robust fashion due to the absence of pregnancy phenotypes in gene-deficient mouse models. These include the proposals that the death ligand FasL, the negative costimulatory molecule PD-L1, and the tryptophan catabolizing enzyme idoleamine 2,3-dioxygenase, which are all expressed at the maternal-fetal interface would respectively kill, render inactive, or starve infiltrating effector T cells (Baban et al., 2004, Chaouat and Clark, 2001, Taglauer et al., 2009). In contrast, there is mounting evidence that the multifunctional carbohydrate-binding protein Galectin-1 may locally regulate decidual T cell behavior to promote a uterine environment supportive of pregnancy (for review see (Barrientos et al., 2013)). Consistent with this possibility, female mice deficient in Galectin-1 show higher rates of fetal loss in allogeneic but not syngeneic pregnancies (Blois et al., 2007). Galectin-1 has also been shown to be co-localized to clusters of T cells within the human decidua that are undergoing apoptosis (Kopcow et al., 2008), suggesting a possible role in regulating intradecidual T cell survival. As discussed further below, decidual NK cells have also recently been shown to repress TH17 cells through their production of IFNγ (Fu et al., 2013). Lastly, as also discussed further below, decidual TReg cells are thought to attenuate the activity of decidual effector T cells.
Functions and Pathological associations
Implantation
At present, it is technically difficult to perform intrauterine T cell ablations in mice, and ethical concerns preclude experimentation on pregnant women. Consequently, our current understanding of the function of decidual T cell subsets during pregnancy has largely been inferential, as it derives from knowledge about the function of each subset in non-uterine tissues and discoveries of associations between human pregnancy pathologies and changes in a subset’s prevalence within the decidua. Examples of this approach will be discussed further below. One emerging exception to the current state of ambiguity, however, comes from work suggesting that TReg cells play an important role in preparing the endometrium for implantation (for review, see (Robertson et al., 2013)). This idea has been gaining support from multiple studies in mice showing that TReg cell deficiency in the peri-implantation period, induced experimentally via a variety of means, causes either implantation failure or embryo resorption soon after implantation. In some studies (Aluvihare et al., 2004, Shima et al., 2010) but not others (Teles et al., 2013a), this failure occurs in allogeneic but not syngeneic mating combinations, suggesting that T cell responses to alloantigens present in semen might be important for implantation. Consistent with this possibility, maternal CD8 T cells undergo a transient wave of proliferation in the uterine LN following copulation in response to a surrogate seminal antigen (Moldenhauer et al., 2009). Interestingly, in this model, seminal fluid rather than spermatozoa was the antigen-containing material.
A specific, intrauterine requirement for TReg cells in implantation was first suggested by the observation that CD4+ CD25+ FOXP3+ TReg cell frequencies also transiently increase in the uterine LN on E3.5 (but not in the non-uterine LN), concurrent with the wave of antigen-induced T cell proliferation described above (Guerin et al., 2011). In addition, FOXP3+ cells, which are presumably also TReg cells, were found to be greater in number in the uteri of mice on E3.5 of pregnancy as compared to the uteri of mice in estrous. Both of these phenomena required seminal plasma, which, provocatively, is rich in the TReg cell-inducing cytokine TGF-β (Sharkey et al., 2012). Copulation also induced expression of the CCL19 chemokine by uterine epithelial cells, which was suggested to be a recruitment factor for uterine TReg cells (Guerin et al., 2011). In another study, mice deficient in CCR7, the receptor for CCL19, both lacked uterine Tregs and also showed reduced fertility (Teles et al., 2013a). This study also showed evidence of uterine fibrosis in mice lacking TReg cells.
Together these data suggest a scenario whereby TReg cells, induced in the uterine LN in response to semen and seminal antigens, home back to the uterus where they prepare the endometrium for implantation (Figure 2) (for further discussion, see (Robertson et al., 2013)). Presumably, the TReg cells in part act to dampen uterine inflammation, which is induced by semen (De et al., 1991). However, several issues still need to be addressed before the existence of this pathway can be firmly established. First, the extent to which antigen recognition is important for post-copulation TReg cell expansion and ultimate TReg cell function in the uterus needs to be determined. This is a key issue because postulating a specific requirement for iTReg cells in the peri-implantation period would need to be reconciled with the absence of a major implantation defect in mice that are specifically deficient in these cells (Samstein et al., 2012). Second, it will be important to determine whether the TReg cells that expand in the uterine LN following insemination selectively home to the uterus, as this would imply the existence of specific, and currently unappreciated, uterine-homing pathways. Indeed, it remains possible that the requirement for CCR7 in generating the cells actually reflects the CCR7 dependence of DC emigration from the reproductive tract (Collins et al., 2009), which is expected to be necessary for the presentation of seminal antigens in the draining LN. Third, the idea that the anti-inflammatory effects of TReg cells are important for preparing the uterus for implantation needs to be reconciled with the growing sense that a modest amount of uterine inflammation promotes implantation (Dekel et al., 2010).
Figure 2. TReg cell generation in response to semen in the pre-implantation mouse uterus.
