Abstract
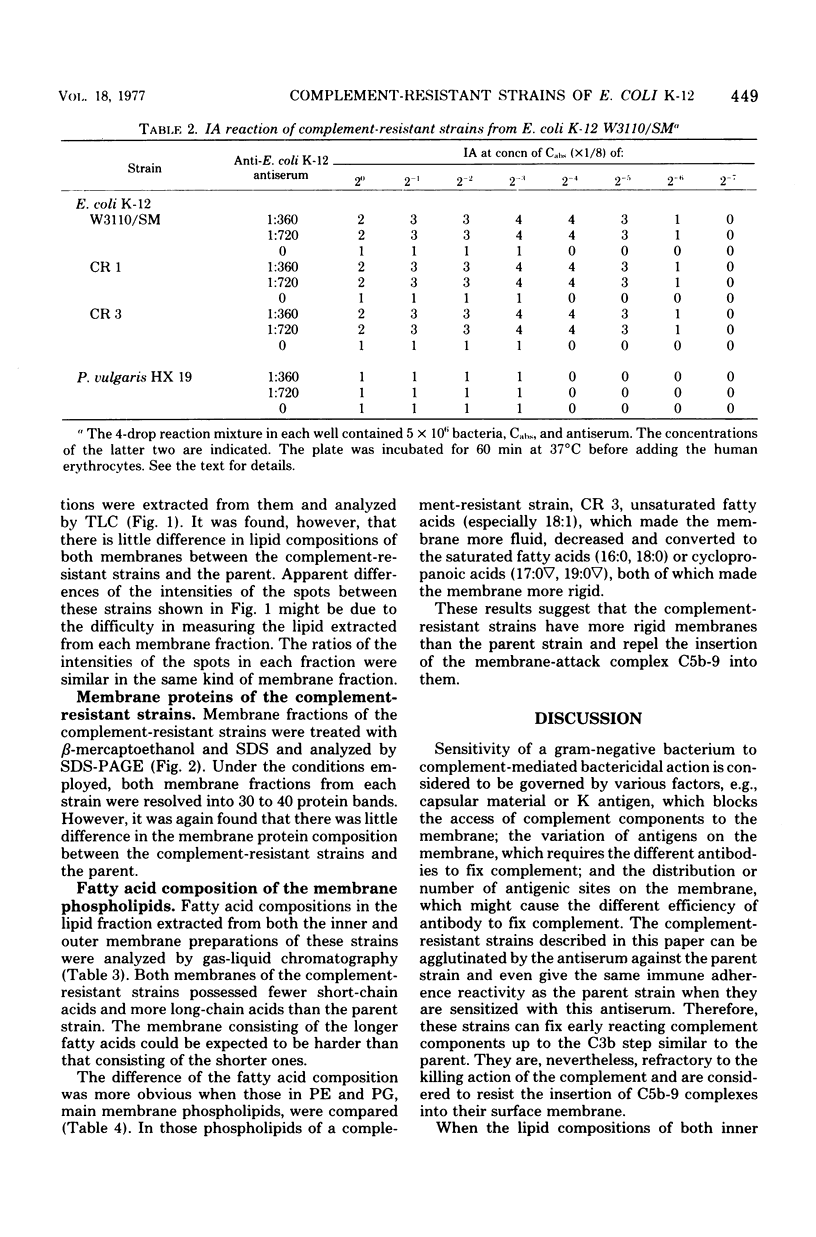
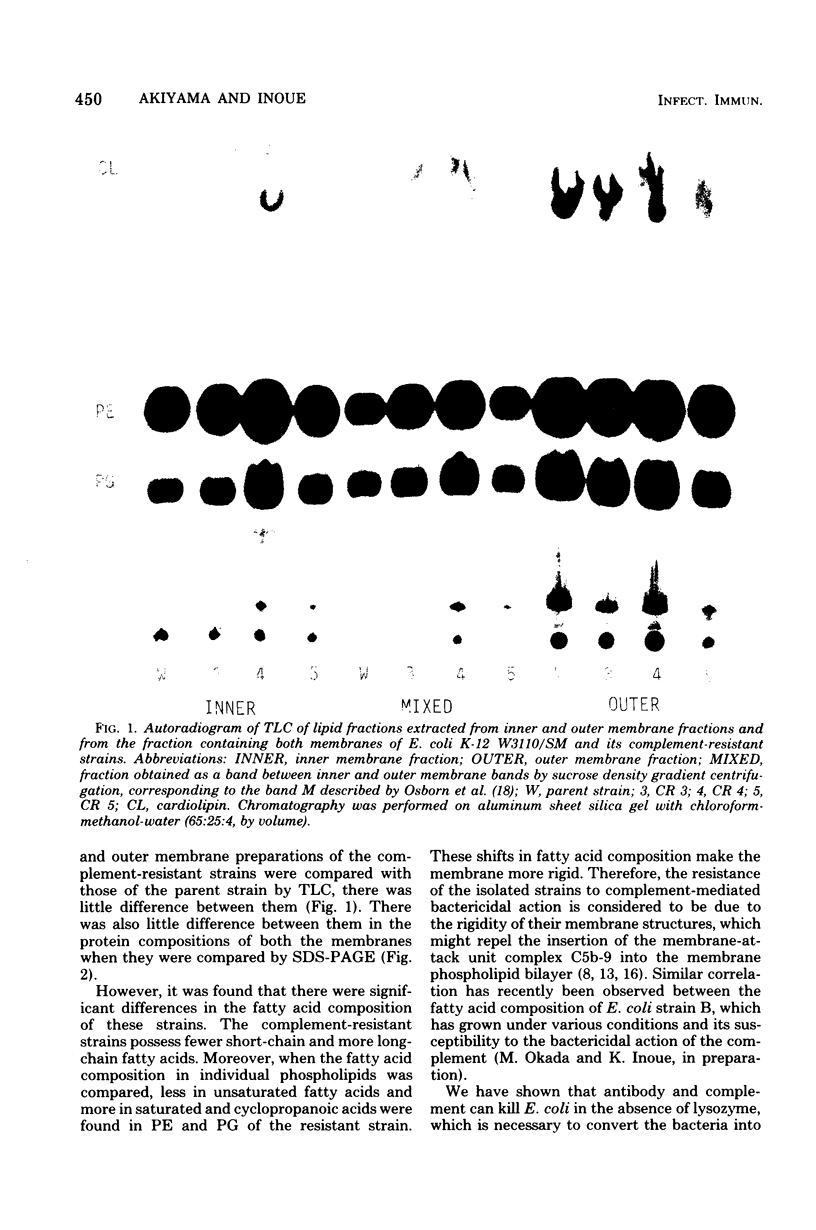
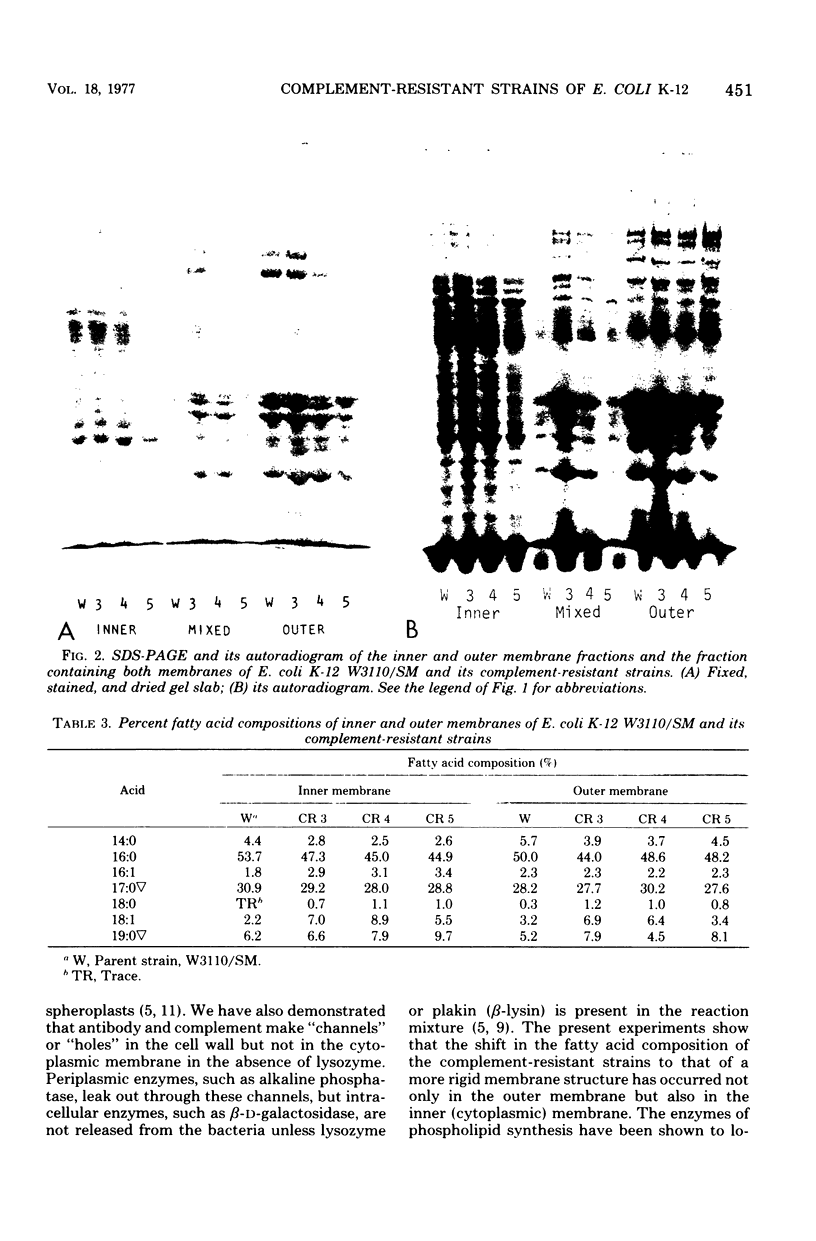
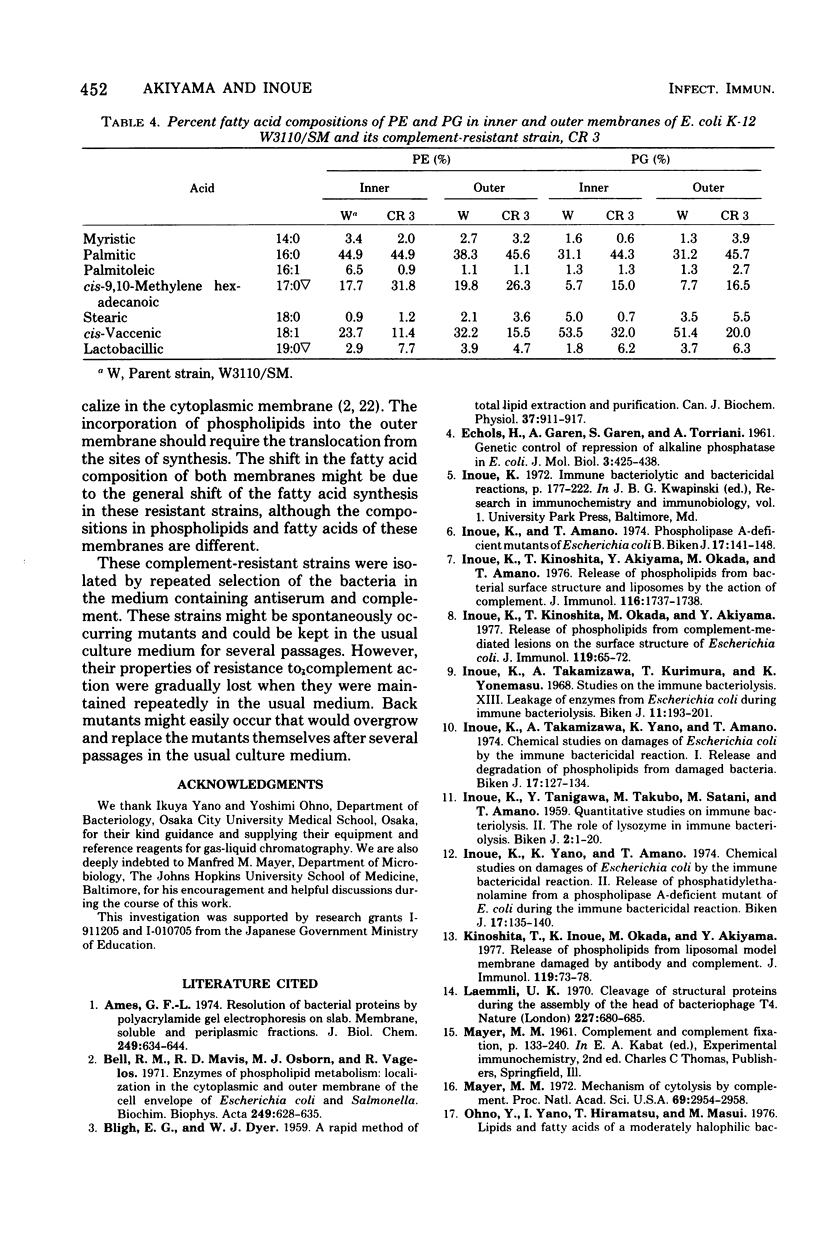
Several strains that were resistant to the bactericidal action of antibody and complement were isolated from Escherichia coli K-12 W3110/SM by selecting them through the medium containing antiserum and complement. They can be agglutinated by antiserum against the parent strain and showed similar immune adherence reactivity to the parent when sensitized with this antiserum. Few differences were found in the compositions of phospholipids and proteins between both inner and outer membranes of these strains and those of the parent. However, there were fewer short-chain and more long-chain fatty acids in these strains than in the parent. It was also found that unsaturated fatty acide decreased and saturated and cyclopropanoic acids increased in phosphatidylethanolamine and phosphatidylglycerol in both inner and outer membranes of one of these strains when compared with those from the parent. Therefore, the resistance of these strains to the