Abstract
Objective
Endothelial dysfunction is an early manifestation of atherosclerosis. Inflammation and vasa vasorum play a pivotal role in the pathophysiology of plaque initiation, development and complications. Optical coherence tomography (OCT) allows high-resolution imaging of tissue microstructure. Therefore, the aim of this study was to test the hypothesis that segments with endothelial dysfunction show macrophages and/or vasa vasorum in patients with early coronary artery disease.
Approach and Results
OCT images were obtained from 40 patients with mild coronary atherosclerosis who underwent coronary endothelial function assessment. OCT findings including macrophages and micorchannels were evaluated in 76 coronary segments corresponding to those in endothelial response to acetylcholine. Coronary artery diameter (CAD) change in response to acetylcholine was more severe in segments showing macrophages (−17.7±14.7% vs. −6.3±13.9%, p<0.01) and microchannels (−15.9±15.9% vs. −6.4±13.5%, p<0.01) than those without. There were increasing trends of the prevalence of macrophages and microchannels with endothelial dysfunction as stratified by quartiles of CAD change (p<0.01 and p=0.02 for trend, respectively). In particular, segments with both macrophages and microchannels (n=12) tended to have worse endothelial function than those with macrophages alone (n=15) and microchannels alone (n=15) (−22.1±14.6% vs.−10.9±15.6% and −10.9±15.6%, p=0.07 and p=0.06, respectively).
Conclusion
Epicardial endothelial dysfunction was associated with OCT-identified macrophages and microchannels in mild coronary atherosclerosis. The current study further supports the role of inflammation and vasa vasorum proliferation in the early stage of coronary atherosclerosis.
Keywords: inflammation, coronary disease, optical coherence tomography, endothelial dysfunction
Introduction
Endothelial dysfunction is a key step in early lesion formation and also involved in plaque progression and the occurrence of atherosclerotic complications.1–3
Alteration in epicardial endothelial function has been considered to precede the development of morphological atherosclerotic change and to contribute to lesion development.4, 5 Previous in vivo human imaging studies using coronary angiography6, 7 and grayscale intravascular ultrasound (IVUS)8 did not detect significant relationship between endothelial function and morphological appearances of atherosclerosis.
However, microstructural manifestations of vasa vasorum neovascularization occur within a few weeks in the initial stage of the coronary atherosclerosis of experimental hypercholesterolemia.9 Moreover, from a pathobiological point of view, inflammatory mechanisms, critical to all stages of cardiovascular disease progression, play a causal role in the pathogenesis of vascular endothelial dysfunction.10 Local and systemic release of inflammatory cytokines promotes lipid-laden foam cell accumulation and impairs endothelium-dependent arterial vasodilation.11
We have previously demonstrated that coronary arterial segments with endothelial dysfunction are associated with specific plaque characteristics consistent with necrotic core12 or lipid core.13 Recently developed optical coherence tomography (OCT) allows in vivo visualization of coronary artery microstructure including macrophages14, 15 and vasa vasorum16, 17 in advanced atherosclerotic plaques, and therefore may have the potential for identification of specific plaque characteristics related to the early stage of atherosclerosis with endothelial dysfunction.
Therefore, the aim of the study was to test the hypothesis that segmental coronary endothelial dysfunction is associated with the presence of macrophages and/or intimal vasa vasorum in patients with early coronary artery disease.
Material and Methods
Material and methods are available in the online-only Data Supplement.
Results
Patients
After screening of 201 patients, a total of 42 patients were enrolled in the study. Patients with early coronary artery disease, defined as diameter stenosis <30% throughout entire coronary arteries on diagnostic coronary angiography, and vasoconstriction in epicardial coronary artery on endothelial function test were consecutively enrolled. Exclusion criteria were low ejection fraction (< 45%), acute coronary syndrome, angioplasty or bypass surgery within 6 months prior to study, uncontrolled hypertension, valvular heart disease, significant endocrine, renal disorder, or pregnancy. From 42 patients, 84 coronary segments were identified. After excluding 8 segments due to poor image quality, 76 segments in 40 patients (2 segments in 36 subjects, and 1 segment in 4 subjects) were analyzed. The clinical characteristics of the study subjects are described in Table 1. The comparisons of coronary artery function assessments, IVUS and OCT findings between two groups stratified according to endothelial dysfunction are shown in Table 2. There was no correlation between plaque burden assessed by IVUS and the percent change of CAD (r2<0.01, p=0.84). The OCT imaging demonstrated 21 lipid-rich plaques (27%) among all segments. Thirteen of 23 (56%) segments with macrophage images and 7 of 27 (26%) segments with microchannels were identified in lipid-rich plaques. Compared with segments with normal endothelial function, those with endothelial dysfunction showed significantly higher prevalence of macrophage images (p=0.03), and higher, but non-significant, prevalence of microchannels (p= 0.07). The maximum angle of macrophage arc was insignificantly greater in segments with endothelial dysfunction compared to those without (Table 2).
