Abstract
We have recently shown that A3 adenosine receptors and P2Y2 purinergic receptors play an important role in neutrophil chemotaxis. Chemotaxis of neutrophils to sites of infections is critical for immune defense. However, excessive accumulation of neutrophils in the lungs can cause acute lung tissue damage. Here we assessed the role of A3 and P2Y2 receptors in neutrophil sequestration to the lungs in a mouse model of sepsis. Sepsis was induced by cecal ligation and puncture (CLP) using adult male C57BL/6J mice (wild type [WT]), homozygous A3 receptor knockout (A3KO) mice, and P2Y2 receptor knockout (P2Y2KO) mice. Animals were killed 2, 4, 6, or 8 h after CLP, and peritoneal lavage fluid and blood were collected. Lungs were removed, and neutrophil infiltration was evaluated using elastase as a marker. Leukocyte and bacterial counts in peritoneal lavage fluid and blood samples were determined. Survival after sepsis was determined in a separate group. Leukocyte counts in the peritoneum were lower in A3KO and P2Y2KO mice than in WT mice. Conversely, initial leukocyte counts in the peripheral blood were higher in KO mice than in WT mice. Neutrophil sequestration to the lungs reached a maximum 2 h after CLP and remained significantly higher in WT mice compared with A3KO and P2Y2KO mice (P < 0.001). Survival after 24 h was significantly lower in WT mice (37.5%) than in A3KO or P2Y2KO mice (82.5%; P < 0.05). These data suggest that A3 and P2Y2 receptors are involved in the influx of neutrophils into the lungs after sepsis. Thus, pharmaceutical approaches that target these receptors might be useful to control acute lung tissue injury in sepsis.
Keywords: A3 adenosine receptor, P2Y2 purinergic receptor, cecal ligation and puncture, sepsis, acute lung injury, neutrophil elastase
INTRODUCTION
Acute lung injury (ALI) is a serious complication that raises mortality after trauma, sepsis, and major surgical procedures (1). Acute lung injury results in acute respiratory distress syndrome, a prominent form of end-organ damage that is frequently observed in patients even in the absence of direct mechanical trauma to the lungs (2, 3). The events leading to ALI are not fully understood. However, it has been shown that activated neutrophils play an important role in this process. Neutrophils infiltrate lung tissues, occlude pulmonary capillaries, release cytotoxic mediators such as neutrophil pro-teases, and thus destroy lung tissues (2, 4–6).
A key feature of neutrophil activation is their ability to migrate toward infected or otherwise compromised tissues by following a concentration gradient of chemotactic substances that are released from bacteria and inflamed tissues. Chemotaxis is a process that involves gradient sensing to detect the orientation of a chemotactic gradient field, cell polarization within the gradient field, and finally directed migration toward the source of the chemoattractant (7–10). Recently, we have found that the polarized release of cellular adenosine triphosphate (ATP) from neutrophils, the formation of adenosine in the extracellular space near the leading edge, and feedback signal amplification through P2Y2 and A3 receptors at the leading edge of migrating cells play important roles in controlling gradient sensing and migration, respectively (11).
Here we investigated the roles of A3 or P2Y2 receptors in the infiltration of neutrophil into the lungs and in the development of ALI in a mouse model of sepsis.
MATERIALS AND METHODS
Animals
The use of laboratory animals was in accordance with National Institutes of Health guidelines and was approved by the Institutional Animal Care and Use Committee. We used adult male C57BL/6J wild-type (WT) mice aged 8 to 10 weeks, weighing 20 to 25 g. The animals were obtained from Jackson Laboratory (Bar Harbor, Me). Homozygous A3−/− mice (A3KO) were a generous gift of Dr Francisco Villarreal (University of California, San Diego, La Jolla, Calif). Homozygous P2Y2−/− mice (P2Y2KO) were obtained from Dr Beverly H. Koller (University of North Carolina, Chapel Hill, NC).
Sepsis model
Sepsis was induced as described in Baker et al. (12) by subjecting mice to cecal ligation and puncture (CLP). Briefly, mice were anesthetized, restrained in supine position, and a 1-cm midline incision was made. The cecum was exposed, ligated distal to the ileocecal valve with 4-0 silk, avoiding intestinal blockade. The cecum was punctured twice with a 22-gauge needle, returned to the abdomen, and the abdominal wall was closed in layers. The mice were then returned to their cages and allowed access to a standard diet and water ad libitum. Animals were euthanized after 2, 4, 6, or 8 h, and leukocyte infiltration into the peritoneal cavity was assessed by lavage of peritoneal cavities with 3 mL ice-cold Hanks’ buffered salt solution (HBSS) containing 1% mouse serum and counting peritoneal leukocytes.
