Abstract
Objectives
Nasopharyngeal carcinoma (NPC) is a rare cancer in the United States. An association between NPC and Epstein-Barr virus (EBV) is well-established for World Health Organization (WHO) types II and III (WHO-II/III) NPC but less well-established for WHO type I (WHO-I) NPC. Given the rise in oropharyngeal tumors positive for high-risk human papillomavirus (HPV) and the unique biology of WHO-I NPC, we examined the relationship between HPV and WHO-I NPC.
Study Design
Retrospective case-comparison study.
Methods
A search of a large multidisciplinary cancer center tumor registry identified 183 patients seen from January 1999 to December 2008 with incident NPC and no prior cancer. Available paraffin-embedded tumor specimens (N=30) were analyzed for oncogenic HPV status by in-situ hybridization (ISH) and polymerase chain reaction (PCR) for HPV-16 and HPV-18; EBV status by ISH; and p16 expression by immunohistochemistry. Demographic parameters, including race and smoking, were obtained from the medical records.
Results
Among the 18 WHO-I NPC patients, 66% (N=12) were smokers and 17% (N=3) Asian; among the 165 WHO-II/III NPC patients, 44% (N=73) were smokers and 24% (N=39) Asian. Eight WHO-I NPC patients had available paraffin blocks; 5 of 6 were HPV-16-positive by PCR and 4 of 8 were HPV-positive by ISH; only 2 of 8 (25%) were EBV-positive. Twenty-two WHO-II/III NPC patients had available paraffin blocks; only 1 was HPV-positive by ISH, and 13 of 22 (60%) were EBV-positive.
Conclusions
These results suggest that WHO-I NPC is associated with oncogenic HPV, though larger studies are needed to verify these findings.
Keywords: Human papillomavirus, Nasopharyngeal Carcinoma, WHO Type, In-situ hybridization, Polymerase chain reaction
Introduction
In the past three decades, the incidence of nasopharyngeal carcinoma (NPC) in the United States has been stable at 0.7 cases per 100,000 individuals, with consistently low rates in non-Hispanic white Americans (0.4 per 100,000), African Americans (0.7), and Hispanic Americans (0.4) but considerably higher rates among Asian Americans (2.6 to 3.8).1 These rates are dramatically lower than in areas where NPC is endemic, such as southern China, where incidence rates can approach 30 cases per 100,000 individuals in males and 15 per 100,000 in females.2
The World Health Organization (WHO) has historically classified NPC into three histologic types: type I (keratinizing), type II (nonkeratinizing), and type III (undifferentiated). In North America, 25% of NPC is type I, 12% is type II, and 63% is type III, whereas in southern China, the corresponding rates are 2%, 3%, and 95%, respectively.3 Recent studies have begun grouping types II and III together, as they share similar clinical features, strong association with Epstein Barr virus (EBV), and lack of keratinization.3 While WHO histologic type has been shown to be an independent prognostic factor, the survival advantage is chiefly seen for nonkeratinizing over keratinizing NPC.4 Asian Americans typically present with undifferentiated and nonkeratinizing carcinomas (type II/III); non-Hispanic white Americans, African Americans, and Hispanic Americans often present with keratinizing squamous cell carcinoma (type I), and Chinese NPC patients have improved survival compared to NPC patients of other ethnicities.4,5 Unlike nonkeratinizing NPC, type I (keratinizing) NPC lacks a clear, consistent association with EBV,6 suggesting that the decreased survival and high locoregional failure rates observed in non-Asians with type I NPC may reflect differences in disease etiology.7
The etiology of NPC appears to involve the interaction of genetic, environmental, and viral factors. For instance, polymorphisms in human leukocyte antigens, diets with high amounts of salted fish and nitrosamines, and heavy cigarette smoking are all associated with increased risk for NPC.8,9,10,11 In areas where NPC is not endemic, there is a closer association between smoking and WHO type I (WHO-I; keratinizing) NPC than for nonkeratinizing NPC.12
Recently, the rise in detection of human papillomavirus (HPV) in oropharyngeal cancers and the histologic similarities between the lymphoid-rich tissues of the oropharynx and nasopharynx have raised the possibility of a role of this epitheliotrophic virus in NPC.13,14 Several authors have proposed a polarized relationship linking EBV to nonkeratinizing NPC and HPV to keratinizing NPC.15-20 Other studies have linked HPV to undifferentiated NPC,21 while yet others suggest that both viruses contribute to carcinogenesis in nonkeratinizing NPC via coinfection.22-24 We investigated the possible relationship between HPV and NPC, with emphasis on WHO-I NPC, which is more prevalent in the United States.