Table 1.
Demographics of study subjects (76 segments from 40 patients)
| Endothelial dysfunction (n=38) |
Normal endothelial function (n=38) |
p | |
|---|---|---|---|
| Age | 57.0±11.1 | 52.0±11.6 | 0.08 |
| Male gender, n (%) | 10(26.3) | 10(26.3) | 0.71 |
| Body mass index, kg/m2 | 30.7±6.6 | 32.0±7.6 | 0.70 |
| Risk factors, n (%) | |||
| Diabetes mellitus | 0(0) | 4(10.5) | - |
| Hypertension | 18(47.4) | 17(44.7) | 0.57 |
| Hypercholesterolemia | 20(52.6) | 14(36.8) | 0.19 |
| Current smoking | 5(13.5) | 3(8.1) | 0.45 |
| Medications, n (%) | |||
| Aspirin | 18(47.4) | 22(57.9) | 0.56 |
| β blockers | 7(18.4) | 12(31.6) | 0.25 |
| ACE inhibitors/ARBs | 5(13.5) | 7(18.9) | 0.68 |
| Calcium channel blockers | 17(44.7) | 18(47.4) | 0.86 |
| Statins | 20(62.6) | 13(34.2) | 0.19 |
| Laboratory data | |||
| Total cholesterol, mg/dl | 185±33 | 189±45 | 0.72 |
| LDL cholesterol, mg/dl | 95±30 | 100±40 | 0.66 |
| HDL cholesterol, mg/dl | 66±20 | 64±18 | 0.88 |
| Triglyceride, mg/dl | 123±65 | 125±57 | 0.97 |
| Glucose, mg/dl | 98±13 | 99±20 | 0.96 |
| Hemoglobin A1c, % | 5.3±0.4 | 5.4±0.4 | 0.23 |
| C-reactive protein, mg/L | 3.3±2.7 | 5.1±5.3 | 0.29 |
Table 2.
Segmental comparison of intravascular ultrasound and optical coherence tomography results according to endothelial function
| Endothelial dysfunction (n=38) |
Normal endothelial function (n=38) |
p | |
|---|---|---|---|
| Coronary artery function assessments | |||
| % CAD change, response to | |||
| Acetylcholine | −18.9 [−29.2, −12.6] | 0.2 [−5.4, 8.9] | - |
| Nitroglycerin | 9.1 [2.1, 15.0] | 14.0 [5.1, 21.5] | 0.05 |
| IVUS findings | |||
| Vessel area (mm2) | 14.2±4.6 | 14.4±4.0 | 0.85 |
| Lumen area (mm2) | 10.7±3.3 | 11.7±3.1 | 0.20 |
| Plaque area (mm2) | 4.5±1.7 | 4.6±1.5 | 0.78 |
| Plaque burden (%) | 30.0±7.5 | 30.0±7.3 | 0.84 |
| OCT findings | |||
| Lipid-rich plaque, n (%) | 11 (29) | 10 (26) | 0.95 |
| Macrophage image, n (%) | 16 (42) | 7 (18) | 0.03 |
| *Maximum angle (degree) | 37.9 [24.9, 49.2] | 25.2 [14.1, 31.0] | 0.05 |
| *Length (µm) | 700 [600, 940] | 600 [400, 800] | 0.24 |
| Microchannels, n (%) | 18 (47) | 9 (24) | 0.07 |
| †Maximum number, n | 1.5 [1, 2] | 1 [1, 2] | 0.16 |
| †Length (µm) | 800 [600, 850] | 800 [600, 1300] | 0.38 |
Measured in lesions with *macrophage image or †microchannels.
