Abstract
Objectives
To determine the effects of a lifestyle intervention on the development of type 2 diabetes mellitus (T2DM) among participants with impaired glucose tolerance (IGT), in particular in the subgroup with baseline glycated hemoglobin (HbA1c) levels ≥5.7%, in primary healthcare settings.
Design
Randomized controlled trial.
Setting
32 healthcare centers in Japan.
Participants
Participants with IGT, aged 30–60 years, were randomly assigned to either an intensive lifestyle intervention group (ILG) or a usual care group (UCG).
Interventions
During the initial 6 months, participants in the ILG received four group sessions on healthy lifestyles by public health providers. An individual session was further conducted biannually during the 3 years. Participants in the UCG received usual care such as one group session on healthy lifestyles.
Outcome measures
The primary endpoint was the development of T2DM based on an oral glucose tolerance test.
Results
The mean follow-up period was 2.3 years. The annual incidence of T2DM were 2.7 and 5.1/100 person-years of follow-up in the ILG (n=145) and UCG (n=149), respectively. The cumulative incidence of T2DM was significantly lower in the ILG than in the UCG among participants with HbA1c levels ≥5.7% (log-rank=3.52, p=0.06; Breslow=4.05, p=0.04; Tarone-Ware=3.79, p=0.05), while this was not found among participants with HbA1c levels <5.7%.
Conclusions
Intensive lifestyle intervention in primary healthcare setting is effective in preventing the development of T2DM in IGT participants with HbA1c levels ≥5.7%, relative to those with HbA1c levels <5.7%.
Trial registration number
UMIN000003136.
Keywords: HbA1c, Type 2 Diabetes, Impaired Glucose Tolerance, Intervention Groups
Key messages.
The results were based on a multicenter design using real-world primary healthcare settings, including existing healthcare resources.
During the initial 6 months, subjects in the intensive lifestyle intervention group received four group sessions on healthy lifestyles by public health providers. An individual session was further conducted biannually during the 3 years.
Intensive lifestyle intervention in primary healthcare setting is more effective in preventing the development of type 2 diabetes mellitus in impaired glucose tolerance subjects with HbA1c levels ≥5.7%, relative to those with HbA1c levels <5.7%.
Introduction
Type 2 diabetes mellitus (T2DM), a disease with a huge medico-social burden affecting economically affluent countries, is gradually afflicting the developing world.1 Prevention of T2DM is a major global public health objective, with 366 million people estimated to have the condition worldwide, and the anticipation that this will increase to 522 million by 2030.2 The Finnish Diabetes Prevention Study (DPS) and the US Diabetes Prevention Program (DPP) demonstrated that the incidence of T2DM could be reduced by 58% in patients with prediabetes through weight loss resulting from changes in diet and physical activity.3 4 Translational studies based on the intensive diabetes prevention programs showed that there is potential for less intensive interventions both to be feasible and to have an impact on future progression to diabetes in at-risk individuals.2 Considering the increasing incidence of T2DM and cost-effectiveness, intervention in existing primary healthcare settings may be one of public health strategies in real-world practice. Japanese with T2DM are not often obese and can show low insulin secretion different to Caucasians with T2DM5; so evidence of preventative diabetology for the Japanese is also required. The American Diabetes Association (ADA) has recommended using glycated hemoglobin (HbA1c) levels to diagnose DM.6 The HbA1c testing in addition to fasting plasma glucose (FPG) can also help predict future diabetes.7 However, there are limited intervention studies that examined the usefulness of HbA1c to predict the development of T2DM.8 The aim of the study is to investigate the effectiveness of lifestyle intervention on the development of T2DM in impaired glucose tolerance (IGT) participants, in particular the effects between those with baseline HbA1c levels ≥5.7% and <5.7%.
Methods
Study design
The study design, protocol, recruitment and interim results have been described in detail previously.9 10 This study was an unmasked, multicenter, randomized controlled trial. The randomized, parallel group trial took place at 32 public health centers in Japan. Participants with IGT were randomly assigned to either an intensive lifestyle intervention group (ILG) or a usual care group (UCG). The participants were followed up for T2DM. The follow up of the participants started in April 1999 and the last case completed in April 2008.
Participants
Participants with IGT, aged 30–60 years, were recruited through health checkups conducted at each collaborative center. The recruitment started in March 1999 and was completed in December 2002. A two-step strategy was adopted for identifying participants with IGT as described previously.9 The definition of IGT using 75 g oral glucose tolerance test (OGTT) was based on the WHO’s criteria.11 Those with (1) a previous diagnosis of any type of DM other than gestational diabetes; (2) a history of gastrectomy; (3) a physical condition such as ischemic heart disease, heart failure, exercise-induced asthma, and orthopedic problems where exercise was not allowed by a doctor; (4) definitive liver and kidney diseases; (5) autoimmune diseases; and (6) a habit of drinking heavily (69 g or more of ethanol per day12) were excluded.
