Abstract
The glycosphingolipid sulfatide (SO3-3Galβ1Cer) is a demonstrated ligand for a subset of CD1d-restricted NKT cells, which could regulate experimental autoimmune encephalomyelitis, a murine model for multiple sclerosis, as well as tumor immunity and experimental hepatitis. Native sulfatide is a mixture of sulfatide isoforms, i.e. sulfatide molecules with different long-chain bases and fatty acid chain lengths and saturation. Here, we demonstrate that sulfatide-specific CD1d-restricted murine NKT hybridomas recognized several different sulfatide isoforms. These included the physiologically relevant isoforms C24:1 and C24:0, major constituents of the myelin sheet of the nervous system, and C16:0, prominent in the pancreatic islet β-cells. The most potent sulfatide isoform was lysosulfatide (lacking a fatty acid). Shortened fatty acid chain length (C24:1 versus C18:1), or saturation of the long fatty acid (C24:0), resulted in reduced stimulatory capacity, and fatty acid hydroxylation abolished the response. Moreover, sulfatide was not responsible for the natural autoreactivity toward splenocytes by XV19 T hybridoma cells. Our results reveal a promiscuity in the recognition of sulfatide isoforms by a CD1d-restricted NKT-cell clone, and suggest that sulfatide, a major component of the myelin sheet and pancreatic β-cells, is one of several natural ligands for type II CD1d-restricted NKT cells.
Keywords: Antigen presentation/processing, Autoimmunity, CD1 molecules, NKT cells Self/non-self discrimination
Introduction
NKT lymphocytes, a population with potent immunoregulatory properties, are activated by glycolipid antigens presented by the MHC class I like CD1d–molecule [1]. CD1d is a non-classical and non-polymorphic antigen-presenting molecule present in most species [2]. A diversity of glycolipids comprise a new previously unacknowledged category of molecular structures from both pathogens and self-tissues that can be recognized by CD1-restricted T lymphocytes [3].
Recently, the glycosphingolipid (GSL) sulfatide (SO3-3Galβ1Cer) was identified as a ligand for type II NKT cells [4–11]. This subset of NKT cells (type II) has characteristic features of NKT cells, but uses a diverse TCR repertoire [12–17] (reviewed in [18,19]). There is so far little information on the GSL ligands for type II NKT cells, but they appear to be different from those identified for type I NKT cells [7, 20–23] that carry a semi-invariant TCR comprising the canonical Vα14-Jα18 rearrangement [24]. Therefore, the two subsets of NKT cells are likely to be activated by different sets of ligands, and they may also display distinct functional characteristics [25, 26], supporting the notion that type I and II NKT cells have different roles within the immune system [19].
Importantly, sulfatide-specific CD1d-restricted NKT cells were found to make up a significant population of T cells in the liver and spleen of normal mice [7]. Several reports have shown that NKT cells can protect against experimentally induced or spontaneous autoimmune disease in the mouse [27–29]. In EAE, a murine model for multiple sclerosis, it was furthermore demonstrated that sulfatide-specific CD1d-restricted NKT cells accumulated in the CNS during EAE, suggesting that these cells play a role in the inflammatory process [7]. T cells reactive with glycolipids, including sulfatide, were also found at higher levels in multiple sclerosis patients [4]. Treatment of mice with sulfatide prevented EAE and led to reduced cytokine production by auto reactive T cells specific for the triggering autoantigen myelin oligodendrocyte glycoprotein. In other studies, sulfatide treatment was found to also promote the suppression of tumor immunity and to prevent experimental inflammatory liver disease, further demonstrating the potent regulatory ability of sulfatide-specific CD1d-restricted NKT cells [10, 11].
Sulfatide consists of a variety of naturally existing sulfatide isoforms with different physicochemical properties [30, 31]. The composition of sulfatide isoforms differs between organs, where sulfatide present in the myelin sheet of the brain is dominated by long (>20 carbon atoms) unsaturated and hydroxylated fatty acids, while sulfatide of the pancreatic β-cells contain a high proportion of sulfatide isoforms with short fatty acid lacking hydroxylation [32]. Several studies provide evidence that the fatty acid chain length of a lipid determines its ability to stimulate CD1d-restricted NKT cells, and potentially also its biological function [33–38]. Our aim in this study was to determine the ability of different sulfatide isoforms to stimulate a single sulfatide reactive TCR, using a CD1d-restricted, sulfatide-specific NKT-cell hybridoma (XV19) that also displays a natural autoreactivity to endogenous CD1d [7, 12]. We further tested the hypothesis that sulfatide is the endogenous ligand underlying the autoreactivity of sulfatide-specific NKT cells. Our results provide the first detailed description of the requirements for TCR stimulation using CD1d-restricted ligand variants for type II NKT cells, and suggest that C24:1 sulfatide, a major component of the myelin sheet, is one of several possible natural ligands for type II CD1d-restricted NKT cells. In contrast, endogenous sulfatide was not required for the autoreactivity of sulfatide-specific CD41d–restricted NKT cells, suggesting that other GSL may contribute to the thymic selection and peripheral activation of sulfatide reactive CD1d-restricted NKT cells.
Results
The GSL sulfatide is a CD1d-restricted ligand for type II NKT cells
To determine the capacity of different sulfatide isoforms (Fig. 1 and Table 1) to stimulate CD1d-restricted T lymphocytes, we took advantage of the sulfatide-reactive CD1d-restricted NKT-cell hybridoma XV19 [7, 12]. This T-cell hybridoma is CD1d–autoreactive but does not express the canonical Vα14-Jα18 TCR, and therefore belongs to what is now termed type II NKT cells [18]. We first used two different cell lines, which naturally express CD1d, for presentation of sulfatides to the hybridoma. Both represent cells that naturally interact with NKT cells in vivo; thymocytes that interact with developing NKT cells during positive thymic selection, and DC important for NKT-cell activation in the periphery. The thymoma line RMA-S does not support CD1d–autoreactivity of XV19 cells ([20] and Fig. 2A). RMA-S cells pre-loaded with the natural sulfatide isoform mixture isolated from pig brain tissue (here termed native sulfatide) exhibited a dose-dependent stimulation of the XV19 NKT-cell hybridoma (Fig. 2A).
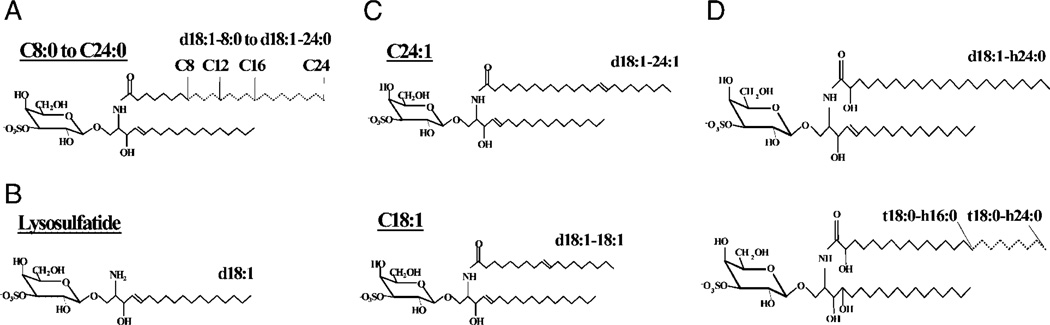
Figure 1.
Sulfatide isoforms. Semi-synthetic sulfatide isoforms with saturated fatty acids of C8–C24 length (A) and lyso (B) were synthesized from native sulfatide. Semi-synthetic C24:1 and C18:1 with unsaturated fatty acids are shown in (C). (D) Sulfatide isoforms were isolated from human intestine. The shorthand nomenclature for the sulfatide isoforms is indicated (see Table 1).
Table 1.