Endothelial dysfunction in segments with macrophage image and/or microchannels
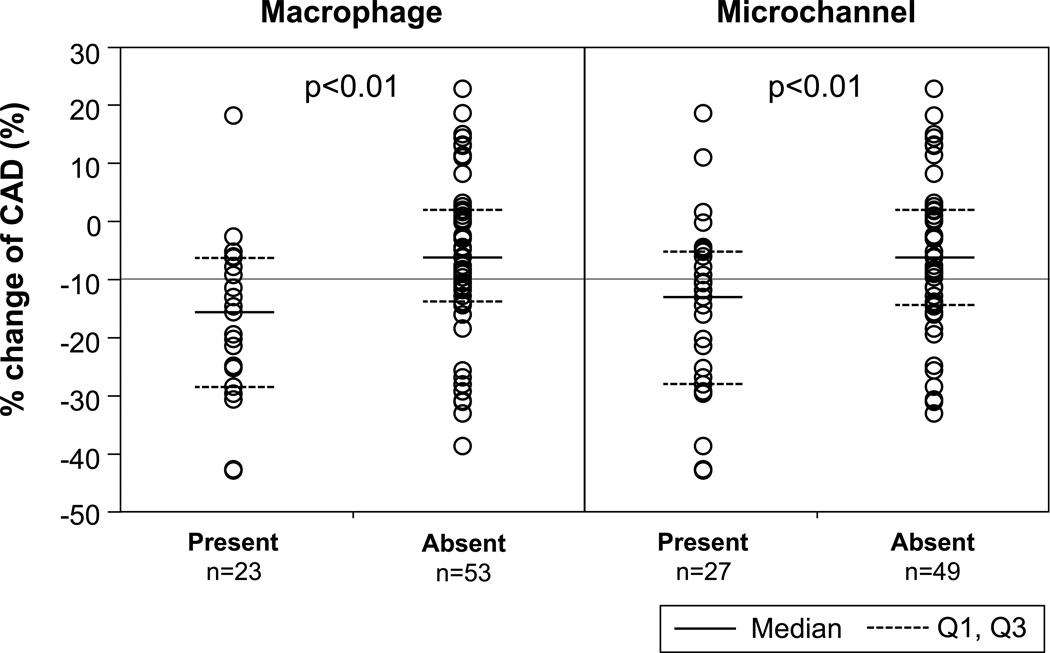
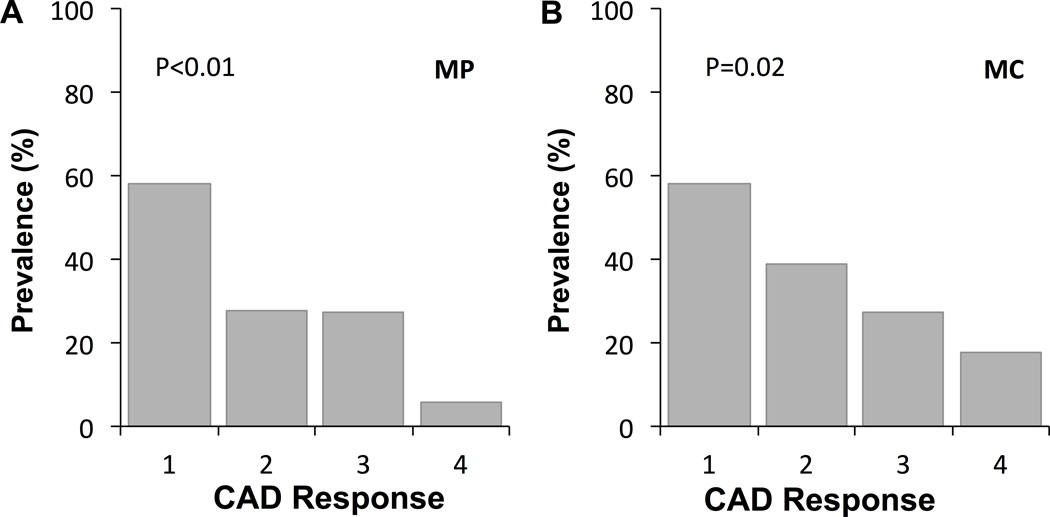
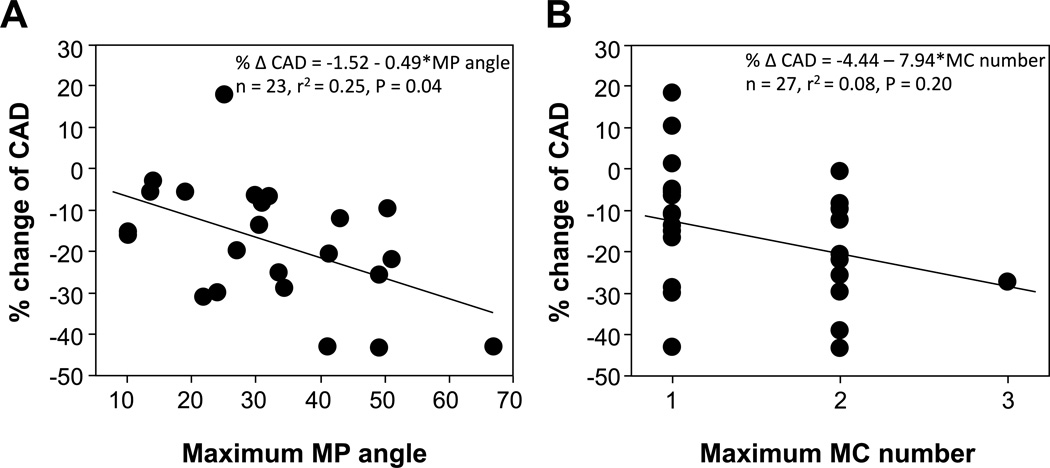
When segments were divided into two groups according to the presence or absence of macrophage images or microchannels, more severe endothelial dysfunction was observed in segments with macrophage images (−17.7±14.7% vs. −6.3±13.9%, p<0.01) and in those with microchannels (−15.9±15.9% vs. −6.4±13.5%, p<0.01) as compared to those without (Figure 1). Furthermore, there was an increasing trend of the prevalence of macrophage image with endothelial dysfunction as stratified by quartiles of the percent change of CAD (p<0.01, Figure 2A). Likewise, there were trends toward higher prevalence of segments with microvessels according to endothelial dysfunction (p=0.02) (Figure 2B). The maximum angle of macrophage arc, not maximum number of microchannels, significantly correlated with the percent CAD (Figure 3A and B).
Figure 1.
Comparison of % change of coronary artery diameter (CAD) according to the presence or absence of macrophage image and microchannels.
Figure 2.
The prevalence of segments with (A) macrophage image (MP) and (B) microchannels (MC) according to coronary artery diameter (CAD) response stratified by quartiles of the percent CAD (Q1 %CAD < −19.3%; n=19, Q2 −19.2 to −9.03%; n=19, Q3 −9.02 to 0%; n=19, Q4 >0.1%; n=19).
Figure 3.
% change of coronary artery diameter (CAD) significantly correlated with the maximum angle of macrophage image (MP) (A), but not with the maximum number of microchannels (MC) (B).
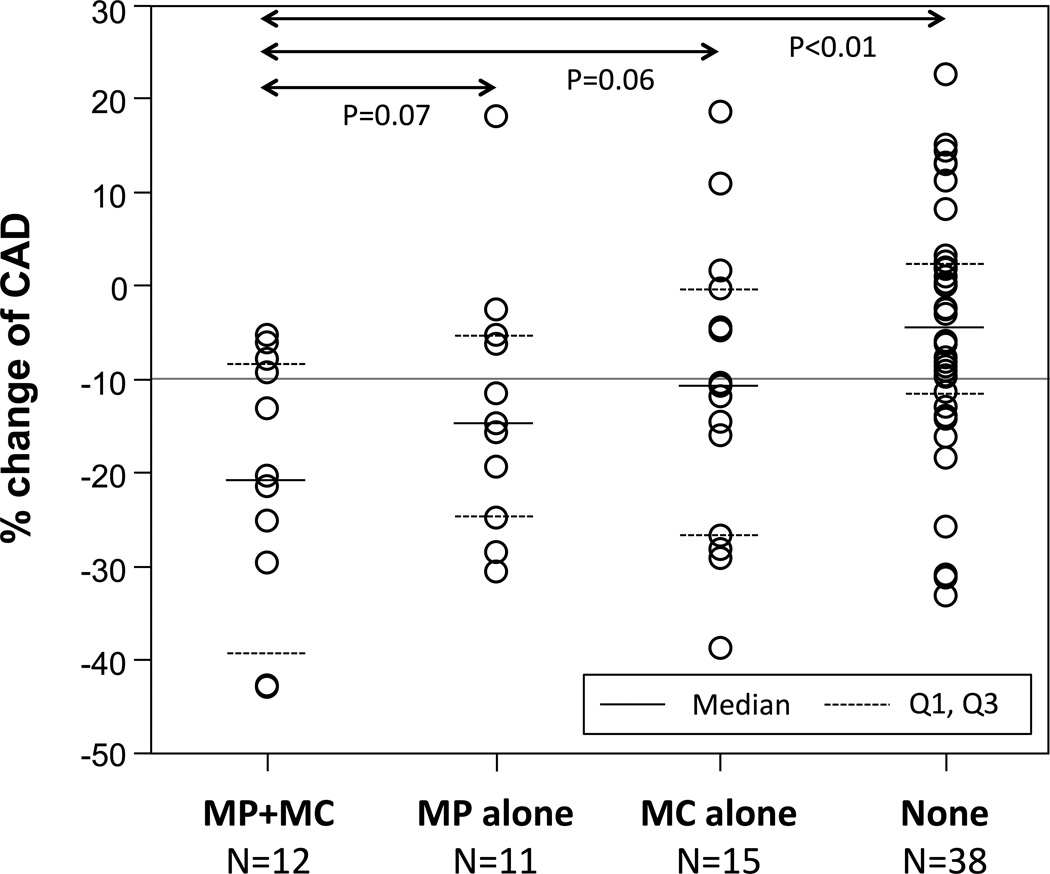