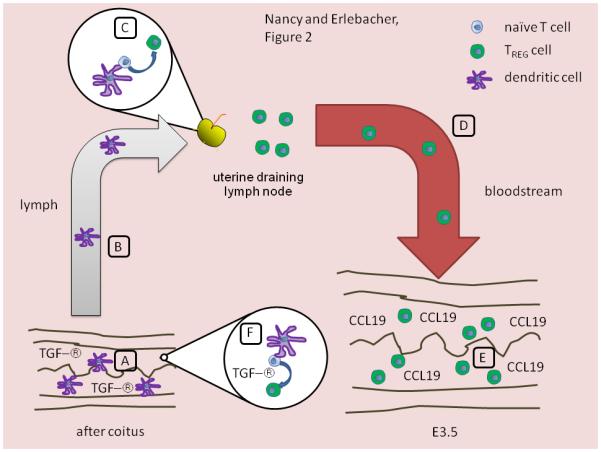
Two complementary mechanisms are shown; the evidence for the first is discussed in the text. (A-E) Under the influence of seminal fluid rich in TGF-β (A), DCs ingest and process seminal fluid antigens and then migrate via lymphatic vessels (B) to the uterine LN. In these LN, they present seminal fluid antigens and convert responding naïve T cells into TReg cells due to the influence of TGF-β (C). Of note, it is unclear whether the TGF-β that acts during this phase of the response is derived from seminal fluid, or is produced by maternal cells. Once generated, the TReg cells migrate via the blood back to the uterus (D), possibly attracted by CCL19 induced by uterine epithelial cells in response to semen (E). (A, F) Alternatively, uterine DCs might present seminal fluid antigens to naïve T cells within the uterus itself, which locally induces iTReg cell generation due to the high levels of TGF-β in seminal fluid.
Lastly, and most importantly, the current set of data does not resolve whether TReg cells act locally within the uterus, or systemically, or both. Indeed, TReg depletion induces systemic inflammation (Kim et al., 2007), and the peri-implantation period is particularly sensitive to inflammation-induced ovarian insufficiency (Erlebacher et al., 2004). Thus, it remains possible that implantation failure in the absence of sufficient TReg cell function systemically is due to impaired progesterone production by the corpus luteum. In fact, the requirement for TReg cells during the peri-implantation period might even reflect a crucial role for TReg cells within the ovary itself, a possibility suggested by work in cows showing that the luteolytic effect of prostaglandin PGF2α is associated with reductions in ovarian TReg cell numbers (Poole and Pate, 2012).
Preeclampsia and spontaneous abortion
Potential roles for decidual TReg cells have also emerged from studies on the pathogenesis of preeclampsia and spontaneous abortion (for review, see (Cerdeira et al., 2012, Ernerudh et al., 2011)). Stimulated by reports of reduced TReg cell frequencies in the peripheral blood of pregnant women with preeclampsia compared to control pregnant women (Darmochwal-Kolarz et al., 2007, Prins et al., 2009, Santner-Nanan et al., 2009, Sasaki et al., 2007, Toldi et al., 2012) (a finding not replicated with other cohorts (Hu et al., 2008, Paeschke et al., 2005)), it was initially suggested that TReg frequencies at the maternal-fetal interface were also reduced in preeclampsia (Quinn et al., 2011, Sasaki et al., 2007). A recent report failed to reproduce this observation, but instead revealed that preeclamptic patients showed specific reductions decidual frequencies of Helios− FOXP3+ CD25+ CD4+ cells, which presumably represent iTReg cells (Hsu et al., 2012). This defect was furthermore linked to an altered phenotype of decidual DC-SIGN+ APCs, which were found to be located in proximity to decidual TReg cells. Reduced decidual TReg cell frequency or function has also been reported in cases of spontaneous abortion (Inada et al., 2013, Sasaki et al., 2004, Schumacher et al., 2009, Wang et al., 2010), sometimes in association with increased TH17 cell frequencies (Wang et al., 2010).
Given the well-known immunosuppressive functions of TReg cells, these data together suggest a scenario in which TReg cells expand within the decidua, potentially as the result of the local presentation of placental antigens by decidual APCs, in order to limit effector T cell activity at the maternal-fetal interface (Ernerudh et al., 2011). Without such a limit, effector T cells (i.e. TH1 cells, TH17 cells and CTLs) are freer to induce pregnancy pathologies ranging from first trimester fetal loss to inadequate spiral artery remodeling. Exact effector mechanisms for a given pathology, however, are unknown and an important area of research. In addition, TReg cells might also generally limit decidual inflammation through antigen non-specific mechanisms. This latter possibility is consistent with the cells’ secretion of anti-inflammatory factors such as IL-10 and TGF-β, which will have broad effects, as well as with emerging data in mice discussed above that uterine TReg cells play an anti-inflammatory role in preparing the uterus for embryo implantation. Such a latter role would obviously be independent of placental antigen exposure. Importantly, elevated proportions of decidual TH17 cells per se have been linked to the pathogenesis of spontaneous abortion (Nakashima et al., 2010, Wang et al., 2010), and recent work in both mice and humans (Fu et al., 2013) suggests that decidual NK cells, through their production of IFNγ, are also capable of suppressing decidual TH17 cell accumulation. Thus, elevated decidual TH17 cell frequencies in spontaneous abortion have also been associated with low NK cell numbers (Fu et al., 2013). Together, these data build upon older work and have led to a revised Th1/Th2/Th17/TReg cell paradigm for pregnancy success (Saito et al., 2010).
VUE and chronic deciduitis
Overt, histologically apparent T cell accumulations, akin to those seen with organ transplant rejection, are not findings typically associated with preeclampsia or spontaneous abortion. Thus, the contribution of T cells to these pathologies is thought to be mainly due to changes in T cell subset proportions and altered T cell functions. In contrast, villitis of unknown etiology (VUE), chronic deciduitis, and chronic chorioamnionitis are three histological findings of the third trimester placenta where maternal T cells do accumulate at the maternal-fetal interface (Erlebacher, 2013a). Given the greater amount of relevant literature, we will discuss the pathogenesis and significance of VUE, with the recognition that the set of questions this lesion raises also applies to chronic deciduitis and chronic chorioamnionitis.