Randomization
Participants were randomly allocated (allocation ratio 1:1) to the ILG or the UCG, using a computer-generated randomization. The Taves method of minimization13 was used to ensure that the groups were balanced for public health centers, gender, age groups (30–39/40–44/45–49/50–54/55–60 years), the body mass index (BMI) levels (<19.8/19.8–24.1/24.2–26.3/≥26.4 kg/m2) and FPG (<100/100–109/110–125 mg/dL).
Sample size
According to prospective studies on the Japanese population, the yearly incidence of T2DM among participants with IGT varies between 1% and 5%.14–16 Therefore, it was assumed that the 6-year cumulative incidence of T2DM would be 30% in the control group. The present study was designed to detect a 50% reduction in the incidence by the intervention. Thus the sample size required was 313 with a type 1 error of 5%, with 80% power (β=20%) at the two-tailed 5% significance level, and allowing for a withdrawal rate of 30%.
Intervention
The goals of intervention were: (1) to reduce initial body weight by 5% in overweight and obese participants and (2) to increase energy expenditure due to leisure time physical activity by 700 kcal/week. The participants in the ILG received four group sessions on a healthy lifestyle for the prevention of T2DM during the initial 6 months, and an individual session was further conducted biannually during the 3 years. The interventions were carried out by the study nurse in each collaborative center in the form of group as well as individual sessions, using the guidelines, curriculum and educational materials provided by the committee of the study group. The session was conducted based on theoretical concepts and techniques for behavioral change, such as self-efficacy, self-monitoring and the transtheoretical model.17
The participants in the UCG received only one group session on a healthy lifestyle for the prevention of T2DM at the outset. No individual guidance was given during the study period. However, the UCG group received anthropometric and blood examinations regularly during the study as did the ILG group.
Outcome measures
The primary endpoint was the development of T2DM, diagnosed and confirmed by two consecutive 75 g OGTTs. Plasma glucose was measured by a glucose oxidase method. The diagnosis of T2DM was based on the WHO’s criteria.11
Measurements
Height and weight were measured with the participants wearing light clothes without shoes, and BMI was calculated as weight (kg)/height2 (m2). Plasma glucose, serum insulin, and HbA1c were measured at a central laboratory (SRL Inc, Tokyo, Japan). HbA1c levels were measured using an automatic high-performance liquid chromatography device.18 19 The area under the curve (AUC) was calculated using FPG and 2 h plasma glucose during OGTT. Pancreatic β-cell function was assessed using the homeostasis model assessment (HOMA-β).20
Blinding
The result of the randomization was unmasked to the participants, those administering the interventions and those assessing the data.
Statistical analyses
All data are presented as the mean±SD. Comparisons between the groups were made with a two-tailed unpaired t test or the χ2 test when applicable. Survival curves were calculated to estimate the cumulative incidence of T2DM. Groups were compared using time-to-event (Kaplan-Meier) curves and statistically evaluated using the log-rank and Breslow (generalized Wilcoxon) test on the intention-to-treat population. The groups based on HbA1c (≥5.7% and <5.7%) were compared in the subgroup analyses. A p value less than 0.05 was considered statistically significant. The statistical analysis was performed using the SPSS program (IBM SPSS Statistics V.20.0).
Results
Main findings
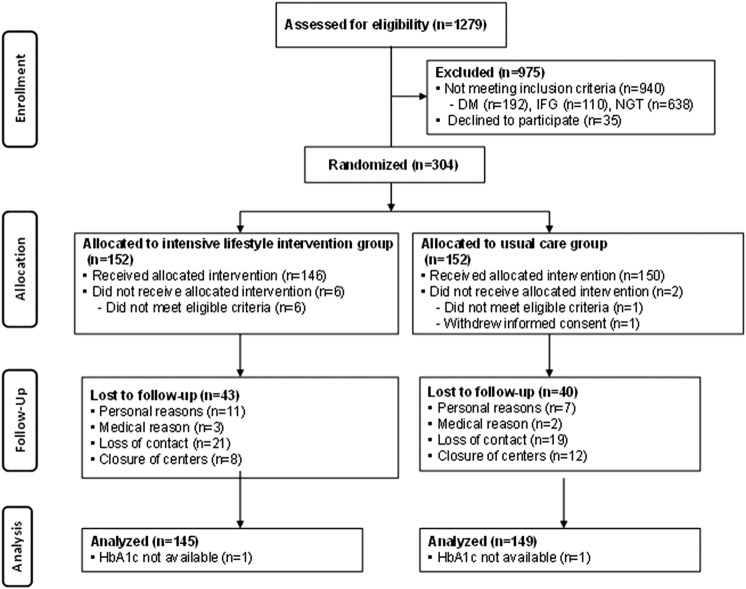
Figure 1 shows a flow diagram for recruiting study participants. The mean follow-up was 2.3 years. Dropout rates during the 3 years were 28% (83 participants). A total of 27 participants were diagnosed with T2DM; 9 in the ILG and 18 in the UCG. The incidence of T2DM was 2.7 and 5.1/100 person-years of follow-up in the ILG and UCG, respectively. For all participants, the ILG tended to show a low cumulative incidence of T2DM compared with the UCG (log-rank=2.70, p=0.10; Breslow=2.98, p=0.08; Tarone-Ware=2.85, p=0.09). There was no significant difference in HbA1c levels at baseline between the groups (table 1). The cumulative incidence of T2DM was significantly lower in the ILG than UCG among participants with baseline HbA1c levels ≥5.7% (log-rank=3.52, p=0.06; Breslow=4.05, p=0.04; Tarone-Ware=3.79, p=0.05), while this was not found among participants with baseline HbA1c levels <5.7% (log-rank=0.31, p=0.58; Breslow=0.56, p=0.46; Tarone-Ware=0.43, p=0.51; figure 2).