Because of a substantial overlap between segments with macrophage images and those with microchannels, all segments were divided into four groups (Figure 4): those with both macrophage image and microchannels (n=12), those with macrophage image alone (n=11), those with microchannels alone (n=15), and those without macrophage image or microchannels (n=38). Segments with both characteristics tended to have worse endothelial function than those with macrophages alone and microchannels alone (−22.1±14.6% vs. −10.9±15.6% and −10.9±15.6%, p=0.07 and p=0.06, respectively).
Figure 4.
Comparison of percent coronary artery diameter (CAD) change among segments with both macrophage image (MP) and microchannels (MC), those with MP alone, those with MC alone, and those without.
Discussion
The present study demonstrates the association of coronary endothelial dysfunction with OCT-identified macrophages and microchannels in patients with early coronary artery disease. The segments with macrophage image or microchannels showed significantly more severe epicardial endothelial dysfunction. In addition, segments with endothelial dysfunction showed significantly higher prevalence of macrophage image and microchannels although they had similar extent of atherosclerosis as assessed by IVUS. Thus, the current study supports the role of inflammation and the vasa vasorum in early atherosclerosis in humans.
In the initial stage of atherosclerosis, the dysfunctional endothelium produces proinflammatory cytokines and reduces nitric oxide activity with anti-inflammatory properties, subsequently facilitating recruitment and transmigration of circulating monocytes into the intima followed by their differentiation into macrophages.18 Taking up oxidized lipoproteins, macrophages form the characteristics of foam cells, a hallmark of early atherosclerosis, which can be detected by OCT.19 These activated macrophages in turn secrete proinflammatory cytokines aggravating endothelial