VUE affects 5-15% of all births and is characterized by focal accumulations of maternal T cells, comprised of both CD4 and CD8 T cells, as well as fetal macrophages (i.e. Hofbauer cells) (for reviews, see (Redline, 2007, Tamblyn et al., 2013)). The lesions are primarily located in the distal placental villi, but it has been suggested that they arise from leukocytes that have first infiltrated into the decidua before spreading vertically via the anchoring villi to reach the villus tree. Its origin is not thought to be infectious. From a clinical viewpoint, VUE is an interesting lesion since its incidence is increased in several complications of human pregnancy, including idiopathic preterm birth and intrauterine growth restriction. Its causal relationship with these pregnancy complications is at present unclear. The lesion is associated with increased placental mRNA expression of CXCL9, CXCL10, CXCL11 and CCL5 (Freitag et al., 2013, Kim et al., 2009), as well as focal expression of CXCL9, CXCL10, and CCL5 protein at the sites of leukocyte accumulation (Kim et al., 2009). TH1 cells are thought to primarily comprise the CD4 T cell component of the response, while the CD8 T cells are thought to be CTLs.
From a basic viewpoint, VUE is also interesting because its histological features are what might be expected if maternal T cells were to be rejecting the placenta. Considering this possibility raises the interesting question of the antigen specificity of the infiltrating T cells, which remains unknown. This is a key question since it speaks to underlying pathogenic mechanisms. If the T cells are specific for placental antigens, then there must have been at some point a breakdown in those pathways that normally prevent the immunogenic presentation of placental antigens to maternal T cells. In addition, there must have been a breakdown in mechanisms that prevent TH1 cell and CTL infiltration into the decidua and placenta. Indeed, the focal nature of VUE in itself points to the potential existence of such mechanisms, since otherwise such activated T cells should show a diffuse pattern of infiltration. Indeed, we have previously conjectured that focal dysregulation of the epigenetic pathways that silence Cxcl9, Cxcl10, and Ccl5 expression in mouse DSCs, if these pathways are also active in human DSCs, might underlie at least the decidual component of VUE (Erlebacher, 2013a). It also is possible that maternal T cell directly enter the placental tree as the result of a focal damage or because syncytiotrophoblasts (which form the cellular layer of the villus tree in contact with maternal blood) have upregulated certain key adhesion molecules (Redline, 2007, Tamblyn et al., 2013)).
If, on the other hand, the infiltrating T cells in VUE are not specific for placental antigens, then work on underlying pathogenic mechanisms needs to consider only how these T cells gain access to the decidua and placenta, and could consider the same set of hypotheses as if they did have placental specificity. Interestingly, the possibility that the infiltrating T cells are bystanders (with respect to fetal and placental antigens) raises the possibility that they were generated at distal sites, perhaps in response to infection.
Importantly, although indirect presentation pathways are thought to mediate T cell recognition of placental antigens under physiological conditions in both mice and humans, as discussed above, it remains possible that the T cells within VUE lesions are specific for intact paternal MHC molecules. In the case of CD8 T cells, the cells might be interacting directly with paternal HLA-C molecules expressed not only by extravillous trophoblasts (for those areas of the lesion within the decidua) but also with all MHC class I molecules expressed by the fetus-derived non-trophoblastic stromal constituents of the villi including fibroblasts, endothelial cells and Hofbauer cells. In the case of CD4 T cells, the cells might be interacting directly with Hofbauer cells, which express MHC class II molecules in VUE lesions. If such directly alloreactive maternal T cells are indeed constituents of VUE lesions, the question again arises of how these cells first become activated.
Conclusions
Importantly, the above pathological scenarios view effector T cells within the decidua as having a negative impact upon pregnancy success. This idea is certainly more likely if a large proportion of these T cells turn out to have specificity for placental antigens. However, recent work suggesting that the human maternal-fetal interface is normally colonized with a wide variety of bacteria (Stout et al., 2013) suggests that effector T cells within the decidua might perform a positive function in controlling infection. If this is true, then this function must be very finely tuned so that the response, on the one hand, is sufficient to prevent progression to pregnancy endpoints such as acute infectious chorioamnionitis, while on the other hand is not so robust that the attendant inflammation in fighting the infection leads to pregnancy complications such as preterm labor secondary to non-infectious chorioamnionitis. As discussed further elsewhere (Erlebacher, 2013a, Tilburgs and Strominger, 2013), the existence of intra-decidual mechanisms to limit maternal T cell responses against the placenta might also explain why the decidua is susceptible to infection by certain organisms such as Listeria monocytogenes and cytomegalovirus.
Indeed, how intradecidual T cell behavior is balanced in order to accommodate the competing demands of reproduction and host defense can now be viewed as a key question facing research on the biology of decidual T cells. As discussed above, other important unresolved questions include the pathways that regulate intradecidual T cell function, the antigen specificity of decidual T cells, and the degree to which dysregulation of decidual T cells function or migration are causative pathogenic factors in human pregnancy complications. It is hoped that the current surge in interest in this critical area of reproductive immunology will lead to insights with potential impact on human reproductive health.
Acknowledgements
Work in the Erlebacher lab is supported by grants from the NIH (RO1-CA168755 and RO1-AI106745), The American Cancer Society (RSG-10-158-01-LIB), and The March of Dimes (#6-FY13-80).