Sulfatide isoforms tested for stimulation of XV19 hybridoma cells.
| No. Trivial name | Structure | Ceramidea) | Effectb) | Source |
|---|---|---|---|---|
| 1. Native sulfatide | SO3-3Galβ1Cer | d18:1-mixture | + + | Porcine brain |
| 2. Lysosulfatide | SO3-3Galβ1Sph | d18:1 | + + + + | Semi-synthetic |
| 3. Sulfatide C8:0 | SO3-3Galβ1Cer | d18:1–8:0 | + | Semi-synthetic |
| 4. Sulfatide C12:0 | SO3-3Galβ1Cer | dl8:1–12:0 | + + | Semi-synthetic |
| 5. Sulfatide C16:0 | SO3-3Galβ1Cer | d18:1–16:0 | + | Semi-synthetic |
| 6. Sulfatide C18:1 | SO3-3Galβ1Cer | d18:1–18:1 | + | Semi-synthetic |
| 7. Sulfatide C24:0 | SO3-3Galβ1Cer | d18:1–24:0 | + + | Semi-synthetic |
| 8. Sulfatide C24:1 | SO3-3Galβ1Cer | d18:1–24:1 | + + + | Semi-synthetic |
| 9. Sulfatide | SO3-3Galβ1Cer | d18:1-h24:0 | − | Human intestine |
| 10. Sulfatide | SO3-3Galβ1Cer | t18:0-h16:0 | − | Human intestine |
| 11. Sulfatide | SO3-3Galβ1Cer | t18:0-h24:0 | − | Human intestine |
In the shorthand nomenclature for fatty acids and bases, the number before the colon refers to the carbon chain length and the number after the colon gives the total number of double bonds in the molecule. For long chain bases, d denotes dihydroxy and t denotes trihydroxy. Thus, d18:1 designates sphingosine (1,3-dihydroxy-2-aminooctadecene) and t18:0 phytosphingosine (1,3,4-trihydroxy-2-aminooctadecane). Fatty acids with a 2-hydroxy group are denoted by the prefix h before the abbreviation e.g. h16:0.
Summary of the relative stimulatory effect in the assays with different APC and XV19 hybridoma responder cells.
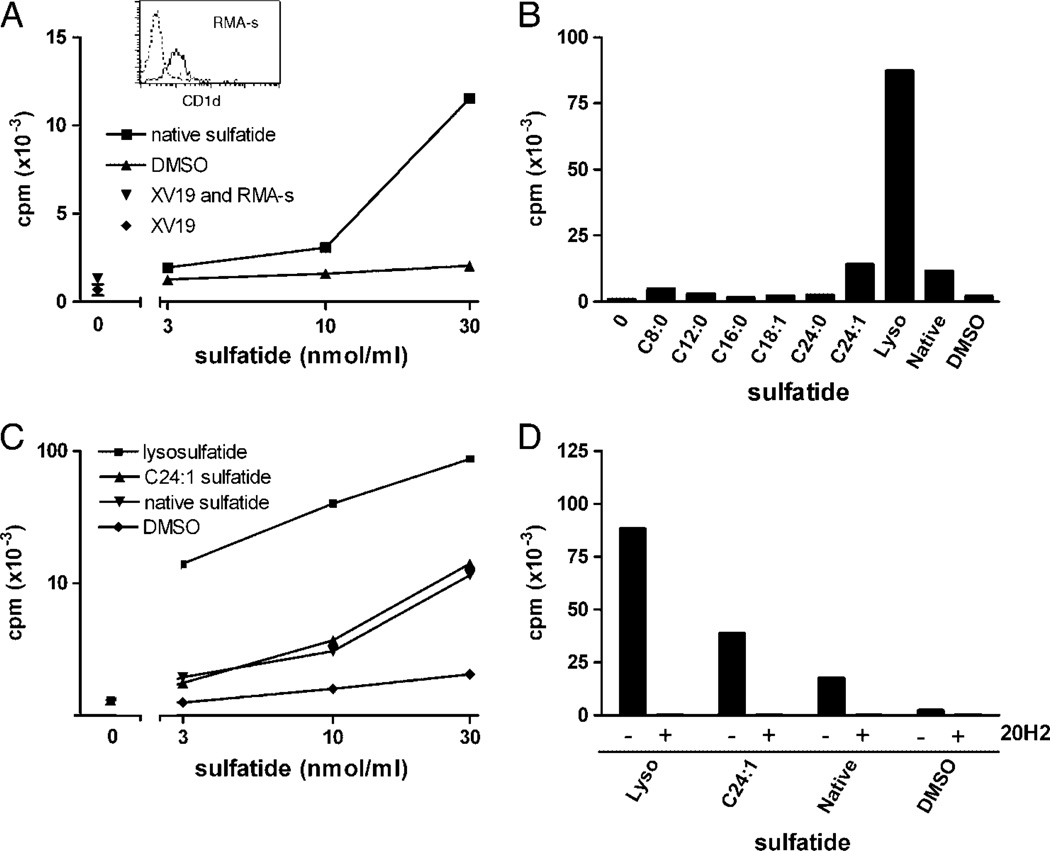
Figure 2.
Activation of the NKT-cell hybridoma XV19 by distinct sulfatide isoforms using RMA-S cells as APC. RMA-S cells (50 × 103 cells/well) were pulsed with the indicated sulfatide isoforms or vehicle (DMSO) before the addition of XV19 NKT hybridoma cells (40 × 103 cells/well). Activation was measured as IL-2 secretion determined in a CTLL-2 bioassay as described in Materials and methods. (A) Different concentrations of native sulfatide (squares) or vehicle (DMSO) were used to stimulate XV19 NKT-cell hybridoma cells. Inset: CD1d expression of RMA-S cells was determined by flow cytometry (dotted line; unstained cells and solid line; CD1d–stained cells). (B) XV19 cells were stimulated with RMA-S cells pre-pulsed with 30 nmol/mL of the indicated sulfatide isoforms (see Fig. 1 and Table 1 for structures of sulfatide isoforms) or vehicle control. (C) XV19 cells were stimulated with a titration of selected sulfatide isoforms presented on RMA-S cells. (D) RMA-S cells were pre-pulsed with lysoe, C24:1 sulfatide or native sulfatide (30 nmol/mL), incubated with or without the CD1d mAb 20H2 [39] for 15 min, followed by addition of the XV19 NKT-cell hybridoma cells. Data shown are from one representative experiment of at least three (A–C) or two (D) (mean of duplicate cultures). Lyso, lysosulfatide.
The fatty acid chain of sulfatide determines its stimulatory effect on type II NKT cells
To elucidate the stimulatory capacity of distinct sulfatide isoforms, including the major isoforms present in mammalian brain, we synthesized a panel of sulfatide isoforms (Fig. 1 and Table 1). These were C24:0, C16:0, C12:0 and C8:0 isoforms with decreasing length of a saturated fatty acid, mono-unsaturated C24:1 and C18:1, and lysosulfatide (lyso) lacking the entire fatty acid, all having a sphingosine long-chain base. The different isoforms were pre-loaded on RMA-S cells, which were then used to stimulate the XV19 NKT-cell hybridoma (Fig. 2). Lyso induced the most efficient stimulation of the XV19 NKT cells in repeated experiments, followed by C24:1, which was somewhat more stimulatory than native sulfatide (Figs. 2B – D). In repetitive experiments using RMA-S cells as APC, a dose-dependent response to the sulfatide isoforms was observed. Also at 3 nmol/mL, lyso showed strong stimulation of the XV19 NKT cells. This concentration of native or C24:1 sulfatide was barely stimulatory, while 10 nmo1/mL or above induced a reproducible response (Fig. 2C). CD1d–dependent stimulation by the sulfatide isoforms was demonstrated by including the anti-CD1d mAb 20H2 antibody [39], which abolished the reactivity (Fig. 2D).