dysfunction.20, 21 The current study extends our previous observations of the correlation between lipoprotein-associated phospholipase A2 (Lp-PLA2) levels in the coronary circulation and coronary endothelial dysfunction.22, 23 In the study, the levels of Lp-PLA2, a protein produced by inflammatory cells including macrophages, was shown to be correlated with plaque volume as assessed by IVUS.23 Given the potential of OCT to specifically identify macrophages in plaques, the present study provides the possibility to directly visualize functional activity of local inflammation of coronary plaques.
Pathology studies on early atherosclerosis demonstrated that even pathological intimal thickening is always vascularized.24, 25 Furthermore, the intimal vasa vasorum originating from adventitial vasa vasora precede the development of epicardial endothelial dysfunction and the appearance of any atherosclerotic features in animal models.9, 26 Hence, intimal vasa vasora have the potential to contribute to the development of endothelial dysfunction in both the conduit vessels and vasa vasorum which results in reduction in oxygen supply to the coronary artery wall.27, 28
There was a substantial overlap between segments with macrophage image and those with microchannels. More severe endothelial dysfunction in segments with both characteristics suggests an incremental effect of inflammation and intimal neovascularization in atherogenesis.29 Intimal neovascularization increases blood flow into the vascular wall and facilitates inflammatory cells to penetrate into plaque.30 Activated macrophages promote angiogenesis and facilitate further macrophage recruitment.31 More investigations are required to better elucidate the link between inflammation and intimal neovascularization and their association with endothelial dysfunction.