Neutrophil recruitment to the lungs
We assessed neutrophil elastase activity in lung tissue samples as a marker of neutrophil infiltration because preliminary experiments have shown that elastase measurements provide more sensitive results compared with myeloperoxidase. After in situ perfusion with normal saline to remove blood, lungs were extracted, weighed and placed in 5 mL of ice-cold potassium phosphate buffer (50 mM, pH 6.0) containing 0.5% hexadecyltrimethylammonium bromide (Sigma, St Louis, Mo). Tissues were homogenized on ice, and the homogenates were centrifuged at 17,000 rpm and 4°C for 30 min. The supernatants (20 μL) were added in duplicates into a 96-well plate followed by 80 μL/well of a buffer consisting of 50 mM Tris-HCl and 100 mM sodium chloride, pH 7.4, containing 0.05% (vol/vol) Triton X-100 (Sigma). Enzymatic reactions were started by the addition of the elastase-specific chromogenic substrate N-methoxysuccinyl-Ala-Ala-Pro-Val p-nitroanilide (Sigma) at a final concentration of 1 mM. After 30 min at room temperature, the change in optical density at a wavelength of 405 nm was measured using a UV-1201 spectrophotometer (Shimazu Corporation, Colombia, Md).
Bacterial cultures in peritoneal and blood samples
Peritoneal lavage fluids and heparinized blood samples were serially diluted in sterile saline, and 100-μL aliquots were plated onto trypticase soy agar plates (Becton Dickinson, Sparks, Md), cultured for 24 h at 37°C, and colonies were counted.
Lung histology
Lungs from each mouse strain were obtained for histological examination 24 hours after the CLP. Animals were anesthetized and killed as described previously. The trachea was exposed, cannulated with a dull 23-gauge needle (Monoject, Tyco Health Care Group, Mansfield, Mass), and 0.5 mL of fixative (10% paraformalde-hyde in HBSS) was instilled. The pulmonary circulation was flushed with 10 mL HBSS through the right ventricle, and the whole lung was carefully removed. Lungs were immersed in fixative solution for 72 h, dehydrated with graded alcohol, and embedded in paraffin. A series of 4-μm-thick tissue slices was obtained and stained with hematoxylin and eosin, and examined under the microscope.
Survival study
Survival after CLP was assessed in a separate set of eight animals per mouse strain.
Statistical analysis
All values were expressed as mean ± SD. Statistical analyses were performed with ANOVA followed by Bonferroni test or Kaplan-Meier tests. Differences between groups were considered statistically significant at P < 0.05.
RESULTS
A3 and P2Y2 receptors are required for neutrophil recruitment to the site of infection
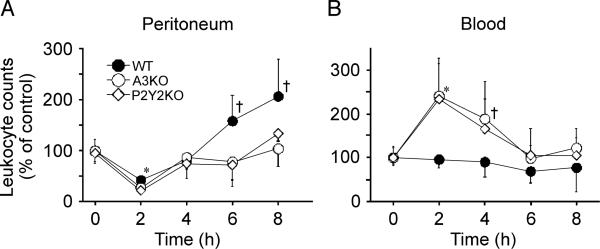
Our previous data have shown that chemotaxis of neutrophils from A3KO and P2Y2KO mice is approximately 50% lower compared with WT mice, suggesting that A3 and P2Y2 receptors play important roles in neutrophil migration (11). Here we tested the roles of these receptors in neutrophil sequestration to the lungs of mice subjected to peritoneal sepsis. Leukocyte counts in the peritoneal cavity of WT, A3KO, and P2Y2KO mice dropped significantly 2 h after CLP (Fig. 1A), probably caused by the rapid consumption of leukocytes during the immune defense. After 6 h, leukocyte counts in the peritoneal cavities of KO mice recovered, whereas cell counts in WT mice increased to levels that were significantly higher than those in KO mice (P < 0.05). Concomitantly, blood leukocyte counts in WT mice remained stable, whereas cell counts in KO mice were increased at 2 h (P < 0.01) and 4 h (P < 0.05) after CLP (Fig. 1B). These data are consistent with the notion that leukocyte recruitment to the site is diminished in A3KO and P2Y2KO mice.