Materials and Methods
Patients
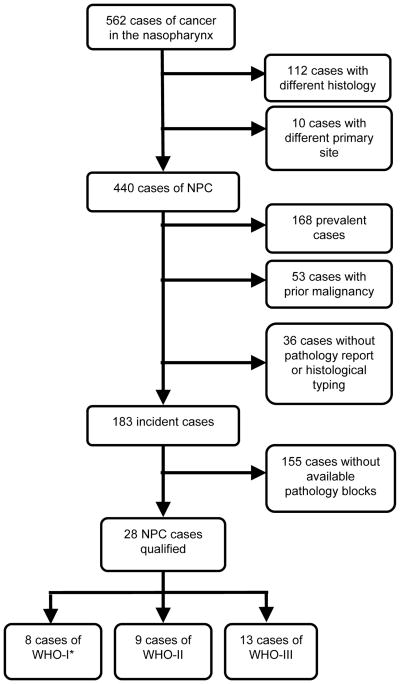
After receiving approval by the institutional review board, we searched the institutional tumor registry for cancer involving the nasopharynx and identified 562 patients seen at our institution during a 10-year period from January 1999 to December 2008 (Figure 1). A thorough review of the electronic medical records excluded histologic types other than NPC (112 cases) and squamous cell carcinomas of other primary sites (10 cases), leaving 440 cases of NPC. Patients were then excluded if they presented with recurrent or previously treated (prevalent) NPC, if they had prior history of cancer other than nonmelanoma skin cancer, if there were no pathology reports in the chart, or if there was no tumor tissue on file. We found that most patients were referred to our institution with a diagnosis of NPC on the basis of biopsy at an outside institution, and tissue from these cases was unavailable. To include more patients with WHO-I NPC, we included two exceptions—one patient who presented with recurrent NPC and one patient with a remote history of bladder cancer (diagnosed 15 years prior to diagnosis of NPC). Consequently, we had 8 patients with WHO-I NPC, 9 with WHO-II NPC, and 13 with WHO-III NPC with tumor specimens available for study.
Figure 1.
Flowchart describing patient selection.
*To increase the number of patients in this group, two patients who did not meet the formal inclusion criteria were added to this category—one who presented with recurrent NPC and one who had a remote history of bladder cancer.
Data on patient characteristics were primarily acquired from the Patient History Database, a questionnaire completed by new patients upon registration at our institution. In the questionnaire, patients answered questions about past medical history and cancer history, race, family history, and smoking and drinking habits. We classified patients into 3 groups: never smokers (<100 cigarettes in lifetime), former smokers (quit at least 1 year previously), and current smokers. Pack-years were defined as number of packs per day multiplied by number of years smoked. We classified alcohol use as never/rare (<1 drink/week), former (stopped drinking at least 1 year previously), and current. Family history was recorded as history of cancer in any first-degree relatives. For charts missing this questionnaire, the data were extracted from the history and physical performed upon initial presentation.
DNA extraction and HPV detection
An experienced head and neck pathologist (D.B.) reevaluated hematoxylin-and-eosin-stained slides of the specimens to confirm the diagnosis and WHO NPC type and selected corresponding paraffin blocks of primary tumor tissue. Two 10-μm sections were sliced from the selected blocks using a microtome. DNA was extracted from these blocks using a modified method as we previously described.25
We used polymerase chain reaction (PCR) to detect the presence of HPV-16 and HPV-18 DNA by amplification of regions of the E6 and E7 genes. The primers we used are shown in Table 1. Assays were run in duplicate using the housekeeper gene β-actin as an internal control for DNA quality; Siha cells (containing 9.2 copies of HPV-16 DNA per 5 μL) as positive controls for HPV-16 DNA; pBR322 plasmid (with the entire HPV-18 genome inserted in the tet gene, 104.5 copies per 5 μL) as a positive control for HPV-18 DNA; MDA-686 cells (HPV-negative cell line) as negative controls; and PCR mixture without template DNA as a control for the PCR process. We excluded tissue samples that failed to amplify β-actin. The MDA-686 cells were purified alongside the samples to ensure that all reagents were free of contamination. PCR was performed in a total reaction volume of 15 μL that contained 5 μL of template DNA, 0.15 μL of 10 mM deoxyribonucelotide triphosphate, 1.5 μL of 10× PCR buffer (containing 15 mM MgCl2, KCl, (NH4)2SO4, Qiagen Inc., Valencia, CA), 0.1 μL of 5 U/μL HotStarTaq DNA Polymerase (Qiagen Inc.), 0.15 μL of a 10 pm/μL mixture of antisense and sense primers (Integrated DNA Technologies, Coralville, IA), and 8.1 μL of ddH2O. Using a PTC-200 Peltier Thermal Cycler (MJ Research, Waltham, MA), amplification conditions were initial denaturation at 95°C for 10 minutes, 45 cycles of denaturation at 95°C for 30 seconds, annealing at 60°C and 58°C for HPV-16 and HPV-18, respectively for 30 seconds, extension at 72°C for 40 seconds, and final extension at 72°C for 10 minutes. Aliquots of 10 μL of DNA were run on a 3% Exclu-sieve high-resolution DNA/RNA agarose gel (The Nest Group, Inc., Southborough, MA) containing 5% ethidium bromide with 3 μL of loading dye alongside a 25-base-pair ladder and visualized using ultraviolet light.
Table 1.