Recently we have demonstrated that coronary endothelial dysfunction is associated with necrotic core as assessed by virtual histology IVUS12 or lipid core plaque by near-infrared spectroscopy.13 However, no association was found between endothelial function and OCT-identified lipid-rich plaque in the current study. These discrepancies may result from differences in stages of atherosclerosis, imaging modality applied to each study, and the definition of atheromatous plaques. Further comparative studies using different imaging modalities are needed to elucidate more precise mechanisms of endothelial dysfunction.
Clinical implications
A recent study has shown that interventions to improve endothelial function contribute to attenuate plaque progression.5 The current study suggests that OCT-identified microstructures such as macrophages and vasa vasorum could be a surrogate marker for the evaluation of new therapeutic strategies. Further studies are needed to demonstrate that inflammation and vasa vasorum imaging by OCT can be used to monitor the effectiveness of the therapy.
Study limitations
We enrolled a relatively small number of patients and only analyzed the LAD. Therefore, caution should be taken in interpreting and generalizing the results to all patient populations. In addition, the definition of macrophage image using visual assessment may introduce bias. However, the validation study demonstrated that macrophage accumulations had distinctive OCT findings and that OCT could evaluate accurately cap macrophages.14, 15 This visual assessment of macrophage has been used in the previous studies17, 32 and also recommended by international working group.33 Finally, OCT has a limitation to evaluate a microstructure, such as vasa vasorum, located in deep site within plaque because of its shallow penetrating depth. Signal can be attenuated by macrophages, lipid pool, or necrotic core. In the present study, OCT images were acquired from early atherosclerosis in which plaque burden at MLA site was comparable in both segments and two independent examiners have evaluated OCT images to minimize bias.
Conclusions
The current study demonstrates that coronary segments with endothelial dysfunction are associated with relative abundance of macrophages and microchannels, which might have an important role in plaque initiation and progression. These observations can refine the current understanding of the link between epicardial coronary endothelial dysfunction and early structural changes in the vascular wall.
Supplementary Material
Significance.
We tested the hypothesis that segments with endothelial dysfunction, an early manifestation of atherosclerosis, show macrophages and/or vasa vasorum detected by optical coherence tomography (OCT), high-resolution and novel imaging modality. A total of 76 segments were studied in 40 patients with endothelial dysfunction. Macrophages and microchannels identified by OCT were evaluated according to epicardial endothelial function. Coronary artery diameter (CAD) change in response to acetylcholine was more severe in segments showing macrophages and microchannels than those without. There were increasing trends of the prevalence of macrophages and microchannels with endothelial dysfunction as stratified by quartiles of CAD change. In particular, segments with both macrophages and microchannels tended to have worse endothelial function than those with macrophages alone and microchannels alone. Coronary endothelial dysfunction was associated with OCT-identified macrophages and microchannels, suggesting that macrophages and vasa vasorum proliferation might play an important role in patients with early coronary atherosclerosis.
Acknowledgements
Sources of founding
This work is supported by the National Institute of Health (NIH Grants HL-92954 and AG-31750 to A.L., and NIH Grants DK-73608, HL-77131, and HL-085307 to L.O.L.) and the Mayo Foundation. T.A. was supported by a research fellowship from Banyu Life Science Foundation International.
Abbreviation
- CAD
coronary artery diameter
- GEE
Generalized estimating equations GEE
- IVUS
intravascular ultrasound
- LAD
left anterior descending artery
- Lp-PLA2
lipoprotein-associated phospholipase A2
- MC
microchannels
- MP
macrophage image
- OCT
optical coherence tomography
Footnotes
Disclosures
None.