Abbreviations
- NK cell
natural killer cell
- TCR
T cell receptor
- NKT cell
natural killer T cell
- DC
dendritic cells
- MHC
histocompatibility complex
- TReg cell
regulatory CD4 T cell
- CTL
cytotoxic T lymphocyte
- IFNγ
interferon-γ
- TNFα
tumor necrosis factor-α
- nTreg cell
natural TReg cell
- iTReg cell
induced TReg cell
- TRM cell
resident memory T cell
- E
embryonic day
- LN
lymph nodes
- APC
antigen presenting cell
- HLA
human leukocyte antigen
- DSC
decidual stromal cell
- VUE
villitis of unknown etiology
References
- AKIMOVA T, BEIER UH, WANG L, LEVINE MH, HANCOCK WW. Helios expression is a marker of T cell activation and proliferation. PLoS One. 2011;6:e24226. doi: 10.1371/journal.pone.0024226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALUVIHARE VR, KALLIKOURDIS M, BETZ AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–71. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- BABAN B, CHANDLER P, MCCOOL D, MARSHALL B, MUNN DH, MELLOR AL. Indoleamine 2,3-dioxygenase expression is restricted to fetal trophoblast giant cells during murine gestation and is maternal genome specific. J Reprod Immunol. 2004;61:67–77. doi: 10.1016/j.jri.2003.11.003. [DOI] [PubMed] [Google Scholar]
- BARRIENTOS G, FREITAG N, TIRADO-GONZALEZ I, UNVERDORBEN L, JESCHKE U, THIJSSEN VL, BLOIS SM. Involvement of galectin-1 in reproduction: past, present and future. Hum Reprod Update. 2013 doi: 10.1093/humupd/dmt040. [DOI] [PubMed] [Google Scholar]
- BLOIS SM, ILARREGUI JM, TOMETTEN M, GARCIA M, ORSAL AS, CORDO-RUSSO R, TOSCANO MA, BIANCO GA, KOBELT P, HANDJISKI B, et al. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med. 2007;13:1450–7. doi: 10.1038/nm1680. [DOI] [PubMed] [Google Scholar]
- BULMER JN, MORRISON L, LONGFELLOW M, RITSON A, PACE D. Granulated lymphocytes in human endometrium: histochemical and immunohistochemical studies. Hum Reprod. 1991;6:791–8. doi: 10.1093/oxfordjournals.humrep.a137430. [DOI] [PubMed] [Google Scholar]
- BULMER JN, WILLIAMS PJ, LASH GE. Immune cells in the placental bed. Int J Dev Biol. 2010;54:281–94. doi: 10.1387/ijdb.082763jb. [DOI] [PubMed] [Google Scholar]
- CAMPBELL DJ, KOCH MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11:119–30. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CERDEIRA AS, KOPCOW HD, KARUMANCHI SA. Regulatory T cells in preeclampsia: some answers, more questions? Am J Pathol. 2012;181:1900–2. doi: 10.1016/j.ajpath.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAOUAT G, CLARK DA. FAS/FAS ligand interaction at the placental interface is not required for the success of allogeneic pregnancy in anti-paternal MHC preimmunized mice. Am J Reprod Immunol. 2001;45:108–15. doi: 10.1111/j.8755-8920.2001.450208.x. [DOI] [PubMed] [Google Scholar]
- COLLINS MK, TAY CS, ERLEBACHER A. Dendritic cell entrapment within the pregnant uterus inhibits immune surveillance of the maternal/fetal interface in mice. J Clin Invest. 2009;119:2062–2073. doi: 10.1172/JCI38714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROY BA, CHEN Z, HOFMANN AP, LORD EM, SEDLACEK AL, GERBER SA. Imaging of vascular development in early mouse decidua and its association with leukocytes and trophoblasts. Biol Reprod. 2012;87:125. doi: 10.1095/biolreprod.112.102830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DARMOCHWAL-KOLARZ D, SAITO S, ROLINSKI J, TABARKIEWICZ J, KOLARZ B, LESZCZYNSKA-GORZELAK B, OLESZCZUK J. Activated T lymphocytes in pre-eclampsia. Am J Reprod Immunol. 2007;58:39–45. doi: 10.1111/j.1600-0897.2007.00489.x. [DOI] [PubMed] [Google Scholar]
- DE M, CHOUDHURI R, WOOD GW. Determination of the number and distribution of macrophages, lymphocytes, and granulocytes in the mouse uterus from mating through implantation. J. Leukocyte Biol. 1991;50:252–262. doi: 10.1002/jlb.50.3.252. [DOI] [PubMed] [Google Scholar]
- DEKEL N, GNAINSKY Y, GRANOT I, MOR G. Inflammation and implantation. Am J Reprod Immunol. 2010;63:17–21. doi: 10.1111/j.1600-0897.2009.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIMOVA T, NAGAEVA O, STENQVIST AC, HEDLUND M, KJELLBERG L, STRAND M, DEHLIN E, MINCHEVA-NILSSON L. Maternal Foxp3 expressing CD4+ CD25+ and CD4+ CD25- regulatory T-cell populations are enriched in human early normal pregnancy decidua: a phenotypic study of paired decidual and peripheral blood samples. Am J Reprod Immunol. 2011;66(Suppl 1):44–56. doi: 10.1111/j.1600-0897.2011.01046.x. [DOI] [PubMed] [Google Scholar]