Multiple isoforms of sulfatide can be presented to NKT cells by DC
DC appear to be the most efficient APC for activation of the primary NKT cells, prompting us investigate whether the DC line JawsII [40] would have superior capacity to present sulfatides to XV19 cells. JawsII cells expressed high levels of CD1d (see inset, Fig. 3A), and similar to spleen cells they induced a basal autoreactivity by XV19 cells. Therefore, a low number of APC was chosen, 5 × 103/well, which alone induced a suboptimal stimulation of the hybridoma. JawsII were indeed potent at presenting sulfatide to XV19 cells. A titration of lyso, C24:1 and native sulfatide demonstrated a low but detectable response to both lyso and C24:1 at 0.3 nmol/mL (Fig. 3A), while native sulfatide was not stimulatory below 3 nmol/mL (data not shown). Further screening of additional sulfatide isoforms employing JawsII as APC revealed recognition of several molecular species that had not been stimulatory with the RMA-S. C24:0 presented on JawsII cells stimulated XV19 cells to a similar extent as native sulfatide, followed by C12:0>C18:1 >/ = C16:0 (Figs. 3B and C). Thus, using the DC line JawsII we demonstrate that many different sulfatide isoforms can be presented and activate a single CD1d-restricted sulfatide-specific TCR.
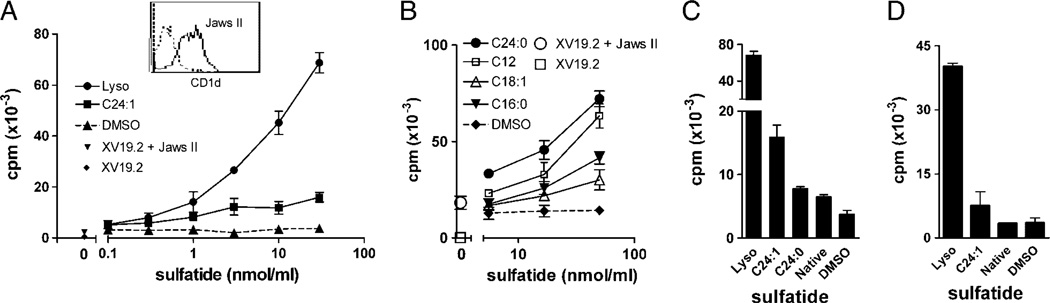
Figure 3.
The DC line JawsII efficiently presented multiple sulfatide isoforms to XV19 T hybridoma cells. JawsII cells (5 × 103 cells per well) were incubated with the indicated sulfatides for 4h, followed by addition of XV19 NKT-cell hybridoma cells (40 × 103 cells per well). After 16 h, IL-2 secretion was assayed using CTLL-2 cells. Data shown are representative of three or more experiments (mean ± SD of triplicate cultures). (A) JawsII cells had been pre-pulsed with lyso or C24:1 at the indicated concentrations, before co-culture with XV19 NKT-cell hybridoma cells. Inset: CD1d expression of JawsII cells was determined by flow cytometry (dotted line; cells stained with isotype control antibody, and solid line; CD1d–stained cells). (B) JawsII cells were incubated together with titrations of indicated sulfatide isoforms, followed by the addition of XV19 NKT-cell hybridoma cells. (C) JawsII cells (5 × 103) were cultured together with selected sulfatide isoforms or native sulfatide (30 nmol/mL) before stimulation of the XV19 NKT-cell hybridoma. (D) Bone marrow-derived DC (10 × 103 cells/well) were incubated with the indicated sulfatide preparations (30 nmol/mL) before stimulation of XV19 cells. Lyso, lysosulfatide.
Using JawsII we also attempted to activate XV19 cells with different purified fractions of sulfatide isolated from human intestine (Fig. 1 and Table 1). These sulfatides had hydroxylated fatty acids (h24:0 or h16:0, see 9–11 in Table 1), and two fractions had phytosphingosine long-chain bases (10 and 11 in Table 1). Neither of these sulfatide isoforms could stimulate XV19 cells (data not shown, see the summary in Table 1). Further, bone marrow-derived DC reproducibly presented lyso and C24:1 sulfatide to XV19 cells, but were not stimulatory when pulsed with native sulfatide (Fig. 3D).
Using a second CD1d-restricted type II NKT-cell hybridoma, 14S.15.5D [21], serendipitously found to recognize sulfatide, we could demonstrate a strong reactivity to lyso and a lower response to native sulfatide, similar to the sulfatide reactivity shown by XV19 cells (Fig. 4). Sulfatide was most efficiently presented to 14S.15.5D cells by the CD1d–transfected macro-phage line RAW264.7 (RAW-mCD1d), while XV19 cells were highly autoreactive to these cells (data not shown).
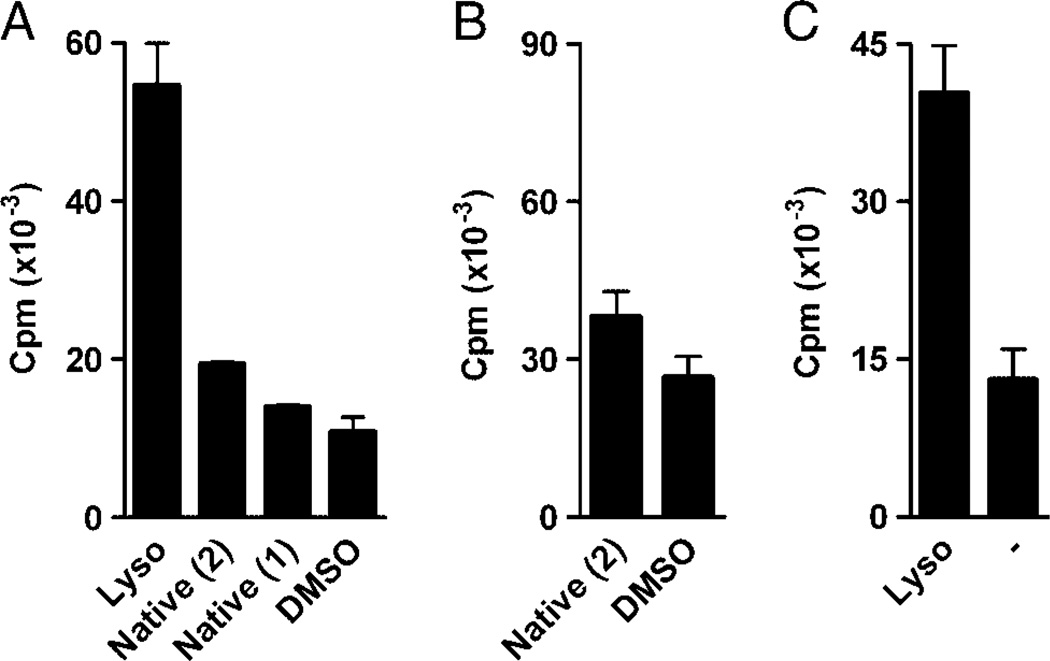
Figure 4.
Lyso strongly stimulated two different sulfatide reactive hybridomas. XV19 cells (A) or 14S.15.5D cells (B, C) were stimulated with APC pre-incubated with native sulfatide isolated from pig brain (Native (1), see Materials and methods) or commercially available native sulfatide from bovine brain (Native (2), Matreya), lyso, all at 30 nmol/ mL, or vehicle control (DMSO (A), or medium, –, (B)). XV19 cells were stimulated using JawsII (5 × 103 cells/well), and 14S.15.5D with RAW-mCD1d cells (10 × 103 cells/well) as APC. Activation was measured in a CTLL-2 bioassay. Data are representative of at least three experiments performed (mean ± SD of triplicate cultures).