Figure 1.
CONSORT flow diagram of Japan Diabetes Prevention Program (JDPP). DM, diabetes mellitus; HbA1c, glycated hemoglobin; IFG, impaired fasting glucose; NGT, normal glucose tolerance.
Table 1.
Baseline parameters according to the group and clinical categories of HbA1c
| Parameters | HbA1c categories |
p Value (HbA1c <5.7% vs ≥5.7%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HbA1c<5.7% |
HbA1c≥5.7% |
||||||||
| All (n=117) | ILG (n=61) | UCG (n=56) | p Value | All (n=177) | ILG (n=84) | UCG (n=93) | p Value | ||
| Age (years) | 50.1±6.8 | 50.0±6.9 | 50.1±6.8 | 0.94 | 51.9±5.8 | 51.9±6.2 | 52.0±5.6 | 0.89 | 0.02 |
| Male (%) | 59.0 | 59.0 | 58.9 | 0.99 | 44.6 | 44.0 | 45.2 | 0.88 | 0.02 |
| Body weight (kg) | 64.6±12.4 | 65.6±12.5 | 63.5±1.6 | 0.36 | 64.4±12.2 | 64.3±12.8 | 64.4±11.6 | 0.95 | 0.89 |
| BMI (kg/m2) | 24.3±3.7 | 24.5±3.5 | 24.1±3.8 | 0.63 | 24.9±3.4 | 24.8±3.4 | 24.9±3.0 | 0.96 | 0.19 |
| Fasting plasma glucose (mmol/L) | 5.8±0.5 | 5.8±0.5 | 5.8±0.6 | 0.66 | 6.1±0.5 | 6.0±0.5 | 6.1±0.5 | 0.06 | <0.01 |
| 2 h plasma glucose (mmol/L) | 8.9±0.8 | 9.0±0.7 | 8.9±0.9 | 0.49 | 9.1±0.9 | 9.2±0.9 | 9.1±0.9 | 0.24 | 0.03 |
| HOMA-β | 68.7±37.3 | 69.7±35.7 | 67.7±39.2 | 0.77 | 56.9±26.3 | 57.8±27.3 | 56.1±25.5 | 0.67 | <0.01 |
| HbA1c (%) | 5.4±0.2 | 5.4±0.2 | 5.4±0.2 | 0.14 | 6.0±0.2 | 6.0±0.2 | 6.0±0.3 | 0.98 | <0.01 |
Data were means±SD.
BMI, body mass index; HbA1c, glycated hemoglobin; HOMA-β, homeostatic model assessment β-cell function; ILG, intensive lifestyle intervention group; UCG, usual care group.
Figure 2.
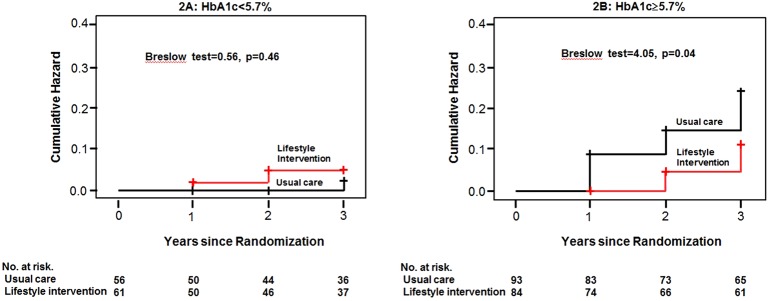
Kaplan-Meier plot of development of diabetes according to glycated hemoglobin (HbA1c) categories.