Primers for Amplification of HPV Genome.
| Primer | Sequence | Location on Virus Genome | Length (bp) |
|---|---|---|---|
| HPV16 E6 S (20 mer) | 5′-GCA ATG TTT CAG GAC CCA CA-3′ (forward) | 101-219 | 119 |
| HPV16 E6 AS (22 mer) | 5′-CGC AGT AAC TGT TGC TTG CAG T-3′ (reverse) | ||
| HPV16 E7 S (23 mer) | 5′-TTG TTG CAA GTG TGA CTC TAC GC-3′ (forward) | 732-824 | 93 |
| HPV16 E7 AS (23 mer) | 5′- CCT AGT GTG CCC ATT AAC AGG TC-3′ (reverse) | ||
| HPV18 E6 S (21 mer) | 5′-TCA CAA CAT AGC TGG GCA CTA-3′ (forward) | 485-576 | 92 |
| HPV18 E6 AS (21 mer) | 5′-CTT GTG TTT CTC TGC GTC GTT-3′ (reverse) | ||
| HPV18 E7 S (21 mer) | 5′-ATG AAA TTC CGG TTG ACC TTC-3′ (forward) | 645-746 | 102 |
| HPV18 E7 AS (21 mer) | 5′-GTC GGG CTG GTA AAT GTT GAT-3′ (reverse) | ||
| β-actin 1-S (20 mer) | 5′-GGC ATC CTC ACC CTG AAG TA-3′ (forward) | 3198-3279 | 82 |
| β-actin 1-AS (20 mer) | 5′-AGG TGT GGT GCC AGA TTT TC-3′ (reverse) |
bp = base pairs.
In situ hybridization
In situ hybridization for HPV and EBV nucleic acids was performed on formalin-fixed, paraffin-embedded tissue sections using the automated BenchMark system (Ventana, Tuscon, AZ) according to the manufacturer's recommended protocols. The Ventana INFORM EBER probe was used to detect early RNA transcripts of EBV. The Ventana INFORM HPV III Family 16 Probe was used to detect positivity of hybridization in the nuclei of high-risk HPV genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 66).
Immunohistochemistry (IHC) and immunoreactivity analysis
Immunohistochemical analysis for p16 was performed using the BOND MAX immunohistochemical staining protocol by Vision Biosystems (Norwell, MA) on 4-μm paraffin sections of the tissue material. In brief, following dewaxing, washing, and rehydration of the slides through xylene and graded alcohols, Tris-EDTA buffer was used for antigen retrieval. Slides were subsequently treated with 3% hydrogen peroxide to block endogenous peroxidase. Following incubation with the primary antibody, p16 (BD Systems, Franklin Lakes, NJ, 1:50 dilution), the secondary conjugate antibody was applied. Finally, each specimen-containing slide was developed using the chromogen DAB and counterstained with hematoxylin. Nuclear and cytoplasmic staining was scored for p16 expression. Staining was recorded as positive when there was strong staining (nuclear and/or cytoplasmic) in at least 30% of tumor cells.
Statistical Analysis
Chi-squared test was used to compare categorical variables between groups, and Fisher's exact test was used when one or more subgroups had fewer than five observations. T-test was used to compare continuous variables (such as age) between groups, and Satterthwaite's adjustment was used for unequal variances where appropriate.
Results
The demographic and exposure characteristics for the entire group are shown in Table 2. WHO-I patients tended to be older (mean age 55.6 years, median age 58 years, range 19-81) than WHO-II/III patients (mean age 47.6 years, median age 47 years, range 12-80, P=.019). Higher proportions of WHO-I patients than WHO-II/III patients were female and were non-Asian, but these differences were not significant (Table 2).
Table 2.
Demographic and Exposure Characteristics for All Patients with Incident NPC (1999-2008).
| No. of Patients (%) | ||||
|---|---|---|---|---|
|
| ||||
| Characteristic | All (n = 183) |
WHO Type I (n = 18) |
WHO Type II/III (n = 165) |
P value* |
| Age, years | ||||
| <30 | 20 (10.9) | 1 (5.6) | 19 (11.5) | .036† |
| 30-59 | 126 (68.9) | 9 (50.0) | 117 (70.9) | |
| ≥60 | 37 (20.2) | 8 (44.4) | 29 (17.6) | |
| Sex | ||||
| Male | 130 (71.0) | 11 (61.1) | 119 (72.1) | .328 |
| Female | 53 (29.0) | 7 (38.9) | 46 (27.9) | |
| Ethnicity | ||||
| Non-Hispanic White | 97 (53.0) | 11 (61.1) | 86 (52.1) | .803† |
| Asian | 42 (23.0) | 3 (16.7) | 39 (23.6) | |
| Other | 44 (24.0) | 4 (22.2) | 40 (24.2) | |
| Smoking | ||||
| Never | 98 (53.6) | 6 (33.3) | 92 (55.8) | .180 |
| Former | 37 (20.2) | 5 (27.8) | 32 (19.4) | |
| Current | 48 (26.2) | 7 (38.9) | 41 (24.8) | |
| Pack-years of ever smokers‡ | ||||
| <30 pack-years | 43 (23.5) | 3 (16.7) | 40 (24.2) | .059† |
| ≥30 pack-years | 39 (21.3) | 9 (50.0) | 30 (18.2) | |
| Alcohol use | ||||
| Never/rare | 104 (56.8) | 11 (61.1) | 93 (56.4) | .001† |
| Former | 12 (6.6) | 5 (27.8) | 7 (4.2) | |
| Current | 67 (36.6) | 2 (11.1) | 65 (39.4) | |
| History of cancer in first-degree relative | ||||
| None | 116 (63.4) | 10 (55.6) | 106 (64.2) | .468 |
| Any cancer | 67 (36.6) | 8 (44.4) | 59 (35.8) | |
WHO- World Health Organization.