Fig 1. Leukocytes in the peritoneum and peripheral blood after CLP.
The WT, A3KO, and P2Y2KO mice were subjected to intraperitoneal sepsis by CLP with a 22-gauge needle, and the time course of leukocyte recruitment in the peritoneal cavity (A) and the peripheral blood (B) was measured after CLP. Data are expressed relative to leukocyte counts of controls without CLP. Data points represent mean ± SD of 5 animals per group. Statistical analyses were performed with ANOVA followed by Bonferroni test, *P < 0.01, † P < 0.05 compared with WT control.
A3 and P2Y2 receptors and bacterial clearance
Reduced leukocyte recruitment into the peritoneal cavity would be expected to decrease the ability of KO mice to clear bacteria from the site of infection and to prevent the spread of bacteria in the systemic circulation. Bacterial counts in the peritoneal cavity after CLP did not differ between WT mice and A3KO and P2Y2KO mice. However, bacterial counts in the systemic circulation rose less rapidly in WT mice than in A3KO and P2Y2KO mice (Fig. 2). These findings may be caused by the reduced availability of leukocytes at the infection site in KO mice. However, it is also possible that the absence of P2Y2 and A3 receptors may impair the ability of neutrophils to phagocytose and kill bacteria.
Fig 2. Bacterial counts in peritoneum and peripheral blood after CLP.
Bacterial counts in peritoneal lavaged fluids (A) and peripheral blood (B) of WT, A3KO, and P2Y2KO mice were assessed at different times after CLP. Data show mean ± SD of colony-forming units determined in five animals for each time point and mouse strain. CFU indicates colony-forming units.
Neutrophil recruitment to the lungs after sepsis depends on A3 and P2Y2 receptors
Sepsis results in a systemic inflammatory response that causes neutrophil sequestration to host organs such as the lungs, which contributes to end-organ damage. Because of its fragile architecture, lung tissue is particularly prone to secondary tissue damage by sequestered phagocytes. We compared the time course of neutrophil accumulation in the lung tissues of WT with that of KO mice using elastase as a marker. As shown in Figure 3, sepsis in WT mice caused a greater than 10-fold increase in lung elastase activity within 2 h after CLP. These levels remained significantly elevated above control values for up to 4 h after the induction of sepsis (P < 0.001). In comparison, during the first 4 h after CLP, neutrophil accumulation in the lungs of A3KO and P2Y2KO mice was significantly lower than that in WT mice (P < 0.01). This result shows that A3 and P2Y2 receptors are important for neutrophil accumulation in lung tissues, but that the absence of one of these receptors does not completely prevent neutrophil sequestration. This is likely a result of the fact that both receptor types play different roles in the control of neutrophil chemotaxis (11). Thus, the absence of either one of these receptors diminishes chemotaxis, but it does not completely prevent chemotaxis.
Fig 3. Neutrophil recruitment to the lungs after CLP.
Neutrophil recruitment to the lungs of WT, A3KO, and P2Y2KO mice after CLP was assessed by measuring elastase activity in lung tissue homogenates. Data are expressed relative to control values in untreated animals. Values are mean ± SD of five animals in each group and at each time point. Statistical analysis between WT, A3KO, or P2Y2KO was performed with 2-way ANOVA followed by Bonferroni test, *P < 0.001, † P < 0.01.
Lung tissue damage in KO and WT mice
Lung tissue damage after sepsis was evaluated with histological samples obtained 24 h after CLP (Fig. 4). Focal atelectasis and hyperemia caused by blood congestion were observed in lung samples from WT mice. In addition, there was clear evidence of numerous neutrophils and debris in alveolar spaces of the lungs in WT mice subjected to CLP. These histological markers of lung injury were less evident in A3KO and P2Y2 KO mice.
Fig 4. Lung histology.
Representative micrographs of histological sections of lung tissue from untreated control WT mice (WT control) and WT mice, A3KO mice, and P2Y2 KO mice 24 h after CLP (hematoxylin-eosin, original magnification [notdef]200). Lung sections of WT mice after CLP showed characteristic abnormalities including focal atelectasis, hyperemia with congestion, and infiltration of erythrocytes and neutrophil within the widened alveolar septae, and with alveolar spaces containing fluid, debris, and inflammatory cells.