References
- 1.Anderson TJ, Gerhard MD, Meredith IT, Charbonneau F, Delagrange D, Creager MA, Selwyn AP, Ganz P. Systemic nature of endothelial dysfunction in atherosclerosis. Am J Cardiol. 1995;75:71B–74B. doi: 10.1016/0002-9149(95)80017-m. [DOI] [PubMed] [Google Scholar]
- 2.Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363–368. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 3.Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Luscher TF, Shechter M, Taddei S, Vita JA, Lerman A. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126:753–767. doi: 10.1161/CIRCULATIONAHA.112.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 5.Yoon MH, Reriani M, Mario G, Rihal C, Gulati R, Lennon R, Tilford JM, Lerman LO, Lerman A. Long-term endothelin receptor antagonism attenuates coronary plaque progression in patients with early atherosclerosis. Int J Cardiol. 2013;168:1316–1321. doi: 10.1016/j.ijcard.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quyyumi AA, Cannon RO, 3rd, Panza JA, Diodati JG, Epstein SE. Endothelial dysfunction in patients with chest pain and normal coronary arteries. Circulation. 1992;86:1864–1871. doi: 10.1161/01.cir.86.6.1864. [DOI] [PubMed] [Google Scholar]
- 7.Zeiher AM, Drexler H, Saurbier B, Just H. Endothelium-mediated coronary blood flow modulation in humans. Effects of age, atherosclerosis, hypercholesterolemia, and hypertension. J Clin Invest. 1993;92:652–662. doi: 10.1172/JCI116634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishimura RA, Lerman A, Chesebro JH, Ilstrup DM, Hodge DO, Higano ST, Holmes DR, Jr, Tajik AJ. Epicardial vasomotor responses to acetylcholine are not predicted by coronary atherosclerosis as assessed by intracoronary ultrasound. J Am Coll Cardiol. 1995;26:41–49. doi: 10.1016/0735-1097(95)00142-m. [DOI] [PubMed] [Google Scholar]
- 9.Herrmann J, Lerman LO, Rodriguez-Porcel M, Holmes DR, Jr, Richardson DM, Ritman EL, Lerman A. Coronary vasa vasorum neovascularization precedes epicardial endothelial dysfunction in experimental hypercholesterolemia. Cardiovasc Res. 2001;51:762–766. doi: 10.1016/s0008-6363(01)00347-9. [DOI] [PubMed] [Google Scholar]
- 10.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 11.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 12.Lavi S, Bae JH, Rihal CS, Prasad A, Barsness GW, Lennon RJ, Holmes DR, Jr, Lerman A. Segmental coronary endothelial dysfunction in patients with minimal atherosclerosis is associated with necrotic core plaques. Heart. 2009;95:1525–1530. doi: 10.1136/hrt.2009.166017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi BJ, Prasad A, Gulati R, Best PJ, Lennon RJ, Barsness GW, Lerman LO, Lerman A. Coronary endothelial dysfunction in patients with early coronary artery disease is associated with the increase in intravascular lipid core plaque. Eur Heart J. 2013;34:2047–2054. doi: 10.1093/eurheartj/eht132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tearney GJ, Yabushita H, Houser SL, Aretz HT, Jang IK, Schlendorf KH, Kauffman CR, Shishkov M, Halpern EF, Bouma BE. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation. 2003;107:113–119. doi: 10.1161/01.cir.0000044384.41037.43. [DOI] [PubMed] [Google Scholar]
- 15.MacNeill BD, Jang IK, Bouma BE, Iftimia N, Takano M, Yabushita H, Shishkov M, Kauffman CR, Houser SL, Aretz HT, DeJoseph D, Halpern EF, Tearney GJ. Focal and multi-focal plaque macrophage distributions in patients with acute and stable presentations of coronary artery disease. J Am Coll Cardiol. 2004;44:972–979. doi: 10.1016/j.jacc.2004.05.066. [DOI] [PubMed] [Google Scholar]
- 16.Kitabata H, Tanaka A, Kubo T, Takarada S, Kashiwagi M, Tsujioka H, Ikejima H, Kuroi A, Kataiwa H, Ishibashi K, Komukai K, Tanimoto T, Ino Y, Hirata K, Nakamura N, Mizukoshi M, Imanishi T, Akasaka T. Relation of microchannel structure identified by optical coherence tomography to plaque vulnerability in patients with coronary artery disease. Am J Cardiol. 2010;105:1673–1678. doi: 10.1016/j.amjcard.2010.01.346. [DOI] [PubMed] [Google Scholar]