Chi-squared test comparing WHO type I to WHO type II/III.
Fisher's exact test comparing WHO type I to WHO type II/III.
Pack-years was not documented for 3 WHO type II/III patients.
WHO-I patients were more likely to be current or former smokers, and WHO-II/III patients were more likely to be never smokers, though these differences were not significant (Table 2). Among the ever smokers, WHO-I patients had greater pack-years of use (mean 42, median 39.5) than WHO-II/III patients (mean 29.9, median 25), but again, these differences were not significant (P=.113). WHO-I patients were significantly more likely to be never or former alcohol drinkers, and WHO-II/III patients were significantly more likely to be current drinkers (P=.001, Table 2).
Among the 67 patients (37%) with a first-degree family history of cancer, no WHO-I patients and six WHO-II/III patients had a first-degree relative with NPC. Regarding HPV or EBV-related cancers, one WHO-I patient and five WHO-II/III patients had a first-degree relative with an HPV-related cancer (all had cervical cancer); no WHO-I patients and two WHO-II/III patients had a first-degree relative with lymphoma. Regarding tobacco-related cancers, two WHO-I patients and five WHO-II/III patients had a first-degree relative with head and neck cancer; one WHO-I patient and 12 WHO-II/III patients had a first-degree relative with lung cancer. The remaining patients with first-degree family history of cancer had first-degree relatives with primarily colon, prostate, breast, and ovarian cancer.
The demographic, exposure, and family history characteristics of the patients for whom pathology specimens were available are presented in Table 3. The trends in age, ethnicity, smoking, and alcohol use were similar to those seen in the larger source sample. WHO-I patients tended to be older (mean age 60.3 years, median age 62.5 years, range 38-74) than WHO-II/III patients (mean age 47.4 years, median age 47 years, range 16-77, P=.051). Additionally, WHO-I patients were more likely to be smokers with greater pack-years of use (mean 53.8, median 51.3) than WHO-II/III patients (mean 22.8, median 15, P=.008).
Table 3.
Demographics and Exposure Characteristics of Patients with Incident NPC (1999-2008) for Whom Pathology Specimens Were Available for HPV Testing.†
| No. of Patients (%) | ||||
|---|---|---|---|---|
|
| ||||
| Characteristic | All (n = 30) |
WHO Type I (n = 8) |
WHO Type II/III (n = 22) |
P value* |
| Age, years | ||||
| <30 | 4 (13.3) | 0 (0) | 4 (18.2) | .230 |
| 30-59 | 15 (50.0) | 3 (37.5) | 12 (54.5) | |
| ≥60 | 11 (36.7) | 5 (62.5) | 6 (27.3) | |
| Sex | ||||
| Male | 22 (73.3) | 6 (75.0) | 16 (72.7) | 1.0 |
| Female | 8 (26.7) | 2 (25.0) | 6 (27.3) | |
| Ethnicity | ||||
| Non-Hispanic White | 19 (63.3) | 6 (75.0) | 13 (59.1) | .851 |
| Asian | 5 (16.7) | 1 (12.5) | 4 (18.2) | |
| Other | 6 (20.0) | 1 (12.5) | 5 (22.7) | |
| Smoking | ||||
| Never | 13 (43.3) | 2 (25.0) | 11 (50.0) | .441 |
| Former | 7 (23.3) | 2 (25.0) | 5 (22.7) | |
| Current | 10 (33.3) | 4 (50.0) | 6 (27.3) | |
| Pack-years of ever smokers | ||||
| <30 pack-years | 7 (23.3) | 0 (0) | 7 (31.8) | .035 |
| ≥30 pack-years | 10 (33.3) | 6 (75.0) | 4 (18.2) | |
| Alcohol use | ||||
| Never/rare | 18 (60.0) | 5 (62.5) | 13 (59.1) | .059 |
| Former | 5 (16.7) | 3 (37.5) | 2 (9.1) | |
| Current | 7 (23.3) | 0 (0) | 7 (31.8) | |
| History of cancer in first-degree relative | ||||
| None | 15 (50.0) | 2 (25.0) | 13 (59.1) | .215 |
| Any cancer | 15 (50.0) | 6 (75.0) | 9 (40.9) | |
WHO = World Health Organization.