Mortality after sepsis in A3 and P2Y2 receptor KO mice
Our previous work indicated that chemotaxis is reduced in A3KO and P2Y2KO mice compared with that in WT controls. Our current data suggest that neutrophil sequestration to the lungs of septic mice and lung tissue damage are decreased in A3KO and P2Y2KO mice compared with those in WT mice. Based on these observations, mortality would be expected to be lower in KO mice compared with WT animals, despite the fact that fewer neutrophils are available to prevent bacterial spreading beyond the site of infection.
To better understand the roles of P2Y2 and A3 receptors in the clinical course of sepsis, we evaluated survival of WT, A3KO, and P2Y2KO mice in a separate set of eight animals per mouse strain. Twenty-four hours after CLP, only 3 of the 8 animals in the WT group survived, whereas 7 of 8 were still alive in the A3KO and P2Y2KO groups. Whereas only 1 of the WT mice was still alive at 36 h, there were 4 survivors in the P2Y2KO group and in the A3KO group. Although A3KO mice and P2Y2KO mice showed significantly reduced mortality rates in the first 36 h after CLP (PG 0.05), these differences in survival were no longer significant at later time points (Fig. 5). Thus, our data suggest that the absence of A3 and P2Y2 receptors in KO animals delays mortality after sepsis, possibly by reducing neutrophil sequestration and damage of the lungs.
Fig 5. Mortality from sepsis is delayed in A3KO and P2Y2KO mice.
Mortality of WT, A3KO, and P2Y2KO mice was monitored for 80 h after CLP. The survival curves for each mouse strain were analyzed and compared using Kaplan-Meier statistics (n = 8 per strain). At the 24-h time point, mortality rates in the A3KO and P2Y2KO groups were significantly lower than in the WT group (P < 0.05).
DISCUSSION
Secondary organ damage is a major complication after sepsis, which results in acute respiratory distress and multiple organ failure syndromes (3). Neutrophils sequestered to host organs are known to play an important role in organ damage (2, 13, 14). Thus, therapeutic approaches aimed at preventing neutrophil activation or the recruitment of neutrophils to host tissues hold promise to reduce organ damage (15, 16). Recently, we have shown that autocrine feedback mechanisms through A3 and P2Y2 receptors control neutrophil chemotaxis (11). We found that neutrophils release ATP at the leading edge, where adenosine is rapidly formed from the released ATP. Adenosine activates A3 adenosine receptors expressed predominantly at the leading edge of migrating cells. We found that the inhibition of A3 adenosine receptors with specific receptor antagonists impaired the ability of neutrophils to undergo chemotaxis toward bacterial products such as formyl peptides and inflammatory mediators, such as platelet activating factor, complement fragment C5a, and IL-8 (11). Others have reported that agonists of A3 receptors can attenuate neutrophil functions and myocardial reperfusion injury by decreasing polymorphonuclear leukocyteendothelial cell interactions (17).
Here we studied the roles of the A3 or P2Y2 receptors in leukocytes recruitment to sites of infection and the sequestration of neutrophils to the lungs in a mouse model of sepsis. Consistent with our previous results, our findings in the sepsis model indicate that the absence of A3 or P2Y2 receptors significantly impairs leukocyte migration into the peritoneal cavity of mice subjected to CLP. This may be one of the reasons for the differences in bacterial counts in the peripheral blood of KO and WT mice (Fig. 2). Diminished mobilization of leukocytes to the peritoneum of KO mice renders these animals more susceptible to sepsis compared with WT mice. Despite the diminished leukocyte response in KO mice, we observed reduced mortality in the early phase after sepsis in A3KO and P2Y2KO compared in WT mice. These data imply that the absence of A3 or P2Y2 receptors can have certain benefits for the host, perhaps by reducing host organ damage by neutrophils sequestered to the lungs. This notion is supported by initial histological data that indicate diminished lung tissue injury in KO mice compared with WT mice (Fig. 4).