- ERLEBACHER A. Immunology of the maternal-fetal interface. Annu Rev Immunol. 2013a;31:387–411. doi: 10.1146/annurev-immunol-032712-100003. [DOI] [PubMed] [Google Scholar]
- ERLEBACHER A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat Rev Immunol. 2013b;13:23–33. doi: 10.1038/nri3361. [DOI] [PubMed] [Google Scholar]
- ERLEBACHER A, VENCATO D, PRICE KA, ZHANG D, GLIMCHER LH. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J Clin Invest. 2007;117:1399–411. doi: 10.1172/JCI28214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERLEBACHER A, ZHANG D, PARLOW AF, GLIMCHER LH. Ovarian insufficiency and early pregnancy loss induced by activation of the innate immune system. J Clin Invest. 2004;114:39–48. doi: 10.1172/JCI20645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERNERUDH J, BERG G, MJOSBERG J. Regulatory T helper cells in pregnancy and their roles in systemic versus local immune tolerance. Am J Reprod Immunol. 2011;1:31–43. doi: 10.1111/j.1600-0897.2011.01049.x. [DOI] [PubMed] [Google Scholar]
- FREEMAN BE, HAMMARLUND E, RAUE HP, SLIFKA MK. Regulation of innate CD8+ T-cell activation mediated by cytokines. Proc Natl Acad Sci U S A. 2012;109:9971–6. doi: 10.1073/pnas.1203543109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREITAG L, VON KAISENBERG C, KREIPE H, HUSSEIN K. Expression analysis of leukocytes attracting cytokines in chronic histiocytic intervillositis of the placenta. Int J Clin Exp Pathol. 2013;6:1103–11. [PMC free article] [PubMed] [Google Scholar]
- FU B, LI X, SUN R, TONG X, LING B, TIAN Z, WEI H. Natural killer cells promote immune tolerance by regulating inflammatory TH17 cells at the human maternal-fetal interface. Proc Natl Acad Sci U S A. 2013;110:E231–40. doi: 10.1073/pnas.1206322110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOTTSCHALK RA, CORSE E, ALLISON JP. Expression of Helios in peripherally induced Foxp3+ regulatory T cells. J Immunol. 2012;188:976–80. doi: 10.4049/jimmunol.1102964. [DOI] [PubMed] [Google Scholar]
- GUERIN LR, MOLDENHAUER LM, PRINS JR, BROMFIELD JJ, HAYBALL JD, ROBERTSON SA. Seminal Fluid Regulates Accumulation of FOXP3+ Regulatory T Cells in the Preimplantation Mouse Uterus Through Expanding the FOXP3+ Cell Pool and CCL19-Mediated Recruitment. Biol Reprod. 2011 doi: 10.1095/biolreprod.110.088591. [DOI] [PubMed] [Google Scholar]
- HSU P, SANTNER-NANAN B, DAHLSTROM JE, FADIA M, CHANDRA A, PEEK M, NANAN R. Altered decidual DC-SIGN+ antigen-presenting cells and impaired regulatory T-cell induction in preeclampsia. Am J Pathol. 2012;181:2149–60. doi: 10.1016/j.ajpath.2012.08.032. [DOI] [PubMed] [Google Scholar]
- HU D, CHEN Y, ZHANG W, WANG H, WANG Z, DONG M. Alteration of peripheral CD4+CD25+ regulatory T lymphocytes in pregnancy and pre-eclampsia. Acta Obstet Gynecol Scand. 2008;87:190–4. doi: 10.1080/00016340701823991. [DOI] [PubMed] [Google Scholar]
- INADA K, SHIMA T, NAKASHIMA A, AOKI K, ITO M, SAITO S. Characterization of regulatory T cells in decidua of miscarriage cases with abnormal or normal fetal chromosomal content. J Reprod Immunol. 2013;97:104–11. doi: 10.1016/j.jri.2012.12.001. [DOI] [PubMed] [Google Scholar]
- KAMMERER U, SCHOPPET M, MCLELLAN AD, KAPP M, HUPPERTZ HI, KAMPGEN E, DIETL J. Human decidua contains potent immunostimulatory CD83(+) dendritic cells. Am J Pathol. 2000;157:159–69. doi: 10.1016/S0002-9440(10)64527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM JM, RASMUSSEN JP, RUDENSKY AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–7. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- KIM MJ, ROMERO R, KIM CJ, TARCA AL, CHHAUY S, LAJEUNESSE C, LEE DC, DRAGHICI S, GOTSCH F, KUSANOVIC JP, et al. Villitis of unknown etiology is associated with a distinct pattern of chemokine up-regulation in the feto-maternal and placental compartments: implications for conjoint maternal allograft rejection and maternal anti-fetal graft-versus-host disease. J Immunol. 2009;182:3919–27. doi: 10.4049/jimmunol.0803834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING A, BURROWS TD, HIBY SE, BOWEN JM, JOSEPH S, VERMA S, LIM PB, GARDNER L, LE BOUTEILLER P, ZIEGLER A, et al. Surface expression of HLA-C antigen by human extravillous trophoblast. Placenta. 2000;21:376–87. doi: 10.1053/plac.1999.0496. [DOI] [PubMed] [Google Scholar]