Sulfatide stimulates XV19 NKT cells above the level of background autoreactivity to APC
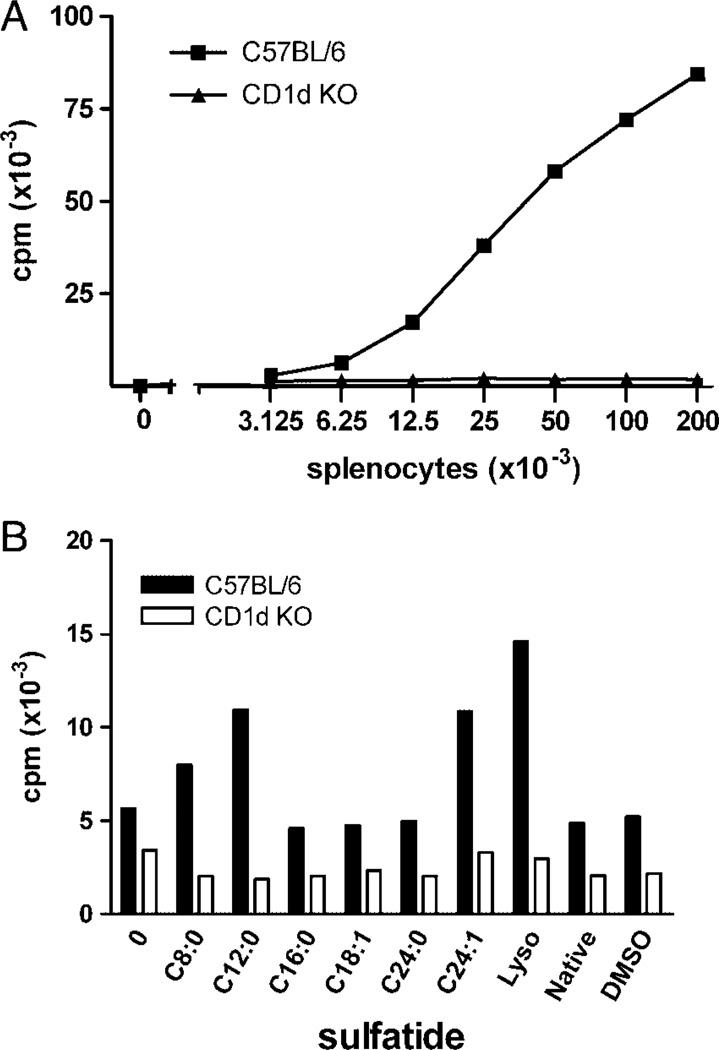
We next investigated whether spleen cells could present sulfatides to XV19 NKT hybridoma cells. The natural autoreactivity of the NKT-cell hybridoma XV19 toward splenocytes isolated from C57BL/6 mice is shown in Fig. 5A. Splenocytes from CD1d−/− mice failed to induce a response by XV19 cells, verifying the CD1d dependence of the autoreactivity. To study the stimulation of XV19 NKT cells by the sulfatide isoforms, we used 12 × 103 splenocytes per well which led to low autoreactivity. Splenocytes from B6 mice were pre-incubated with the sulfatide isoforms, followed by co-culture with the XV19 hybridoma. In general, spleen cells were inferior to JawsII, but slightly better than RMA-S, as APC for sulfatide. Again, addition of lyso resulted in the strongest stimulation (Fig. 5B). The C24:1 and C12:0 sulfatide isoforms reproducibly induced a 2–3-fold increase in stimulation of the XV19 NKT-cell hybridoma above the background autoreactivity to spleen cells, while C8:0 produced a weak stimulation above background in some experiments. In contrast, when splenocytes from CD1d−/−mice were used as APC, no stimulatory effect was obtained by the sulfatides (Fig. 5B).
Figure 5.
Spleen cells presented exogenous sulfatide to XV19 T hybridoma cells. (A) A titration of splenocytes was cultured together with the XV19 NKT-cell hybridoma, and IL-2 secretion assayed by CTLL-2 cells. Autoreactivity of the NKT-cell hybridoma XV19 was demonstrated to C57BL/6 (squares), but not CD1d−/− (CD1d KO, triangles) splenocytes. Data shown are representative of more than five experiments (mean of duplicate cultures). (B) Sulfatide pulsed CD1d+ splenocytes could stimulate XV19 cells above background autoreactivity. Splenocytes (12 × 103 cells/well) from C57BL/6 (black bars) or CD1d−/− (CD1d KO, white bars) mice were cultured together with sulfatide isoforms (30 nmol/mL) for 4 h before co-culture with the XV19 NKT-cell hybridoma (40 × 103 cells per well). Data shown are one representative experiment of three (mean of duplicate cultures). Lyso, lysosulfatide.
Autoreactivity to spleen cells is not dependent on endogenous sulfatide
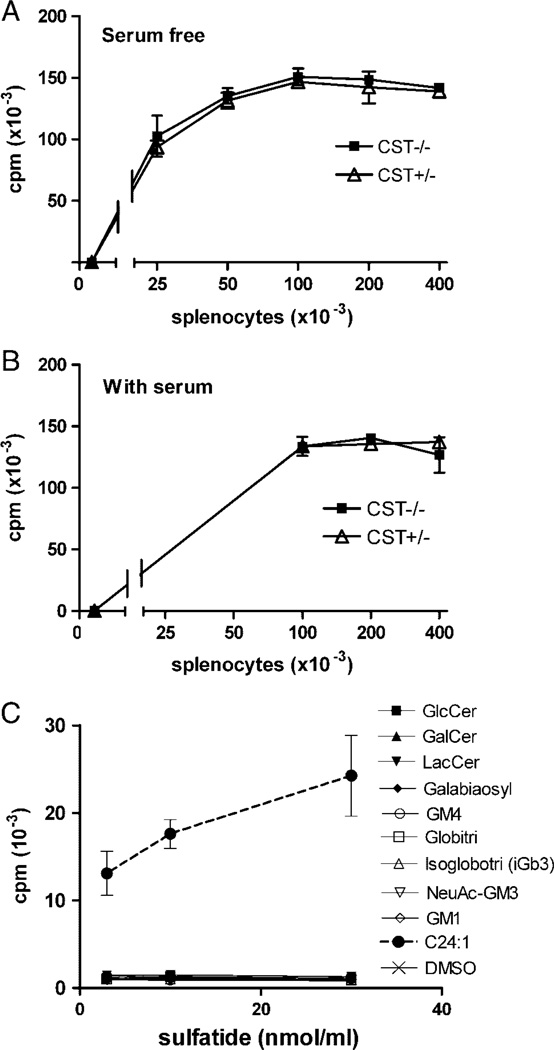
As sulfatide is present in certain tissues, including blood, it was possible that endogenous sulfatide was the CD1d–presented self-antigen underlying the autoreactivity of XV19 cells toward spleen cell APC. To investigate this, we tested the ability of splenocytes from mice lacking cerebroside sulfotransferase (CST), and thereby sulfoglycolipids including sulfatide [41], to stimulate the XV19 hybridoma. Further, to exclude serum as a potential source of sulfatide or other endogenous ligands, the experiment was performed both in regular serum supplemented medium, and defined serum-free medium. Figure 6 shows that under both culture conditions, the auto reactivity of XV19 NKT cells toward splenocytes from the CST−/− and wild-type littermate mice was comparable. Thus, the natural CD1d–presented ligands of the XV19 NKT hybridoma TCR are not restricted to sulfatide, but may include other endogenous GSL. We therefore tested some potential endogenous GSL ligands for their ability to stimulate XV19 cells, employing RMA-S and JawsII cells as APC. However, none of these GSL was able to stimulate XV19 cells (Fig. 6C and Table 2).
Figure 6.