However, our study has certain limitations that must be considered. For example, the complicated dynamics of neutrophil recruitment from the bone marrow and the influx of neutrophils into the peritoneal cavity and their sequestration into the lungs make it difficult to determine which, if not all, of these events depend on A3 and P2Y2 receptors. Similarly, the numbers of bacteria in the peripheral blood and in the peritoneal cavity depend on the dynamics of neutrophil recruitment and flux mentioned previously. In addition, other factors might influence the numbers of bacteria. It is likely that the absence of A3 and P2Y2 receptors may not only affect the ability of neutrophils to undergo chemotaxis, but that it may also impair the cells’ ability to killing and phagocytose bacteria.
Nevertheless, our current findings suggest an important role for A3 and P2Y2 receptors in leukocyte responses that are involved in the complex sequence of events that lead to lung tissue damage in response to acute sepsis. P2Y2 receptors, and in particular, A3 receptors, for which numerous specific pharmacological inhibitors are available, thus seem possible targets for therapeutic interventions to reduce the risk of host tissue damage during sepsis. Several reports have shown that inflammatory complications after stroke (18, 19), myocardial infarction (20, 21), asthma (22, 23), and rheumatoid disorders (24, 25) are diminished in A3 receptorYdeficient mice. A3 agonists have been shown to worsen I/R-induced renal failure (26) and to attenuate I/R injury of heart (21, 27) and lung tissues (28, 29). Moreover, A3 antagonists have been reported to reduce myocardial ischemic injury (30) and airway inflammation (31). Our findings presented here show improvements in the early survival after sepsis of A3KO and P2Y2KO mice. However, despite the initial survival benefits, long-term survival was not improved in A3KO or P2Y2KO mice.
In addition to A3 receptors, other members of the adenosine receptor family, in particular the A2 receptor subclass, also control neutrophil responses and they mediate lung tissue damage in response to endotoxemia, I/R, and trauma/hemorrhagic shock (28, 32, 33). The available pharmacological agents used to inhibit these receptors can vary considerably in their effectiveness to block human and murine receptors. This should be taken into account when considering therapeutic strategies to reduce lung tissue damage in sepsis.
To our knowledge, this study is the first to show that A3 and P2Y2 receptors control neutrophil sequestration to the lungs after peritoneal sepsis. Our findings conflict with those of a recent report by Lee et al. (29), who showed that the mortality after CLP is more severe in A3KO mice compared with WT controls. It is likely that these differences are caused by differences in the severity of the sepsis in the two models. Our sepsis model caused 100% mortality in 40 h. The CLP procedure described by Lee et al. (29) resulted in approximately 20% mortality within 24 h, whereas our model caused twice that mortality in the same period.
In summary, our results indicate that A3 and P2Y2 receptors contribute to the sequestration of neutrophils into the lungs after sepsis, suggesting that these receptors may represent suitable pharmaceutical targets to control neutrophil host tissue damage after sepsis.
Acknowledgments
This study was supported in part by a Pfizer Fellowship (to Y.I.) and National Institute of General Medical Sciences (grant no. R01 GM-51477 to W.G.J., grant no. GM-60475 to W.G.J., and CDMRP grant no. PR043034 to W.G.J.).
REFERENCES
- 1.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- 2.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 3.Durham RM, Moran JJ, Mazuski JE, Shapiro MJ, Baue AE, Flint LM. Multiple organ failure in trauma patients. J Trauma. 2003;55:608–616. doi: 10.1097/01.TA.0000092378.10660.D1. [DOI] [PubMed] [Google Scholar]