- 17.Uemura S, Ishigami K, Soeda T, Okayama S, Sung JH, Nakagawa H, Somekawa S, Takeda Y, Kawata H, Horii M, Saito Y. Thin-cap fibroatheroma and microchannel findings in optical coherence tomography correlate with subsequent progression of coronary atheromatous plaques. Eur Heart J. 2012;33:78–85. doi: 10.1093/eurheartj/ehr284. [DOI] [PubMed] [Google Scholar]
- 18.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 19.Kashiwagi M, Kitabata H, Ozaki Y, Imanishi T, Akasaka T. Fatty streak assessed by optical coherence tomography: early atherosclerosis detection. Eur Heart J Cardiovasc Imaging. 2013;14:109. doi: 10.1093/ehjci/jes182. [DOI] [PubMed] [Google Scholar]
- 20.Steinberg D. Lipoproteins and the pathogenesis of atherosclerosis. Circulation. 1987;76:508–514. doi: 10.1161/01.cir.76.3.508. [DOI] [PubMed] [Google Scholar]
- 21.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 22.Yang EH, McConnell JP, Lennon RJ, Barsness GW, Pumper G, Hartman SJ, Rihal CS, Lerman LO, Lerman A. Lipoprotein-associated phospholipase A2 is an independent marker for coronary endothelial dysfunction in humans. Arterioscler Thromb Vasc Biol. 2006;26:106–111. doi: 10.1161/01.ATV.0000191655.87296.ab. [DOI] [PubMed] [Google Scholar]
- 23.Lavi S, McConnell JP, Rihal CS, Prasad A, Mathew V, Lerman LO, Lerman A. Local production of lipoprotein-associated phospholipase A2 and lysophosphatidylcholine in the coronary circulation: association with early coronary atherosclerosis and endothelial dysfunction in humans. Circulation. 2007;115:2715–2721. doi: 10.1161/CIRCULATIONAHA.106.671420. [DOI] [PubMed] [Google Scholar]
- 24.Williams JK, Heistad DD. Structure and function of vasa vasorum. Trends Cardiovasc Med. 1996;6:53–57. doi: 10.1016/1050-1738(96)00008-4. [DOI] [PubMed] [Google Scholar]
- 25.Moreno PR, Purushothaman KR, Sirol M, Levy AP, Fuster V. Neovascularization in human atherosclerosis. Circulation. 2006;113:2245–2252. doi: 10.1161/CIRCULATIONAHA.105.578955. [DOI] [PubMed] [Google Scholar]
- 26.Heistad DD, Armstrong ML. Blood flow through vasa vasorum of coronary arteries in atherosclerotic monkeys. Arteriosclerosis. 1986;6:326–331. doi: 10.1161/01.atv.6.3.326. [DOI] [PubMed] [Google Scholar]
- 27.Kwon HM, Sangiorgi G, Ritman EL, McKenna C, Holmes DR, Jr, Schwartz RS, Lerman A. Enhanced coronary vasa vasorum neovascularization in experimental hypercholesterolemia. J Clin Invest. 1998;101:1551–1556. doi: 10.1172/JCI1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson SH, Herrmann J, Lerman LO, Holmes DR, Jr, Napoli C, Ritman EL, Lerman A. Simvastatin preserves the structure of coronary adventitial vasa vasorum in experimental hypercholesterolemia independent of lipid lowering. Circulation. 2002;105:415–418. doi: 10.1161/hc0402.104119. [DOI] [PubMed] [Google Scholar]
- 29.Herrmann J, Lerman A. Atherosclerosis in the back yard. J Am Coll Cardiol. 2007;49:2102–2104. doi: 10.1016/j.jacc.2007.02.042. [DOI] [PubMed] [Google Scholar]
- 30.Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, Wrenn SP, Narula J. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2005;25:2054–2061. doi: 10.1161/01.ATV.0000178991.71605.18. [DOI] [PubMed] [Google Scholar]
- 31.Polverini PJ, Cotran PS, Gimbrone MA, Jr, Unanue ER. Activated macrophages induce vascular proliferation. Nature. 1977;269:804–806. doi: 10.1038/269804a0. [DOI] [PubMed] [Google Scholar]
- 32.Kato K, Yonetsu T, Kim SJ, Xing L, Lee H, McNulty I, Yeh RW, Sakhuja R, Zhang S, Uemura S, Yu B, Mizuno K, Jang IK. Nonculprit plaques in patients with acute coronary syndromes have more vulnerable features compared with those with non-acute coronary syndromes: a 3-vessel optical coherence tomography study. Circ Cardiovasc Imaging. 2012;5:433–440. doi: 10.1161/CIRCIMAGING.112.973701. [DOI] [PubMed] [Google Scholar]
- 33.Tearney GJ, Regar E, Akasaka T, et al. International Working Group for Intravascular Optical Coherence Tomography. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012;59:1058–1072. doi: 10.1016/j.jacc.2011.09.079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.