The NKT-cell hybridoma XV19 was autoreactive toward splenocytes lacking sulfatide. Indicated numbers of splenocytes from CST−/− (black squares) and CST+/− littermate control mice (white triangles) were cultured together with the XV19 NKT-cell hybridoma for 16 h, after which IL-2 secretion was assayed using CTLL-2 cells. The cultures were performed in the absence of serum (A) or in regular supplemented RPMI with 5% FBS (B). Data shown are one representative experiment of three (B) or one (A) performed (mean ± SD of triplicate cultures). (C) XV19 cells were tested for their reactivity to a number of different GSL (see Table 2) by pulsing JawsII cells (5 × 103 cells/well) with the indicated GSL concentrations, before the addition of hybridoma cells. IL-2 secretion was assayed using CTLL-2 cells. Data are representative of two experiments using JawsII and two using RMA-S as APC.
Table 2.
Additional GSL tested for stimulation of XV19 hybridoma cells.
| No. | Trivial name | Structure | Ceramidea) | Effectb) | Source |
|---|---|---|---|---|---|
| 1. | GlcCer | Glcβ1Cer | d18:1/t18:0–16:0–24:0c)d) | − | Porcine kidney |
| 2. | GalCer | Galβ1Cer | D18:1-h18:0-h24:0d | − | Sigma |
| 3. | LacCer | Galβ4Glcβ1Cer | d18:1–16:0 and 24:1e) | − | Human neutrophils |
| 4. | Sulf-LacCer | SO3-3Galβ4Glcβ1Cer | D18:1-h16:0-h24:0d) | − | Human kidney |
| 5. | Galabiaosyl | Galα4Galβ1Cer | d18:1–16:0–24:0d) | − | Human kidney |
| 6. | GM4 | NeuAcα3Galβ1Cer | D18:1/d20:1–18:0c) | − | Human brain |
| 7. | Globotri (Gb3) | Galα4Galβ4Glcβ1Cer | d18:1–16:0 and 24:0e) | − | Human erythrocytes |
| 8. | Isoglobotri (iGb3) | Galα3Galβ4Glcβ1Cer | t18:0-h22:0 and h24: e) | − | Cat intestine |
| 9. | GM3 | NeuAcα3Galβ4Glcβ1Cer | d18:1/d20:1–18:0c) | − | Human brain |
| 10. | GM1 | Galβ3GalNAcβ4(NeuAcα3)Galβ4Glcβ1Cer | d18:1/d20:1–18:0c) | − | Human brain |
In the shorthand nomenclature for fatty acids and bases, the number before the colon refers to the carbon chain length and the number after the colon gives the total number of double bonds in the molecule. For long chain bases, d denotes dihydroxy and t denotes trihydroxy. Thus, d18:1 designates sphingosine (1,3-dihydroxy-2-aminooctadecene) and tl8:0 phytosphingosine (l,3,4-trihydroxy-2-aminooctadecane). Fatty acids with a 2-hydroxy group are denoted by the prefix h before the abbreviation e.g. h16:0.
Summary of the relative stimulatory effect in the assays with different APC and XV19 hybridoma responder cells (see also Table 1).
The GSL contains isoforms differing in the long chain base.
The fatty acid of the GSL varies in length between the indicated numbers.
The fatty acid of the GSL has two dominant lengths as indicated.
Discussion
The seminal discovery of α-galactosylceramide (α-GalCer) as a ligand for type I NKT cells [42] has been followed by many studies addressing the stimulatory potential of this ligand and its modifications. Subsequently, several endogenous and exogenous GSL ligands for type I NKT cells have been identified, greatly facilitating a wide spectrum of investigations of this NKT-cell subset (see [1, 3, 43] for reviews) The demonstration that sulfatide acts as a ligand for type II NKT cells represents an important advance in the studies of these cells [7, 10, 11]. In this study we set out to determine the TCR-ligand requirement of sulfatide-specific type II NKT cells, and show that several different isoforms of sulfatide can activate the same CD1d-restricted TCR.
Lyso was the most stimulatory sulfatide isoform tested using three different APC. Importantly, the preferential activation by lyso over native sulfatide was demonstrated for two independent NKT-cell hybridomas, the XV19 cells and 14S.15.5D cells, suggesting that this may be a common pattern of reactivity to sulfatide. In concordance with our results, a recent study also identified lyso to be a ligand for the XV19 hybridoma, while the inability to detect XV19 reactivity to other isoforms than lyso in these studies could possibly result from a lower sensitivity in the experimental system used [9]. We find that among the isoforms which contain a fatty acid chain, sulfatide C24:1 induced the strongest stimulation of XV19 cells. C24:0 with a long but saturated fatty acid resulted in a markedly reduced activity, showing that a double bond was required for optimal stimulation. Further, sulfatides with a shorter fatty acid (C18:1 versus C24:1, and C12:0, C8:0 and C16:0 versus C24:0) were less stimulatory, demonstrating the importance of the fatty acid chain length. Hydroxylation of the fatty acid abolished stimulation with the C24:0 isoform (compare isoforms 7 and 9, Table 1). Neither of the sulfatide isoforms with a phytosphingosine base were stimulatory (isoforms 10 and 11, Table 1), however, they also carried hydroxylated fatty acids that may prevent stimulation. Lack of stimulation of XV19 cells by GalCer, the core GSL of sulfatide indicated that the sulfate group was necessary. There was also an inability to respond to Sulf-LacCer, which is sulfated but has a longer core carbohydrate. A negative charge was not sufficient as GM4 was inactive as a ligand. Thus, the optimal antigen for the XV19 NKT-cell hybridoma had a sulfated galactose, a long (24 carbon) nonhydroxylated fatty acid chain with one unsaturation and a sphingosine long-chain base.
An increased degree of unsaturation and a shorter fatty acid chain of sulfatide will result in less hydrophobic molecules, which may alter their uptake and/or loading on CD1d, as well as the stability of the CD1d–sulfatide complex. The isoforms C24:1, C24:0 and C16:0 have been shown to load onto plate-bound CD1d molecules at neutral pH, while the loading of lyso required reduced pH and was further improved by the presence of saposin C [9]. In addition, changes in the hydrophobic chains which anchor sulfatide in the CD1d molecule may alter the conformation of the GSL-CD1d surface facing the TCR potentially affecting its binding to the GSL-CD1d complex [43]. Either, or a combination of, these mechanisms may lie behind the diverse capacities of the sulfatide isoforms to stimulate the NKT-cell hybridomas. Interestingly, studies using α-GalCer analogues have shown that changes in the sphingosine long chain or the fatty acid chain could strongly influence the pattern of cytokines released when stimulating primary type I NKT cells [33, 35, 36]. Such effects were not possible to determine in our experiments using a T-cell hybridoma, as the cytokine pattern of T-cell hybridomas does not reflect that of the original primary T-cell it was derived from. Thus, further studies will be required to reveal whether the different sulfatide species can modulate the cytokine profile of primary sulfatide-specific NKT cells.
Previous studies by Zajonc et al. demonstrated a significant stimulation of murine spleen cells by C24:1 but not to C24:0, C16:0 nor lyso in a CD1d–dependent manner [8]. We show that lyso was a highly efficient sulfatide species in activating both XV19 and 14S.15.5D hybridoma cells. This indicates that there exists some diversity in the natural repertoire of sulfatide-specific CD1d-restricted TCR used by NKT cells, with distinct differences in their TCR fine specificities. In the myelin of the nervous system, sulfatide C24:1 is a major component of the native sulfatide mixture which, in this tissue, is dominated by isoforms with long (more than 20 carbon atoms) unsaturated and hydroxylated fatty acids. The strong response of XV19 cells to C24:1 sulfatide suggests that this isoform is indeed the major active component in the native, pig brain derived, sulfatide which stimulated XV19 cells. Thus, in agreement with Zajonc et al. [8], we suggest that C24:1 sulfatide is a possible myelin-derived endogenous ligand for a subset of type II NKT cells, which may be activated during demyelinating inflammatory diseases of the CNS [7]. Outside the nervous system, sulfatide with short fatty acids such as C16:0 are dominating. This isoform is found in the kidney, but also at high levels in β-cells in the pancreatic islets of Langerhans, suggesting that this sulfatide isoform could contribute to NKT-cell activation during type 1 diabetes [44]. In contrast, the sulfatide isoforms with short fatty acid chains (C8 and C12) and C18:1, which showed a moderate and weak stimulatory ability of XV19 cells, respectively, are not known to exist naturally. Such isoforms might be of relevance as artificial ligands since they are less hydrophobic and might be developed as sulfatide-ligand analogues for the development of NKT-cell modulatory therapeutic treatments. Lyso is merely present in low amounts in mammals, so far only detected in brain and kidney tissues [45]. However, in a study by Toda et al. [46] elevated levels of lyso were found in subjects suffering from metachromatic leukodystrophy, a lysosomal storage disorder in which sulfatide accumulates. In these subjects, lyso could also be detected in the liver. Furthermore, lysosphingolipids, including lyso, associated with serum HDL have been suggested to have anti-atherogenic and anti-inflammatory properties in the development of arteriosclerosis (reviewed in [47]). It is therefore tempting to speculate that also lyso could play a role in vivo as an endogenous ligand for type II NKT cells.