Fisher's exact test comparing WHO type I to WHO type II/III.
Includes one WHO-I NPC patient with prevalent disease.
Table 4 summarizes the rates of detection of HPV, p16, and EBV in the 30 NPC cases for which pathology specimens were available. Of the eight WHO-I cases, 83% were HPV-16 positive by PCR, and 50% were high-risk HPV positive by in situ hybridization. In contrast, of the 22 WHO-II/III cases, 46% were HPV-16 positive by PCR, and only one was HPV positive by in situ hybridization. HPV-18 DNA was not identified in any specimen from any patient. Similar proportions of WHO-I and WHO-II/III tumors overexpressed p16 by immunohistochemistry. Finally, a greater proportion of WHO-I tumors than WHO-II/III tumors were positive for HPV by in situ hybridization and PCR and overexpressed p16. Only 25% of WHO-I tumors but 60% of WHO-II/III tumors were EBV positive. None of the EBV-positive tumors were HPV positive by in situ hybridization.
Table 4.
Detection of HPV, p16, and EBV in Pathology Specimens.
| No. of Patients (%) with Positive Results | P value† | |||
|---|---|---|---|---|
|
|
||||
| All* (n = 30) |
WHO Type I (n = 8) |
WHO Type II/III (n = 22) |
||
| HPV type 16 by PCR | ||||
| E6 positive | 13/28 (46.4) | 4/6 (66.7) | 9/22 (40.9) | .372 |
| E7 positive | 15/28 (53.6) | 5/6 (83.3) | 10/22 (45.5) | .173 |
| Both E6 & E7 positive | 12/28 (42.9) | 4/6 (66.7) | 8/22 (36.4) | .354 |
| HPV type 18 by PCR | ||||
| E6 positive | 0 (0) | 0 (0) | 0 (0) | 1.0 |
| E7 positive | 0 (0) | 0 (0) | 0 (0) | 1.0 |
| Both E6 & E7 positive | 0 (0) | 0 (0) | 0 (0) | 1.0 |
| HPV positive by ISH‡ | 5/28 (17.9) | 4/8 (50.0) | 1/20 (5.0) | .015 |
| p16 positive by IHC | 25/28 (89.3) | 7/8 (87.5) | 18/20 (90.0) | 1.0 |
| HPV positive by PCR & ISH‡ and p16 positive | 4/26 (15.4) | 3/6 (50.0) | 1/20 (5.0) | .028 |
| EBV positive by ISH | 14/28 (50.0) | 2/8 (25.0) | 12/20 (60.0) | .209 |
PCR = polymerase chain reaction, ISH = in situ hybridization, and IHC = immunohistochemistry.
PCR was not successful for 2 WHO-I cases and ISH was not successful for 2 WHO-II/III cases.
Fisher's exact test comparing WHO type I to WHO type II/III.
ISH probe detects high-risk HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, & 66).
Discussion
Our findings suggest that WHO-I NPC may be associated with oncogenic HPV. Oncogenic HPV was detected by in situ hybridization in half of the WHO-I NPCs but only 5% of the WHO-II/III NPCs in our series.
There is extensive literature investigating viral etiologies to explain the increased risk of NPC in certain populations.8-12 EBV has been detected in a large proportion of patients with WHO-II/III NPC, but a significant subset of patients with WHO-I NPC are EBV negative.17,23,26 We speculated that high-risk HPV may contribute to the development of NPC, given HPV's acknowledged role in the pathogenesis of oropharyngeal carcinomas.
Recent decades have seen a shift in the epidemiology of head and neck squamous cell carcinomas, likely in association with the rise in prevalence of HPV infection. Despite a decreasing prevalence of cigarette smoking and a subsequent decline in overall head and neck cancer incidence rates, the incidences of tonsillar and base-of-tongue cancer are rising.27,28 HPV has been detected in the genomic DNA of 26% of head and neck squamous cell carcinomas overall but 50-70% of oropharyngeal carcinomas; 90% of HPV-associated oropharyngeal carcinomas are positive for high-risk HPV types, overwhelmingly type 16.27,29
The lack of consensus on the relationship between HPV and NPC may be due to the small numbers of patients in the studies published to date, geographic variations, and racial differences between study populations. Most studies have used only PCR methods to detect the virus. In Tyan et al's study of 30 Asian patients with WHO-II/III NPC, 46% of these tumors were positive for high-risk HPV, and consistently these tumors were also positive for EBV.24 In Mirzamani et al's study of 20 Iranian patients with WHO-II/III NPC whose tumor samples were studied using in situ hybridization, 95% were EBV positive and 10% were also positive for high-risk HPV.22 Hørding et al looked at 38 Danish and Inuit patients and found that 3 (20%) of 15 WHO-I NPC were high-risk HPV positive and EBV negative while all 23 WHO-II/III NPC were HPV negative and EBV positive.17 Giannoudis et al's study of 63 Greek patients showed that 9 (33%) of 27 WHO-I NPC but only 3 (8%) of 36 WHO-II/III NPC were high-risk HPV positive, and no HPV positive tumors were also positive for EBV.16 Rassekh et al found that high-risk HPV was present only in association with EBV and was present in 3 (30%) of 10 WHO-I NPC and 4 (57%) of 7 WHO-III NPC.23 A study of a mixed Caucasian and Asian population by Punwaney et al. showed that 2 (50%) of 4 WHO-I NPC and 5 (19%) of 26 WHO-II/III NPC were high-risk HPV positive; co-presence of EBV was found in two Caucasians with WHO-II/III.15 Finally, in a recent publication, Maxwell et al reported one Asian patient with WHO-II/III NPC who was high-risk HPV negative and EBV positive and four Caucasian patients with WHO-II/III NPC who were high-risk HPV positive and EBV negative.21 In summary, 17 (30%) of 56 WHO-I NPC and 32 (22%) of 147 WHO-II/III NPC reported in the literature to date are positive for oncogenic HPV, and only half of HPV positive tumors were also positive for EBV.