- 4.Abraham E. Neutrophils and acute lung injury. Crit Care Med. 2003;31:S195–S199. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- 5.Inoue Y, Seiyama A, Tanaka H, Isao U, Akimau P, Nishino M, Shimazu T, Sugimoto H. Protective effects of a selective neutrophil elastase inhibitor (sivelestat) on LSP-induced acute dysfunction of the pulmonary micro-circulation. Crit Care Med. 2005;33:1814–1822. doi: 10.1097/01.ccm.0000172547.54086.ad. [DOI] [PubMed] [Google Scholar]
- 6.Inoue Y, Tanaka H, Ogura H, Ukai I, Fujita K, Hosotsubo H, Shimazu T, Sugimoto H. A neutrophil elastase inhibitor, sivelestat, improves leukocyte deformability in patients with acute lung injury. J Trauma. 2006;60:936–943. doi: 10.1097/01.ta.0000217271.25809.a0. [DOI] [PubMed] [Google Scholar]
- 7.Weiner OD, Rentel MC, Ott A, Brown GE, Jedrychowski M, Yaffe MB, Gygi SP, Cantley LC, Bourne HR, Kirschner MW. Hem-1 complexes are essential for Rac activation, actin polymerization, and myosin regulation during neutrophil chemotaxis. PLoS Biol. 2006;4:e38. doi: 10.1371/journal.pbio.0040038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charest PG, Firtel RA. Feedback signaling controls leading-edge formation during chemotaxis. Curr Opin Genet Dev. 2006;16:339–347. doi: 10.1016/j.gde.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 9.Franca-Koh J, Kamimura Y, Devreotes P. Navigating signaling networks: chemotaxis in Dictyostelium discoideum. Curr Opin Genet Dev. 2006;16:333–338. doi: 10.1016/j.gde.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Srinivasan S, Wang F, Glavas S, Ott A, Hofmann F, Aktories K, Kalman D, Bourne HR. Rac and Cdc42 play distinct roles in chemotaxis. J Cell Biol. 2003;160:375–385. doi: 10.1083/jcb.200208179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2-2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 12.Baker CC, Chaudry IH, Gaines HO, Baue AE. Evaluation of factors affecting mortality rate after sepsis in a murine cecal ligation and puncture model. Surgery. 1983;94:331–335. [PubMed] [Google Scholar]
- 13.Razavi HM, Wang le F, Weicker S, Rohan M, Law C, McCormack DG, Mehta S. Pulmonary neutrophil infiltration in murine sepsis role of inducible nitric oxide synthase. Am J Respir Crit Care Med. 2004;170:227–233. doi: 10.1164/rccm.200306-846OC. [DOI] [PubMed] [Google Scholar]
- 14.Hirsh MI, Hashiguchi N, Chen Y, Yip L, Junger WG. Surface expression of HSP72 by LPS-stimulated neutrophils facilitates gammadeltaT cell-mediated killing. Eur J Immunol. 2006;36:712–721. doi: 10.1002/eji.200535422. [DOI] [PubMed] [Google Scholar]
- 15.Shenkar R, Abraham EJ. Mechanisms of lung neutrophil activation after hemorrhage or endotoxemia: roles of reactive oxygen intermediates, NF-kappa B, and cyclic AMP response element binding protein. J Immunol. 1999;163:954–962. [PubMed] [Google Scholar]
- 16.Homma Homma H, Deitch EA, Feketeova E, Lu Q, Berezina TL, Zaets SB, Machiedo GW, Xu DZ. Small volume resuscitation with hypertonic saline is more effective in ameliorating trauma-hemorrhagic shock-induced lung injury, neutrophil activation and red blood cell dysfunction than pancreatic protease inhibition. J Trauma. 2005;59:266–272. doi: 10.1097/01.ta.0000184582.55417.77. [DOI] [PubMed] [Google Scholar]
- 17.Jordan JE, Thourani VH, Auchampach JA, Robinson JA, Wang NP, Vinten JJ. A(3) adenosine receptor activation attenuates neutrophil function and neutrophil-mediated reperfusion injury. Am J Physiol. 1999;277:H1895–H1905. doi: 10.1152/ajpheart.1999.277.5.H1895. [DOI] [PubMed] [Google Scholar]
- 18.Von Lubitz DK, Ye W, McClellan J, Lin RC. Stimulation of adenosine A3 receptors in cerebral ischemia: neuronal death, recovery, or both? Ann N Y Acad Sci. 1999;890:93–106. doi: 10.1111/j.1749-6632.1999.tb07984.x. [DOI] [PubMed] [Google Scholar]
- 19.Von Lubitz DK, Simpson KL, Lin RC. Right thing at a wrong time? Adenosine A3 receptors and cerebroprotection in stroke. Ann N Y Acad. 2001;939:85–96. doi: 10.1111/j.1749-6632.2001.tb03615.x. [DOI] [PubMed] [Google Scholar]