- KOPCOW HD, ROSETTI F, LEUNG Y, ALLAN DS, KUTOK JL, STROMINGER JL. T cell apoptosis at the maternal-fetal interface in early human pregnancy, involvement of galectin-1. Proc Natl Acad Sci U S A. 2008;105:18472–7. doi: 10.1073/pnas.0809233105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRUSE A, MERCHANT MJ, HALLMANN R, BUTCHER EC. Evidence of specialized leukocyte-vascular homing interactions at the maternal/fetal interface. Eur J Immunol. 1999;29:1116–26. doi: 10.1002/(SICI)1521-4141(199904)29:04<1116::AID-IMMU1116>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- LISSAUER D, PIPER K, GOODYEAR O, KILBY MD, MOSS PA. Fetal-specific CD8+ cytotoxic T cell responses develop during normal human pregnancy and exhibit broad functional capacity. J Immunol. 2012;189:1072–80. doi: 10.4049/jimmunol.1200544. [DOI] [PubMed] [Google Scholar]
- MADEJA Z, YADI H, APPS R, BOULENOUAR S, ROPER SJ, GARDNER L, MOFFETT A, COLUCCI F, HEMBERGER M. Paternal MHC expression on mouse trophoblast affects uterine vascularization and fetal growth. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1005342108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAO G, WANG J, KANG Y, TAI P, WEN J, ZOU Q, LI G, OUYANG H, XIA G, WANG B. Progesterone increases systemic and local uterine proportions of CD4+CD25+ TReg cells during midterm pregnancy in mice. Endocrinology. 2010;151:5477–88. doi: 10.1210/en.2010-0426. [DOI] [PubMed] [Google Scholar]
- MARLIN R, NUGEYRE MT, DURIEZ M, CANNOU C, LE BRETON A, BERKANE N, BARRE-SINOUSSI F, MENU E. Decidual soluble factors participate in the control of HIV-1 infection at the maternofetal interface. Retrovirology. 2011;8:58. doi: 10.1186/1742-4690-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MJOSBERG J, BERG G, JENMALM MC, ERNERUDH J. FOXP3+ regulatory T cells and T helper 1, T helper 2, and T helper 17 cells in human early pregnancy decidua. Biol Reprod. 2010;82:698–705. doi: 10.1095/biolreprod.109.081208. [DOI] [PubMed] [Google Scholar]
- MOLDENHAUER LM, DIENER KR, THRING DM, BROWN MP, HAYBALL JD, ROBERTSON SA. Cross-presentation of male seminal fluid antigens elicits T cell activation to initiate the female immune response to pregnancy. J Immunol. 2009;182:8080–93. doi: 10.4049/jimmunol.0804018. [DOI] [PubMed] [Google Scholar]
- MOLDENHAUER LM, HAYBALL JD, ROBERTSON SA. Utilising T cell receptor transgenic mice to define mechanisms of maternal T cell tolerance in pregnancy. J Reprod Immunol. 2010;87:1–13. doi: 10.1016/j.jri.2010.05.007. [DOI] [PubMed] [Google Scholar]
- MUELLER SN, GEBHARDT T, CARBONE FR, HEATH WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. 2013;31:137–61. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- NAGAMATSU T, BARRIER BF, SCHUST DJ. The regulation of T-cell cytokine production by ICOS-B7H2 interactions at the human fetomaternal interface. Immunol Cell Biol. 2011;89:417–25. doi: 10.1038/icb.2010.101. [DOI] [PubMed] [Google Scholar]
- NAGAMATSU T, SCHUST DJ. The contribution of macrophages to normal and pathological pregnancies. Am J Reprod Immunol. 2010;63:460–71. doi: 10.1111/j.1600-0897.2010.00813.x. [DOI] [PubMed] [Google Scholar]
- NAKANISHI Y, LU B, GERARD C, IWASAKI A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462:510–3. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKASHIMA A, ITO M, SHIMA T, BAC ND, HIDAKA T, SAITO S. Accumulation of IL-17-positive cells in decidua of inevitable abortion cases. Am J Reprod Immunol. 2010;64:4–11. doi: 10.1111/j.1600-0897.2010.00812.x. [DOI] [PubMed] [Google Scholar]
- NANCY P, TAGLIANI E, TAY CS, ASP P, LEVY DE, ERLEBACHER A. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science. 2012;336:1317–21. doi: 10.1126/science.1220030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAESCHKE S, CHEN F, HORN N, FOTOPOULOU C, ZAMBON-BERTOJA A, SOLLWEDEL A, ZENCLUSSEN ML, CASALIS PA, DUDENHAUSEN JW, VOLK HD, et al. Pre-eclampsia is not associated with changes in the levels of regulatory T cells in peripheral blood. Am J Reprod Immunol. 2005;54:384–9. doi: 10.1111/j.1600-0897.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- POOLE DH, PATE JL. Luteal microenvironment directs resident T lymphocyte function in cows. Biol Reprod. 2012;86:29. doi: 10.1095/biolreprod.111.092296. [DOI] [PubMed] [Google Scholar]
- PRINS JR, BOELENS HM, HEIMWEG J, VAN DER HEIDE S, DUBOIS AE, VAN OOSTERHOUT AJ, ERWICH JJ. Preeclampsia is associated with lower percentages of regulatory T cells in maternal blood. Hypertens Pregnancy. 2009;28:300–11. doi: 10.1080/10641950802601237. [DOI] [PubMed] [Google Scholar]
- QUINN KH, LACOURSIERE DY, CUI L, BUI J, PARAST MM. The unique pathophysiology of early-onset severe preeclampsia: role of decidual T regulatory cells. J Reprod Immunol. 2011;91:76–82. doi: 10.1016/j.jri.2011.05.006. [DOI] [PubMed] [Google Scholar]