complement-mediated bactericidal action was considered to be due to the rigidity of their membrane structures which might repel the insertion of membrane-attack complement complex C5b-9, although they could fix the earlier complement components up to the step of the formation of C4b,2a,3b complex enzyme.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bell R. M., Mavis R. D., Osborn M. J., Vagelos P. R. Enzymes of phospholipid metabolism: localization in the cytoplasmic and outer membrane of the cell envelope of Escherichia coli and Salmonella typhimurium. Biochim Biophys Acta. 1971 Dec 3;249(2):628–635. doi: 10.1016/0005-2736(71)90144-1. [DOI] [PubMed] [Google Scholar]
- ECHOLS H., GAREN A., GAREN S., TORRIANI A. Genetic control of repression of alkaline phosphatase in E. coli. J Mol Biol. 1961 Aug;3:425–438. doi: 10.1016/s0022-2836(61)80055-7. [DOI] [PubMed] [Google Scholar]
- Inoue K., Amano T. Phospholipase A-deficient mutants of Escherichia coli B. Biken J. 1974 Dec;17(4):141–148. [PubMed] [Google Scholar]
- Inoue K., Kinoshita T., Okada M., Akiyama Y. Release of phospholipids from complement-mediated lesions on the surface structure of Escherichia coli. J Immunol. 1977 Jul;119(1):65–72. [PubMed] [Google Scholar]
- Inoue K., Takamizawa A., Kurimura T., Yomemasu K. Studies on the immune bacteriolysis. 13. Leakage of enzymes from Escherichia coli during immune bacteriolysis. Biken J. 1968 Sep;11(3):193–201. [PubMed] [Google Scholar]
- Inoue K., Takamizawa A., Yano K., Amano T. Chemical studies on damages of Escherichia coli by the immune bactericidal reaction. I. Release and degradation of phospholipids from damaged bacteria. Biken J. 1974 Dec;17(4):127–134. [PubMed] [Google Scholar]
- Inoue K., Yano K., Amano T. Chemical studies on damages of Escherichea coli by the immune bactericidal reaction. II. Release of phosphatidylethanolamine from a phospholipase A-deficient mutant of E. coli during the immune bactericidal reaction. Biken J. 1974 Dec;17(4):135–140. [PubMed] [Google Scholar]
- Kinoshita T., Inoue K., Okada M., Akiyama Y. Release of phospholipids from liposomal model membrane damaged by antibody and complement. J Immunol. 1977 Jul;119(1):73–76. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. M. Mechanism of cytolysis by complement. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2954–2958. doi: 10.1073/pnas.69.10.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno Y., Yano I., Hiramatsu T., Masui M. Lipids and fatty acids of a moderately halophilic bacterium, No. 101. Biochim Biophys Acta. 1976 Mar 26;424(3):337–350. doi: 10.1016/0005-2760(76)90024-2. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short S. A., Kaback H. R., Kohn L. D. Localization of D-lactate dehydrogenase in native and reconstituted Escherichia coli membrane vesicles. J Biol Chem. 1975 Jun 10;250(11):4291–4296. [PubMed] [Google Scholar]
- Siroták L., Inoue K., Okada M., Amano T. Immune bactericidal reactions by guinea-pig gamma1 and gamma2 antibodies. Immunology. 1976 Mar;30(3):435–441. [PMC free article] [PubMed] [Google Scholar]
- White D. A., Albright F. R., Lennarz W. J., Schnaitman C. A. Distribution of phospholipid-synthesizing enzymes in the wall and membrane subfractions of the envelope of Escherichia coli. Biochim Biophys Acta. 1971 Dec 3;249(2):636–642. doi: 10.1016/0005-2736(71)90145-3. [DOI] [PubMed] [Google Scholar]