The intact capacity of sulfatide-deficient CST−/− spleen cells to stimulate an autoreactive response by the XV19 T hybridoma cells demonstrates that sulfatide is not the only endogenous GSL ligand for these cells. Further, it has been shown that another sulfatide-deficient mouse, ceramide galactosyl transferase knockout mice, has a significant population of cells reactive with sulfatide loaded CD1d–tetramers [7]. Taken together, this suggests that other GSL ligands may contribute to the thymic selection and/or peripheral activation of sulfatide-specific CD1d-restricted NKT cells. In such a scenario sulfatide released from for example damaged CNS tissue may induce the activation of these type II NKT cells, which may contribute to a modulation of an inflammatory disease process in this tissue as suggested from the mouse model [7]. Additional studies are required to establish whether sulfatide released from the CNS, or other tissues, can naturally activate sulfatide reactive NKT cells in vivo, and to determine if these cells play a natural regulatory role also during other immune responses. Together with recent publications, describing the regulatory potential of these cells in other immune responses upon sulfatide administration [10, 11], this encourages further studies that exploit sulfatide-reactive NKT cells in the development of novel immunotherapies [19].
Materials and methods
Mice
C57BL/6 mice were purchased from Taconic (Ry, Denmark). The CD1d−/− mice were kindly been provided by Chyung-Ru Wang (University of Chicago) [48] and were bred and maintained in a specific pathogen-free animal facility at the University of Göteborg. CST−/− mice [41] were bred and maintained at Bartholin Instituttet, Rigshospitalet, Copenhagen, Denmark. Mice were used at the age of 8–18 wk and were matched for sex and age.
Cells and flow cytometry
The CD1d-restricted NKT-cell hybridoma clone XV19 had been generated from CD4+ T cells from MHC class II° mice, and was originally subcloned twice to ensure clonality [12]. The same T-cell hybridoma has been used by V. Kumar and co-workers and was termed as Hy19.3 [7, 9]. The CD1d-restricted hybridoma 14S.15.5D was derived from a C57BL/6 mouse [21]. CD1d–expressing RMA-S cells [20, 49] or the macrophage line RAW264.7 [50] transfected with murine CD1d (RAW-mCD1d, kindly provided by Dr. Manuela Cernadas, Harvard Medical School, Boston, MA), used as APC, were maintained in RPMI medium supplemented with 5% FBS, 1 mM sodium pyruvate, 10 mM HEPES, 50 µM 2-ME and 100U/mL penicillin/streptomycin. The CTLL-2 cells were maintained in ISCOVE’s medium supplemented with 10% FBS, 10 mM HEPES, penicillin/streptomycin, 50 µM 2-ME and IL-2 containing supernatant [51]. JawsII cells [40] were obtained from ATCC and maintained in ISCOVE’s medium supplemented with 20% FBS, 5 ng/mL murine GM-CSF (Sigma-Aldrich, Germany), 50 µg/mL gentamycin (Sigma-Aldrich) and 50 µM 2-ME. To obtain bone marrow-derived DC, bone marrow cells were isolated from C57BL/6 mice and grown in complete RPMI 1640 medium with 10% FBS in the presence of 20 ng/mL GM-CSF (Sigma-Aldrich) for 6 days, replacing the medium every second day. On day 6 of culture, cells were matured with 1 µg/mL LPS (Sigma-Aldrich) for 24 h before they were pulsed with sulfatide and used for hybridoma stimulation. For flow cytometry analysis, cells were first incubated with the 2.4G2 anti-FcγR antibody followed by PE-conjugated anti-mouse CD1d (clone 1B1, BD Pharmingen) or control antibody and analyzed with a FACSCalibur using CellQuest software.
GSL
Native sulfatide at a purity of > 95% (determined by thin layer chromatography and mass spectometry) had been isolated from pig brain, as described previously [45], and was used throughout the experiments unless otherwise stated. Native sulfatide from bovine brain (Matreya) elicited a similar T hybridoma response (see Fig. 4A). Lyso and sulfatide isoforms containing the fatty acids of caprylic acid (C8:0), lauric acid (C12:0), palmitic acid (C16:0), oleic acid (C18:1), lignoceric acid (C24:0) and nervonic acid (C24:1) were produced from pig brain-derived native sulfatide, as described previously [52]. The synthesized isoforms were free of detectable contaminations in the analysis by thin layer chromatography and mass spectometry, revealing a purity of >99%. The other reference GSL (listed in Tables 1 and 2) were isolated by the method described by Karlsson [53], and characterized by mass spectrometry [54] and proton NMR [55] and had a purity of 90–95%. The solubility in culture medium at final concentration of the various sulfatide isoforms was verified by thin layer chromatography in selected experiments, and was found to be > 80%.
T-cell hybridoma assay
For the sulfatide stimulation assays, 12.5 × 103 splenocytes, 5 × 103 JawsII cells, or 50 × 103 RMA-S cells per well, were used as APC, unless stated otherwise. The APC were incubated in the presence of graded concentrations of sulfatide isoforms for 4–5 h at 37°C, washed twice in supplemented RPMI medium, where after the T-cell hybridoma cells (40 × 103 cells/well) were added. After 16–18 h of culture, IL-2 secretion was determined in culture supernatants in a bioassay using CTLL-2 cells as described previously [12], and hybridoma stimulation is expressed as cpm determined in the CTLL assay. A titration of IL-2 was included as reference to ensure that the responses were in the linear response range. In some figures only results using the highest sulfatide concentration are shown. For serum-free cultures, UltraCULTURE medium (Lonza Walkersville, USA) was utilized, supplemented with 2 mM L-glutamine, 1 mM sodium pyruvate, 10 mM HEPES, 50 µM 2-ME and 100U/mL penicillin/streptomycin. Using thin layer chromatography-ELISA analysis [56] the concentration of sulfatide in UltraCULTURE medium was determined to be 30pmol/mL, which was 100-fold below the lowest stimulatory concentration of C24:1 using spleen cell APC. XV19 cells were washed twice in serum-free medium, before dilution in the supplemented serum-free medium. CST−/− and CST+/− spleen cells were prepared in the absence of serum and diluted in the supplemented serum-free medium, or regular supplemented RPMI medium with 5% FBS. To examine CD1d–dependent stimulation, the anti-CD1d mAb 20H2 (kindly provided by Albert Bendelac, Howard Hughes Medical Institute, University of Chicago) was included at 2 µg/mL [39]. In these experiments, the antibody was pre-incubated with sulfatide loaded RMA-S cells for 15 min before the XV19 NKT-cell hybridoma cells were added. For the demonstration of autoreactivity, twofold serial dilutions of spleen cells were used as indicated to stimulate XV19 T hybridoma cells.