- REDLINE RW. Villitis of unknown etiology: noninfectious chronic villitis in the placenta. Hum Pathol. 2007;38:1439–46. doi: 10.1016/j.humpath.2007.05.025. [DOI] [PubMed] [Google Scholar]
- ROBERTSON SA, PRINS JR, SHARKEY DJ, MOLDENHAUER LM. Seminal fluid and the generation of regulatory T cells for embryo implantation. Am J Reprod Immunol. 2013;69:315–30. doi: 10.1111/aji.12107. [DOI] [PubMed] [Google Scholar]
- ROWE JH, ERTELT JM, XIN L, WAY SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490:102–6. doi: 10.1038/nature11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITO S, NAKASHIMA A, SHIMA T, ITO M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol. 2010;63:601–10. doi: 10.1111/j.1600-0897.2010.00852.x. [DOI] [PubMed] [Google Scholar]
- SAITO S, NISHIKAWA K, MORII T, NARITA N, ENOMOTO M, ITO A, ICHIJO M. A study of CD45RO, CD45RA and CD29 antigen expression on human decidual T cells in an early stage of pregnancy. Immunol Lett. 1994;40:193–7. doi: 10.1016/0165-2478(93)00019-a. [DOI] [PubMed] [Google Scholar]
- SAMSTEIN RM, JOSEFOWICZ SZ, ARVEY A, TREUTING PM, RUDENSKY AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012;150:29–38. doi: 10.1016/j.cell.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANTNER-NANAN B, PEEK MJ, KHANAM R, RICHARTS L, ZHU E, FAZEKAS DE ST GROTH B, NANAN R. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol. 2009;183:7023–30. doi: 10.4049/jimmunol.0901154. [DOI] [PubMed] [Google Scholar]
- SASAKI Y, DARMOCHWAL-KOLARZ D, SUZUKI D, SAKAI M, ITO M, SHIMA T, SHIOZAKI A, ROLINSKI J, SAITO S. Proportion of peripheral blood and decidual CD4(+) CD25(bright) regulatory T cells in pre-eclampsia. Clin Exp Immunol. 2007;149:139–45. doi: 10.1111/j.1365-2249.2007.03397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SASAKI Y, SAKAI M, MIYAZAKI S, HIGUMA S, SHIOZAKI A, SAITO S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod. 2004;10:347–53. doi: 10.1093/molehr/gah044. [DOI] [PubMed] [Google Scholar]
- SCHENKEL JM, FRASER KA, VEZYS V, MASOPUST D. Sensing and alarm function of resident memory CD8(+) T cells. Nat Immunol. 2013;14:509–13. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHUMACHER A, BRACHWITZ N, SOHR S, ENGELAND K, LANGWISCH S, DOLAPTCHIEVA M, ALEXANDER T, TARAN A, MALFERTHEINER SF, COSTA SD, et al. Human chorionic gonadotropin attracts regulatory T cells into the fetal-maternal interface during early human pregnancy. J Immunol. 2009;182:5488–97. doi: 10.4049/jimmunol.0803177. [DOI] [PubMed] [Google Scholar]
- SHARKEY DJ, MACPHERSON AM, TREMELLEN KP, MOTTERSHEAD DG, GILCHRIST RB, ROBERTSON SA. TGF-beta mediates proinflammatory seminal fluid signaling in human cervical epithelial cells. J Immunol. 2012;189:1024–35. doi: 10.4049/jimmunol.1200005. [DOI] [PubMed] [Google Scholar]
- SHIMA T, SASAKI Y, ITOH M, NAKASHIMA A, ISHII N, SUGAMURA K, SAITO S. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J Reprod Immunol. 2010;85:121–9. doi: 10.1016/j.jri.2010.02.006. [DOI] [PubMed] [Google Scholar]
- SLUKVIN II, MERKULOVA AA, VODYANIK MA, CHERNYSHOV VP. Differential expression of CD45RA and CD45RO molecules on human decidual and peripheral blood lymphocytes at early stage of pregnancy. Am J Reprod Immunol. 1996;35:16–22. [PubMed] [Google Scholar]
- SOMERSET DA, ZHENG Y, KILBY MD, SANSOM DM, DRAYSON MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004;112:38–43. doi: 10.1111/j.1365-2567.2004.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOUT MJ, CONLON B, LANDEAU M, LEE I, BOWER C, ZHAO Q, ROEHL KA, NELSON DM, MACONES GA, MYSOREKAR IU. Identification of intracellular bacteria in the basal plate of the human placenta in term and preterm gestations. Am J Obstet Gynecol. 2013;208:226 e1–7. doi: 10.1016/j.ajog.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAGLAUER ES, YANKEE TM, PETROFF MG. Maternal PD-1 regulates accumulation of fetal antigen-specific CD8+ T cells in pregnancy. J Reprod Immunol. 2009;80:12–21. doi: 10.1016/j.jri.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAGLIANI E, SHI C, NANCY P, TAY CS, PAMER EG, ERLEBACHER A. Coordinate regulation of tissue macrophage and dendritic cell population dynamics by CSF-1. J Exp Med. 2011;208:1901–16. doi: 10.1084/jem.20110866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAMBLYN JA, LISSAUER DM, POWELL R, COX P, KILBY MD. The immunological basis of villitis of unknown etiology - review. Placenta. 2013;34:846–55. doi: 10.1016/j.placenta.2013.07.002. [DOI] [PubMed] [Google Scholar]