Consistent with this summary of the literature, we found in our study that a greater proportion of WHO-I than WHO-II/III were HPV positive. Also, nearly all of the WHO-II/III NPCs were HPV negative with the majority being EBV positive by ISH, also consistent with the literature. Additionally, there was no co-presence of EBV detected by ISH, arguing for a central role of HPV in WHO-I NPC carcinogenesis.
In general, our patient demographic data are similar to those previously reported in the literature on NPC in regions where it is not endemic. Both WHO-I disease and WHO-II/III disease were more common in males. WHO-I patients tended to be older and to be non-Hispanic whites, trends that have previously been seen in the United States.5
We also found that WHO-I NPC may share the risk factors of cigarette smoking and alcohol use common to other head and neck squamous cell carcinomas. Both patients with WHO-I disease and patients with WHO-II/III disease tended to be ever smokers, but WHO-I patients had a longer and heavier smoking history. Prior studies in both NPC-endemic and NPC-nonendemic areas have found that a long history of cigarette smoking is associated with increased NPC risk.11,12,30,31 Vaughan et al suggested that cigarette smoking and heavy alcohol intake have little influence on the risk of nonkeratinizing NPC but that two-thirds of cases of keratinizing NPC in patients older than 50 years can be accounted for by smoking and alcohol intake.12
Our results highlight a discrepancy in rates of HPV detection between in situ hybridization and PCR. In situ hybridization is more specific as it identifies HPV DNA in the nucleus, which may indicate integration into the genome leading to malignant transformation. However, PCR is more sensitive and theoretically requires only one copy of the viral genome, whether intranuclear or cytoplasmic. We found several WHO-II/III cases that were HPV positive by PCR but HPV negative by in situ hybridization. In cases of the co-presence of EBV this finding of HPV positivity by PCR but HPV negativity by in situ hybridization may indicate a noncontributory process, with PCR detecting the episomal presence of HPV. Several studies have investigated the physical state of HPV in head and neck squamous cell carcinoma and have noted integrated, episomal, and mixed forms.32,33 Some investigators have suggested that HPV-16 is predominantly episomal in tonsillar cancers, and a similar pattern in our NPC cases may explain our findings of tumors that were HPV positive by PCR but HPV negative by in situ hybridization.34
Most of our samples, whether WHO-I or WHO-II/III, overexpressed the tumor suppressor p16. With integration of high-risk HPV into the host genome, the oncoprotein E7 inactivates pRb protein, leading to overexpression of the p16 protein, a finding consistently demonstrated in our HPV positive cases.35 In NPC, studies have shown reduced p16 expression associated with tumors that overexpress EBV-encoded RNA but not in EBV-negative WHO-I tumors and have suggested a multistep process of carcinogenesis for nonkeratinizing NPC beginning with EBV infection.36 Interestingly, we found that the majority of samples in our study, including those negative for HPV by both PCR and in situ hybridization, demonstrated overexpression of p16. This may be explained by mechanisms unrelated to HPV or by the presence of other undetected types of HPV or episomal forms.
The central limitation of our study is the small sample size. Larger numbers of samples will be needed to confirm the associations that we observed and to permit conclusions regarding survival. Additionally, referral biases may have affected our comparisons of the patient demographics and exposure characteristics. Another limitation may be the high sensitivity of PCR, which may lead to false-positives despite careful measures to avoid contamination. In addition, the formalin fixation process or storage time may have damaged DNA and proteins, rendering HPV sequences undetectable or affecting HPV in situ hybridization or p16 immunohistochemical assays.
Conclusions
Our results suggest that WHO-I NPC may be associated with HPV rather than EBV. Future work to better define the prevalence of HPV in NPC will require much larger pathologic series and collaboration between institutions.
Acknowledgments
The authors wish to thank Ms. Yan Cai for paraffin block preparation and cutting, Dr. Chong Zhao for laboratory assistance, and Ms. Stephanie Deming for manuscript editing.