Acknowledgements
We acknowledge Britt-Marie Rynmark for producing the sulfatide isoforms, Dr. Albert Bendelac for generously providing anti-CD1d antibody, Dr. Sam Behar for generously providing the 14S.15.5D cells and Dr. Manuela Cernadas generously providing the RAW-mCD1d cells. This work was supported by the Strategic Research Center for Mucosal Immunobiology and Vaccines, and grants from the Swedish Cancer Foundation, the Swedish Research Council, LUA-ALF Göteborg, the Novo Nordisk Foundation, the Swedish Diabetes Society, the Swedish Brain Foundation (Hjärnfonden), the Children’s Diabetes Society, and the foundations of Adlebert and W and M Lundgren.
Abbreviations
- CST
cerebroside sulfotransferase
- α-galcer
α-galactosylceramide
- GSL
glycosphingolipid
- lyso
lysosulfatide
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu. Rev. Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 2.Bricard G, Porcelli SA. Antigen presentation by CD1 molecules and the generation of lipid-specific T cell immunity. Cell. Mol. Life Sci. 2007;64:1824–1840. doi: 10.1007/s00018-007-7007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barral DC, Brenner MB. CD1 antigen presentation: how it works. Nat. Rev. Immunol. 2007;7:929–941. doi: 10.1038/nri2191. [DOI] [PubMed] [Google Scholar]
- 4.Shamshiev A, Donda A, Carena I, Mori L, Kappos L, De Libero G. Self glycolipids as T-cell autoantigens. Eur. J. Immunol. 1999;29:1667–1675. doi: 10.1002/(SICI)1521-4141(199905)29:05<1667::AID-IMMU1667>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 5.Shamshiev A, Gober HJ, Donda A, Mazorra Z, Mori L, De Libero G. Presentation of the same glycolipid by different CD1 molecules. J. Exp. Med. 2002;195:1013–1021. doi: 10.1084/jem.20011963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zajonc DM, Elsliger MA, Teyton L, Wilson IA. Crystal structure of CD1a in complex with a sulfatide self antigen at a resolution of 2.15 A. Nat. Immunol. 2003;4:808–815. doi: 10.1038/ni948. [DOI] [PubMed] [Google Scholar]
- 7.Jahng A, Maricic I, Aguilera C, Cardell S, Halder RC, Kumar V. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d–reactive T cell population reactive to sulfatide. J. Exp. Med. 2004;199:947–957. doi: 10.1084/jem.20031389. Epub 2004 Mar 2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zajonc DM, Maricic I, Wu D, Halder R, Roy K, Wong CH, Kumar V, Wilson IA. Structural basis for CD1d presentation of a sulfatide derived from myelin and its implications for autoimmunity. J. Exp. Med. 2005;202:1517–1526. doi: 10.1084/jem.20051625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roy KC, Maricic I, Khurana A, Smith TR, Halder RC, Kumar V. Involvement of secretory and endosomal compartments in presentation of an exogenous self-glycolipid to type II NKT cells. J. Immunol. 2008;180:2942–2950. doi: 10.4049/jimmunol.180.5.2942. [DOI] [PubMed] [Google Scholar]
- 10.Ambrosino E, Terabe M, Halder RC, Peng J, Takaku S, Miyake S, Yamamura T, et al. Cross-regulation between type I and type II NKT cells in regulating tumor immunity: a new immunoregulatory axis. J. Immunol. 2007;179:5126–5136. doi: 10.4049/jimmunol.179.8.5126. [DOI] [PubMed] [Google Scholar]
- 11.Halder RC, Aguilera C, Maricic I, Kumar V. Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease. J. Clin. Invest. 2007;117:2302–2312. doi: 10.1172/JCI31602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardell S, Tangri S, Chan S, Kronenberg M, Benoist C, Mathis D. CD1-restricted CD4+ T cells in major histocompatibility complex class II-deficient mice. J. Exp. Med. 1995;182:993–1004. doi: 10.1084/jem.182.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park SH, Roark JH, Bendelac A. Tissue-specific recognition of mouse CD1 molecules. J. Immunol. 1998;160:3128–3134. [PubMed] [Google Scholar]
- 14.Behar SM, Podrebarac TA, Roy CJ, Wang CR, Brenner MB. Diverse TCRs recognize murine CD1. J. Immunol. 1999;162:161–167. [PubMed] [Google Scholar]
- 15.Park SH, Weiss A, Benlagha K, Kyin T, Teyton L, Bendelac A. The mouse CD1d-restricted repertoire is dominated by a few autoreactive T cell receptor families. J. Exp. Med. 2001;193:893–904. doi: 10.1084/jem.193.8.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skold M, Faizunnessa NN, Wang CR, Cardell S. CD1d–specific NK1.1+ T cells with a transgenic variant TCR. J. Immunol. 2000;165:168–174. doi: 10.4049/jimmunol.165.1.168. [DOI] [PubMed] [Google Scholar]
- 17.Duarte N, Stenstrom M, Campino S, Bergman M-L, Lundholm M, Holmberg D, Cardell S. Prevention of diabetes in non obese diabetic (NOD) mice mediated by non-classical NKT cells. J. Immunol. 2004;173:3112–3118. doi: 10.4049/jimmunol.173.5.3112. [DOI] [PubMed] [Google Scholar]
- 18.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what’s in a name? Nat. Rev. Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 19.Kadri N, Blomqvist M, Cardell SL. Type II natural killer T cells: a new target for immunomodulation? Expert Rev. Clin. Immunol. 2008;4:615–627. doi: 10.1586/1744666X.4.5.615. [DOI] [PubMed] [Google Scholar]
- 20.Makowska A, Kawano T, Taniguchi M, Cardell S. Differences in the ligand specificity between CD1d-restricted T cells with limited and diverse T-cell receptor repertoire. Scand. J. Immunol. 2000;52:71–79. doi: 10.1046/j.1365-3083.2000.00754.x. [DOI] [PubMed] [Google Scholar]
- 21.Gumperz JE, Roy C, Makowska A, Lum D, Sugita M, Podrebarac T, Koezuka Y, et al. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity. 2000;12:211–221. doi: 10.1016/s1074-7613(00)80174-0. [DOI] [PubMed] [Google Scholar]
- 22.Brossay L, Tangri S, Bix M, Cardell S, Locksley R, Kronenberg M. Mouse CD1-autoreactive T cells have diverse patterns of reactivity to CD1+ targets. J. Immunol. 1998;160:3681–3688. [PubMed] [Google Scholar]
- 23.Chiu YH, Jayawardena J, Weiss A, Lee D, Park SH, Dautry-Varsat A, Bendelac A. Distinct subsets of CD1d-restricted T cells recognize self-antigens loaded in different cellular compartments. J. Exp. Med. 1999;189:103–110. doi: 10.1084/jem.189.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268:863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 25.Stenstrom M, Skold M, Ericsson A, Beaudoin L, Sidobre S, Kronenberg M, Lehuen A, Cardell S. Surface receptors identify mouse NK1.11 T cell subsets distinguished by function and T cell receptor type. Eur. J. Immunol. 2004;34:56–65. doi: 10.1002/eji.200323963. [DOI] [PubMed] [Google Scholar]
- 26.Rolf J, Berntman E, Stenstrom M, Smith EM, Mansson R, Stenstad H, Yamagata T, et al. Molecular profiling reveals distinct functional attributes of CD1d-restricted natural killer (NK) T cell subsets. Mol. Immunol. 2008;45:2607–2620. doi: 10.1016/j.molimm.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 27.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d–dependent NKT cells. J. Clin. Invest. 2004;114:1379–1388. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cardell SL. The natural killer T lymphocyte: a player in the complex regulation of autoimmune diabetes in non-obese diabetic mice. Clin. Exp. Immunol. 2006;143:194–202. doi: 10.1111/j.1365-2249.2005.02942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halder RC, Jahng A, Maricic I, Kumar V. Mini review: immune response to myelin-derived sulfatide and CNS-demyelination. Neurochem. Res. 2007;32:257–262. doi: 10.1007/s11064-006-9145-4. [DOI] [PubMed] [Google Scholar]