- TELES A, SCHUMACHER A, KUHNLE MC, LINZKE N, THUERE C, REICHARDT P, TADOKORO CE, HAMMERLING GJ, ZENCLUSSEN AC. Control of uterine microenvironment by foxp3(+) cells facilitates embryo implantation. Front Immunol. 2013a;4:158. doi: 10.3389/fimmu.2013.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TELES A, ZENCLUSSEN AC, SCHUMACHER A. Regulatory T cells are baby's best friends. Am J Reprod Immunol. 2013b;69:331–9. doi: 10.1111/aji.12067. [DOI] [PubMed] [Google Scholar]
- TILBURGS T, CLAAS FH, SCHERJON SA. Elsevier Trophoblast Research Award Lecture: Unique properties of decidual T cells and their role in immune regulation during human pregnancy. Placenta. 2010a;31:S82–6. doi: 10.1016/j.placenta.2010.01.007. Suppl. [DOI] [PubMed] [Google Scholar]
- TILBURGS T, ROELEN DL, VAN DER MAST BJ, DE GROOT-SWINGS GM, KLEIJBURG C, SCHERJON SA, CLAAS FH. Evidence for a selective migration of fetus-specific CD4+CD25bright regulatory T cells from the peripheral blood to the decidua in human pregnancy. J Immunol. 2008;180:5737–45. doi: 10.4049/jimmunol.180.8.5737. [DOI] [PubMed] [Google Scholar]
- TILBURGS T, ROELEN DL, VAN DER MAST BJ, VAN SCHIP JJ, KLEIJBURG C, DE GROOT-SWINGS GM, KANHAI HH, CLAAS FH, SCHERJON SA. Differential distribution of CD4(+)CD25(bright) and CD8(+)CD28(−) T-cells in decidua and maternal blood during human pregnancy. Placenta. 2006;27(Suppl A):S47–53. doi: 10.1016/j.placenta.2005.11.008. [DOI] [PubMed] [Google Scholar]
- TILBURGS T, SCHERJON SA, VAN DER MAST BJ, HAASNOOT GW, VERSTEEG VDV-MM, ROELEN DL, VAN ROOD JJ, CLAAS FH. Fetal-maternal HLA-C mismatch is associated with decidual T cell activation and induction of functional T regulatory cells. J Reprod Immunol. 2009a;82:148–57. doi: 10.1016/j.jri.2009.05.003. [DOI] [PubMed] [Google Scholar]
- TILBURGS T, SCHONKEREN D, EIKMANS M, NAGTZAAM NM, DATEMA G, SWINGS GM, PRINS F, VAN LITH JM, VAN DER MAST BJ, ROELEN DL, et al. Human decidual tissue contains differentiated CD8+ effector-memory T cells with unique properties. J Immunol. 2010b;185:4470–7. doi: 10.4049/jimmunol.0903597. [DOI] [PubMed] [Google Scholar]
- TILBURGS T, STROMINGER JL. CD8+ effector T cells at the fetal-maternal interface, balancing fetal tolerance and antiviral immunity. Am J Reprod Immunol. 2013;69:395–407. doi: 10.1111/aji.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TILBURGS T, VAN DER MAST BJ, NAGTZAAM NM, ROELEN DL, SCHERJON SA, CLAAS FH. Expression of NK cell receptors on decidual T cells in human pregnancy. J Reprod Immunol. 2009b;80:22–32. doi: 10.1016/j.jri.2009.02.004. [DOI] [PubMed] [Google Scholar]
- TOLDI G, SAITO S, SHIMA T, HALMOS A, VERESH Z, VASARHELYI B, RIGO J, JR., MOLVAREC A. The frequency of peripheral blood CD4+ CD25high FoxP3+ and CD4+ CD25− FoxP3+ regulatory T cells in normal pregnancy and pre-eclampsia. Am J Reprod Immunol. 2012;68:175–80. doi: 10.1111/j.1600-0897.2012.01145.x. [DOI] [PubMed] [Google Scholar]
- TRUNDLEY A, MOFFETT A. Human uterine leukocytes and pregnancy. Tissue Antigens. 2004;63:1–12. doi: 10.1111/j.1399-0039.2004.00170.x. [DOI] [PubMed] [Google Scholar]
- VASSILIADOU N, BULMER JN. Quantitative analysis of T lymphocyte subsets in pregnant and nonpregnant human endometrium. Biol Reprod. 1996;55:1017–22. doi: 10.1095/biolreprod55.5.1017. [DOI] [PubMed] [Google Scholar]
- VERHAGEN J, WRAITH DC. Comment on "Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells". J Immunol. 2010;185:7129. doi: 10.4049/jimmunol.1090105. author reply 7130. [DOI] [PubMed] [Google Scholar]
- WANG WJ, HAO CF, YI L, YIN GJ, BAO SH, QIU LH, LIN QD. Increased prevalence of T helper 17 (Th17) cells in peripheral blood and decidua in unexplained recurrent spontaneous abortion patients. J Reprod Immunol. 2010;84:164–70. doi: 10.1016/j.jri.2009.12.003. [DOI] [PubMed] [Google Scholar]
- WELSH AO, ENDERS AC. Light and electron microscopic examination of the mature decidual cells of the rat with emphasis on the antimesometrial decidua and its degeneration. Am J Anat. 1985;172:1–29. doi: 10.1002/aja.1001720102. [DOI] [PubMed] [Google Scholar]
- ZHAO JX, ZENG YY, LIU Y. Fetal alloantigen is responsible for the expansion of the CD4(+)CD25(+) regulatory T cell pool during pregnancy. J Reprod Immunol. 2007;75:71–81. doi: 10.1016/j.jri.2007.06.052. [DOI] [PubMed] [Google Scholar]
- ZHU J, YAMANE H, PAUL WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–89. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]