Sources of Support: NIH grant K-12 88084 (to E.M.S., faculty trainee; to R.C. Bast, P.I.); NIH grant R03 CA 128110-01A1 (to E.M.S.); and NIH grant P-30 CA-16672 (to The University of Texas M. D. Anderson Cancer Center).
Footnotes
No authors have potential conflicts of interest, including financial interests or relationships and affiliations relevant to the subject of this manuscript. Erich M. Sturgis had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Presented as a poster at the Triological Society Combined Sections Meeting, Orlando, Florida, U.S.A., February 4-7, 2010.
References
- 1.Surveillance, Epidemiology, and End Results (SEER) Program, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch. SEER*Stat Database: Incidence - SEER 9 Regs Limited-Use, Nov 2007 Sub (1973-2005) and SEER 13 Regs Limited-Use, Nov 2007 Sub (1992-2005), released April 2008, based on the November 2007 submission. Available at http://www.seer.cancer.gov. Accessed August 5, 2009.
- 2.Jia WH, Huang QH, Liao J, et al. Trends in incidence and mortality of nasopharyngeal carcinoma over a 20-25 year period (1978/1983-2002) in Sihui and Cangwu counties in southern China. BMC Cancer. 2006;6:178. doi: 10.1186/1471-2407-6-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet. 2005;365:2041–2054. doi: 10.1016/S0140-6736(05)66698-6. [DOI] [PubMed] [Google Scholar]
- 4.Sun LM, Li CI, Huang EY, Vaughan TL. Survival differences by race in nasopharyngeal carcinoma. Am J Epidemiology. 2007;165:271–278. doi: 10.1093/aje/kwk008. [DOI] [PubMed] [Google Scholar]
- 5.Ou SH, Zell JA, Ziogas A, Anton-Culver H. Epidemiology of nasopharyngeal carcinoma in the United States: improved survival of Chinese patients within the keratinizing squamous cell carcinoma histology. Ann Oncol. 2007;18:29–35. doi: 10.1093/annonc/mdl320. [DOI] [PubMed] [Google Scholar]
- 6.Niedobitek G. Epstein-Barr virus infection in the pathogenesis of nasopharyngeal carcinoma. Mol Pathol. 2000;53:248–254. doi: 10.1136/mp.53.5.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy SP, Raslan WF, Gooneratne S, Kathuria S, Marks JE. Prognostic significance of keratinization in nasopharyngeal carcinoma. Am J Otolaryngol. 1995;16:103–108. doi: 10.1016/0196-0709(95)90040-3. [DOI] [PubMed] [Google Scholar]
- 8.Lu CC, Chen JC, Jin YT, Yang HB, Chan SH, Tsai ST. Genetic susceptibility to nasopharyngeal carcinoma within the HLA-A locus in Taiwanese. Int J Cancer. 2003;103:745–751. doi: 10.1002/ijc.10861. [DOI] [PubMed] [Google Scholar]
- 9.Gallicchio L, Matanoski G, Tao X, et al. Adulthood consumption of preserved and nonpreserved vegetables and the risk of nasopharyngeal carcinoma: a systematic review. Int J Cancer. 2006;119:1125–1135. doi: 10.1002/ijc.21946. [DOI] [PubMed] [Google Scholar]
- 10.Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15:1765–1777. doi: 10.1158/1055-9965.EPI-06-0353. [DOI] [PubMed] [Google Scholar]
- 11.Hsu WL, Chen JY, Chien YC, et al. Independent effect of EBV and cigarette smoking on nasopharyngeal carcinoma: a 20-year follow-up study on 9,622 males without family history in Taiwan. Cancer Epidemiol Biomarkers Prev. 2009;18:1218–1226. doi: 10.1158/1055-9965.EPI-08-1175. [DOI] [PubMed] [Google Scholar]
- 12.Vaughan TL, Shapiro JA, Burt RD, et al. Nasopharyngeal cancer in a low-risk population: defining risk factors by histological type. Cancer epidemio Biomarkers Prev. 1996;5:587–593. [PubMed] [Google Scholar]
- 13.D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;355:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 14.Frisch M, Biggar RJ. Aetiological parallel between tonsillar and anogenital squamous-cell carcinomas. Lancet. 1999;354:1442–1443. doi: 10.1016/S0140-6736(99)92824-6. [DOI] [PubMed] [Google Scholar]
- 15.Punwaney R, Brandwein MS, Zhang DY, et al. Human papillomavirus may be common within nasopharyngeal carcinoma of Caucasian Americans: investigation of Epstein-Barr virus and human papillomavirus in eastern and western nasopharyngeal carcinoma using ligation-dependent polymerase chain reaction. Head Neck. 1999;21:21–29. doi: 10.1002/(sici)1097-0347(199901)21:1<21::aid-hed3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 16.Giannoudis A, Ergazaki M, Segas J, et al. Detection of Epstein-Barr virus and human papillomavirus in nasopharyngeal carcinoma by the polymerase chain reaction technique. Cancer Letters. 1995;89:177–181. doi: 10.1016/0304-3835(94)03667-8. [DOI] [PubMed] [Google Scholar]