- 20.Liu GS, Richards SC, Olsson RA, Mullane K, Walsh RS, Downey JM. Evidence that the adenosine A3 receptor may mediate the protection afforded by preconditioning in the isolated rabbit heart. Cardiovasc Res. 1994;28:1057–1061. doi: 10.1093/cvr/28.7.1057. [DOI] [PubMed] [Google Scholar]
- 21.Headrick JP, Peart J. A3 adenosine receptor-mediated protection of the ischemic heart. Vasc Pharmacol. 2005;42:271–279. doi: 10.1016/j.vph.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Young HW, Molina JG, Dimina D, Zhong H, Jacobson M, Chan LN, Chan TS, Lee JJ, Blackburn MR. A3 adenosine receptor signaling contributes to airway inflammation and mucus production in adenosine deaminase-deficient mice. J Immunol. 2004;173:1380–1389. doi: 10.4049/jimmunol.173.2.1380. [DOI] [PubMed] [Google Scholar]
- 23.Young HW, Sun CX, Evans CM, Dickey BF, Blackburn MR. A3 adenosine receptor signaling contributes to airway mucin secretion after allergen challenge. Am J Respir Cell Mol Biol. 2006;35:549–558. doi: 10.1165/rcmb.2006-0060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szabo C, Scott GS, Virag L, Egnaczyk G, Salzman AL, Shanley TP, Hasko G. Suppression of macrophage inflammatory protein (MIP)-1alpha production and collagen-induced arthritis by adenosine receptor agonists. Br J Pharmacol. 1998;125:379–387. doi: 10.1038/sj.bjp.0702040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joshi BV, Jacobson KA. Purine derivatives as ligands for A3 adenosine receptors. Curr Top Med Chem. 2005;5:1275–1295. doi: 10.2174/156802605774463079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee HT, Ota-Setlik A, Xu H, D'Agati VD, Jacobson MA, Emala CW. A3 adenosine receptor knockout mice are protected against ischemia- and myoglobinuria-induced renal failure. Am J Physiol Renal Physiol. 2003;284:F267YF273. doi: 10.1152/ajprenal.00271.2002. [DOI] [PubMed] [Google Scholar]
- 27.Hill RJ, Oleynek JJ, Magee W, Knight DR, Tracey WR. Relative importance of adenosine A1 and A3 receptors in mediating physiological or pharmacological protection from ischemic myocardial injury in the rabbit heart. J Mol Cell Cardiol. 1998;30:579–585. doi: 10.1006/jmcc.1997.0621. [DOI] [PubMed] [Google Scholar]
- 28.Matot I, Weiniger CF, Zeira E, Galun E, Joshi BV, Jacobson KA. A3 adenosine receptors and mitogen-activated protein kinases in lung injury following in vivo reperfusion. Crit Care. 2006;10:1–11. doi: 10.1186/cc4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee HT, Kim M, Joo JD, Gallos G, Chen JF, Emala CW. A3 adenosine receptor activation decreases mortality and renal and hepatic injury in murine septic peritonitis. Am J Physiol Regul Integr Comp Physiol. 2006;291:R959–R969. doi: 10.1152/ajpregu.00034.2006. [DOI] [PubMed] [Google Scholar]
- 30.Guo Y, Bolli R, Bao W, Wu WJ, Black RG, Murphree SS, Salvatore CA, Jacobson MA, Auchampach JA. Targeted deletion of the A3 adenosine receptor confers resistance to myocardial ischemic injury and does not prevent early preconditioning. J Mol Cell Cardiol. 2001;33:825–830. doi: 10.1006/jmcc.2001.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tilley SL, Tsai M, Williams CM, Wang ZS, Erikson CJ, Galli SJ, Koller BH. Identification of A3 receptorY and mast cellYdependent and Yindependent components of adenosine-mediated airway responsiveness in mice. J Immunol. 2003;171:331–337. doi: 10.4049/jimmunol.171.1.331. [DOI] [PubMed] [Google Scholar]
- 32.Neely CF, Jin J, Keith IM. A1-adenosine receptor antagonists block endotoxin-induced lung injury. Am J Physiol. 1997;272:353–361. doi: 10.1152/ajplung.1997.272.2.L353. [DOI] [PubMed] [Google Scholar]
- 33.Haskó G, Xu DZ, Lu Q, Németh ZH, Jabush J, Berezina TL, Zaets SB, Csóka B, Deitch EA. Adenosine A2A receptor activation reduces lung injury in trauma/hemorrhagic shock. Crit Care Med. 2006;34:1119–1125. doi: 10.1097/01.CCM.0000206467.19509.C6. [DOI] [PubMed] [Google Scholar]