- 30.Ishizuka I. Chemistry and functional distribution of sulfoglycolipids. Prog. Lipid Res. 1997;36:245–319. doi: 10.1016/s0163-7827(97)00011-8. [DOI] [PubMed] [Google Scholar]
- 31.Buschard K, Josefsen K, Hansen SV, Horn T, Marshall MO, Persson H, Mansson JE, Fredman P. Sulphatide in islets of Langerhans and in organs affected in diabetic late complications: a study in human and animal tissue. Diabetologia. 1994;37:1000–1006. doi: 10.1007/BF00400463. [DOI] [PubMed] [Google Scholar]
- 32.Fredman P, Mansson JE, Rynmark BM, Josefsen K, Ekblond A, Halldner L, Osterbye T, et al. The glycosphingolipid sulfatide in the islets of Langerhans in rat pancreas is processed through recycling: possible involvement in insulin trafficking. Glycobiology. 2000;10:39–50. doi: 10.1093/glycob/10.1.39. [DOI] [PubMed] [Google Scholar]
- 33.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413:531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 34.Rauch J, Gumperz J, Robinson C, Skold M, Roy C, Young DC, Lafleur M, et al. Structural features of the acyl chain determine self-phospholipid antigen recognition by a CD1d-restricted invariant NKT (iNKT) cell. J. Biol. Chem. 2003;278:47508–47515. doi: 10.1074/jbc.M308089200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goff RD, Gao Y, Mattner J, Zhou D, Yin N, Cantu C, Teyton L, III, et al. Effects of lipid chain lengths in alpha-galactosylceramides on cytokine release by natural killer T cells. J. Am. Chem. Soc. 2004;126:13602–13603. doi: 10.1021/ja045385q. [DOI] [PubMed] [Google Scholar]
- 36.Yu KO, Im JS, Molano A, Dutronc Y, Illarionov PA, Forestier C, Fujiwara N, et al. Modulation of CD1d-restricted NKT cell responses by using N-acyl variants of alpha-galactosylceramides. Proc. Natl. Acad. Sci. USA. 2005;102:3383–3388. doi: 10.1073/pnas.0407488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hava DL, Brigl M, van den Elzen P, Zajonc DM, Wilson IA, Brenner MB. CD1 assembly and the formation of CD1-antigen complexes. Curr. Opin. Immunol. 2005;17:88–94. doi: 10.1016/j.coi.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 38.McCarthy C, Shepherd D, Fleire S, Stronge VS, Koch M, Illarionov PA, Bossi G, et al. The length of lipids bound to human CD1d molecules modulates the affinity of NKT cell TCR and the threshold of NKT cell activation. J. Exp. Med. 2007;204:1131–1144. doi: 10.1084/jem.20062342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roark JH, Park SH, Jayawardena J, Kavita U, Shannon M, Bendelac A. CD1.1 expression by mouse antigen-presenting cells and marginal zone B cells. J. Immunol. 1998;160:3121–3127. [PubMed] [Google Scholar]
- 40.Jorgensen TN, Haase C, Michelsen BK. Treatment of an immortalized APC cell line with both cytokines and LPS ensures effective T-cell activation in vitro. Scand. J. Immunol. 2002;56:492–503. doi: 10.1046/j.1365-3083.2002.01166.x. [DOI] [PubMed] [Google Scholar]
- 41.Honke K, Hirahara Y, Dupree J, Suzuki K, Popko B, Fukushima K, Fukushima J, et al. Paranodal junction formation and spermatogenesis require sulfoglycolipids. Proc. Natl. Acad. Sci. USA. 2002;99:4227–4232. doi: 10.1073/pnas.032068299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 43.Zajonc DM, Kronenberg M. CD1 mediated T cell recognition of glycolipids. Curr. Opin. Struct. Biol. 2007;17:521–529. doi: 10.1016/j.sbi.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buschard K, Blomqvist M, Osterbye T, Fredman P. Involvement of sulfatide in beta cells and type 1 and type 2 diabetes. Diabetologia. 2005;48:1957–1962. doi: 10.1007/s00125-005-1926-9. [DOI] [PubMed] [Google Scholar]
- 45.Rosengren B, Fredman P, Mansson JE, Svennerholm L. Lysosulfatide (galactosylsphingosine-3-O-sulfate) from metachromatic leukodystrophy and normal human brain. J. Neurochem. 1989;52:1035–1041. doi: 10.1111/j.1471-4159.1989.tb01844.x. [DOI] [PubMed] [Google Scholar]
- 46.Toda K, Kobayashi T, Goto I, Ohno K, Eto Y, Inui K, Okada S. Lysosulfatide (sulfogalactosylsphingosine) accumulation in tissues from patients with metachromatic leukodystrophy. J. Neurochem. 1990;55:1585–1591. doi: 10.1111/j.1471-4159.1990.tb04942.x. [DOI] [PubMed] [Google Scholar]
- 47.Nofer JR, Assmann G. Atheroprotective effects of high-density lipoprotein-associated lysosphingolipids. Trends Cardiovasc. Med. 2005;15:265–271. doi: 10.1016/j.tcm.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 48.Chen YH, Chiu NM, Mandal M, Wang N, Wang CR. Impaired NK1+ T cell development and early IL-4 production in CD1-deficient mice. Immunity. 1997;6:459–467. doi: 10.1016/s1074-7613(00)80289-7. [DOI] [PubMed] [Google Scholar]
- 49.Ljunggren HG, Karre K. Host resistance directed selectively against H-2-deficient lymphoma variants. Analysis of the mechanism. J. Exp. Med. 1985;162:1745–1759. doi: 10.1084/jem.162.6.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raschke WC, Baird S, Ralph P, Nakoinz I. Functional macrophage cell lines transformed by Abelson leukemia virus. Cell. 1978;15:261–267. doi: 10.1016/0092-8674(78)90101-0. [DOI] [PubMed] [Google Scholar]
- 51.Karasuyama H, Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur. J. Immunol. 1988;18:97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- 52.Blomqvist M, Carrier M, Andrews T, Pettersson K, Mansson JE, Rynmark BM, Fredman P, Buschard K. In vivo administration of the C16:0 fatty acid isoform of sulfatide increases pancreatic sulfatide and enhances glucose-stimulated insulin secretion in Zucker fatty (fa/fa) rats. Diabetes Metab. Res. Rev. 2005;21:158–166. doi: 10.1002/dmrr.519. [DOI] [PubMed] [Google Scholar]
- 53.Karlsson KA. Preparation of total nonacid glycolipids for overlay analysis of receptors for bacteria and viruses and for other studies. Methods Enzymol. 1987;138:212–220. doi: 10.1016/0076-6879(87)38018-8. [DOI] [PubMed] [Google Scholar]
- 54.Samuelsson BE, Pimlott W, Karlsson KA. Mass spectrometry of mixtures of intact glycosphingolipids. Methods Enzymol. 1990;193:623–646. doi: 10.1016/0076-6879(90)93442-n. [DOI] [PubMed] [Google Scholar]
- 55.Koerner TA, Jr, Prestegard JH, Demou PC, Yu RK. High-resolution proton NMR studies of gangliosides. 1. Use of homonuclear two-dimensional spin-echo J-correlated spectroscopy for determination of residue composition and anomeric configurations. Biochemistry. 1983;22:2676–2687. doi: 10.1021/bi00280a014. [DOI] [PubMed] [Google Scholar]
- 56.Davidsson P, Fredman P, Collins VP, von Holst H, Mansson JE, Svennerholm L. Ganglioside composition in human meningiomas. J. Neurochem. 1989;53:705–709. doi: 10.1111/j.1471-4159.1989.tb11761.x. [DOI] [PubMed] [Google Scholar]