- 17.Hørding U, Nielsen HW, Daugaard S, Albeck Hs. Human papillomavirus types 11 and 16 detected in nasopharyngeal carcinoma by the polymerase chain reaction. Laryngoscope. 1994;104:99–102. doi: 10.1288/00005537-199401000-00018. [DOI] [PubMed] [Google Scholar]
- 18.Della Torre G, Pilotti S, Donghi R, et al. Epstein-barr virus genomes in undifferentiated and squamous cell nasopharyngeal carcinomas in Italian patients. Diagn Mol Pathol. 1994;3:32–37. doi: 10.1097/00019606-199403010-00006. [DOI] [PubMed] [Google Scholar]
- 19.Shi W, Pataki I, MacMillan C, et al. Molecular pathology parameters in human nasopharyngeal carcinoma. Cancer. 2002;94:1997–2006. doi: 10.1002/cncr.0679. [DOI] [PubMed] [Google Scholar]
- 20.Murono S, Yoshizaki T, Tanaka S, Takeshita H, Park CS, Furukawa M. Detection of Epstein-Barr virus in nasopharyngeal carcinoma by in situ hybridization and polymerase chain reaction. Laryngoscope. 1997;107:523–526. doi: 10.1097/00005537-199704000-00017. [DOI] [PubMed] [Google Scholar]
- 21.Maxwell JH, Kumar B, Feng FY, et al. HPV-positive/EBV-negative nasopharyngeal carcinoma in white North Americans. Head Neck. 2009 doi: 10.1002/hed.21216. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mirzamani N, Salehian P, Farhadi M, Tehran EA. Detection of EBV and HPV in nasopharyngeal carcinoma by in situ hybridization. Exp Mol Pathol. 2006;81:231–234. doi: 10.1016/j.yexmp.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Rassekh CH, Rady PL, Arany I, et al. Combined Epstein-Barr virus and human papillomavirus infection in nasopharyngeal carcinoma. Laryngoscope. 1998;108:362–367. doi: 10.1097/00005537-199803000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Tyan YS, Liu ST, Ong WR, Chen ML, Shu CH, Chang YS. Detection of EBV and HPV in head and neck tumors. J Clin Microbiol. 1993;31:53–56. doi: 10.1128/jcm.31.1.53-56.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji X, Sturgis EM, Zhao C, Etzel CJ, Wei Q, Li G. Association of p73 G4C14-to-A4T14 polymorphism with human papillomavirus type 16 status in squamous cell carcinoma of the head and neck in non-Hispanic whites. Cancer. 2009;115:1660–1668. doi: 10.1002/cncr.24184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai S, Jin Y, Mann RB, Ambinder RF. Epstein-Barr virus detection in nasopharyngeal tissues of patients with suspected nasopharyngeal carcinoma. Cancer. 1998;82:1449–1453. [PubMed] [Google Scholar]
- 27.Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers? Cancer. 2007;110:429–435. doi: 10.1002/cncr.22963. [DOI] [PubMed] [Google Scholar]
- 28.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 29.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467–75. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 30.Guo X, Johnson RC, Den H, et al. Evaluation of nonviral risk factors for nasopharyngeal carcinoma in a high-risk population of Southern China. Int J Cancer. 2009;124:2942–2947. doi: 10.1002/ijc.24293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu K, Levine RS, Brann EA, Hall HI, Caplan LS, Gnepp DR. Case-control study evaluating the homogeneity and heterogeneity of risk factors between sinonasal and nasopharyngeal cancers. Int J Cancer. 2002;99:119–123. doi: 10.1002/ijc.10311. [DOI] [PubMed] [Google Scholar]
- 32.Syrajänen S. Human papillomavirus (HPV) in head and neck cancer. J Clin Virol. 2005;32(Suppl 1):S59–66. doi: 10.1016/j.jcv.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 33.Badaracco G, Rizzo C, Mafera B, et al. Molecular analyses and prognostic relevance of HPV in head and neck tumours. Oncol Rep. 2007;17:931–939. [PubMed] [Google Scholar]
- 34.Mellin H, Dahlgren L, Munck-Wikland E, et al. Human papillomavirus type 16 is episomal and a high viral load may be correlated with better prognosis. Int J Cancer. 2002;102:152–158. doi: 10.1002/ijc.10669. [DOI] [PubMed] [Google Scholar]
- 35.Klussmann JP, Gültekin E, Weissenborn SJ, et al. Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirus. Am J Pathol. 2003;162:747–753. doi: 10.1016/S0002-9440(10)63871-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan SQ, Ma J, Zhou J, et al. Differential expression of Epstein-Barr virus-encoded RNA and several tumor-related genes in various types of nasopharyngeal epithelial lesions and nasopharyngeal carcinoma using tissue microarray analysis. Hum Pathol. 2006;37:593–605. doi: 10.1016/j.humpath.2006.01.010. [DOI] [PubMed] [Google Scholar]