The mean age, FPG, 2 h plasma glucose and HbA1c levels at the baseline in all those in the HbA1c ≥5.7% group were significantly higher than in the HbA1c <5.7% group, while HOMA-β in all those in the HbA1c ≥5.7% group was significantly lower than in the HbA1c <5.7% group (table 1). There was no difference in the body weight or BMI between the groups. After the 1-year intervention, weight loss in ILG tended to be greater than UCG in both categories of HbA1c. Also, 2 h plasma glucose and the glucose AUC of ILG with HbA1c≥5.7% were significantly decreased compared with those of UCG (table 2). There was no difference in changes of FPG and HbA1c levels between the groups.
Table 2.
Changes in parameters according to the group and clinical categories of HbA1c
| Parameters | Categories of HbA1c |
|||||||
|---|---|---|---|---|---|---|---|---|
| HbA1c<5.7% |
HbA1c≥5.7% |
|||||||
| ILG (n=61) |
UCG (m=56) |
ILG (n=84) |
UCG (n=93) |
|||||
| 1 year | End of trial | 1 year | End of trial | 1 year | End of trial | 1 year | End of trial | |
| Weight (kg) | −1.4±2.2 | −1.1±3.3 | −0.5±2.4 | −0.6±2.8 | −1.7±2.7 | −1.3±2.7 | −0.9±2.5 | −0.7±2.6 |
| Fasting plasma glucose (mmol/L) | −0.1±0.5 | −0.1±0.5 | −0.0±0.5 | −0.1±0.6 | −0.1±0.5 | 0.20±0.7 | −0.1±0.6 | 0.2±1.1 |
| 2 h plasma glucose (mmol/L) | −1.2±1.9 | −1.2±1.9 | −1.3±1.6 | −1.2±2.0 | −1.1±2.1* | −0.17±2.5 | −0.4±2.0 | 0.1±2.8 |
| Glucose AUC during OGTT | −87.3±124.3 | −76.2±123.9 | −74.7±100.3 | −78.5±136.4 | −74.9±140.0* | 1.8±176.9 | −28.9±137.7 | 18.3±206.4 |
| HbA1c (%) | 0.09±0.2 | −0.07±0.3 | −0.12±0.1 | −0.00±0.3 | −0.07±0.2 | −0.02±0.3 | −0.01±0.2 | −0.08±0.4 |
Data are mean±SD.
*p<0.05 (vs UCG).
AUC, area under the curve; HbA1c, glycated hemoglobin; ILG, intensive lifestyle intervention group; OGTT, oral glucose tolerance test; UCG, usual care group.
Adverse events
Adverse events occurred in three participants in the ILG and two participants in the UCG. One death due to subarachnoid hemorrhage and one cervical spinal cord injury occurred in the ILG, but these adverse events were unrelated to the study. Other adverse events were non-serious (eg, injury) and also unrelated to the study.
Discussion
Main findings
Of note, the cumulative incidence of T2DM was significantly lower in the ILG than UCG among participants with baseline HbA1c levels ≥5.7%. Elevated HbA1c levels ≥41 mmol/mol (≥5.9%) were associated with substantial reductions in insulin secretion, insulin sensitivity and β-cell dysfunction in Japanese individuals not treated for diabetes.21 Saito et al2,2 reported that in overweight participants with IGT, the HR of developing T2DM was significantly reduced by lifestyle intervention among those with baseline HbA1c levels ≥5.6% (the Japan Diabetes Society method), although no such reduction was observed among those with isolated impaired fasting glucose or baseline HbA1c levels <5.6%. The results of our study seem to be similar to that of the study of overweight participants with IGT.22
A previous study on non-diabetic American adults showed that preventive intervention for participants with an HbA1c cut-off of 5.7% were cost-effective, but the interventions were less cost-effective when lowering the cut-off level.23 While we acknowledge that IGT is a high-risk state for future T2DM,3 4 IGT participants with higher HbA1c levels may be a better target population for preventing T2DM in primary healthcare settings, where funds and human resources are often limited.
An older age, higher FPG and 2 h plasma glucose, and lower HOMA-β levels were noted in participants with HbA1c ≥5.7% compared with those with HbA1c <5.7%. FPG and 2 h plasma glucose were markers associated with the development of diabetes.24 Aging25 and low HOMA-β26 are also risk factors for T2DM. Glucose tolerance test and HOMA-β measurements are unfamiliar in primary healthcare settings, while HbA1c has long been a commonly used marker of glucose metabolism because of its straightforward and stable features. Thus, the use of HbA1c is suitable for primary healthcare settings.
Conclusion
Intensive lifestyle intervention in primary healthcare setting is effective in preventing the development of T2DM in IGT participants with HbA1c levels ≥5.7%, relative to those with HbA1c levels <5.7%.
Acknowledgments
The following individuals are part of the Japan Diabetes Prevention Program (JDPP) research group, besides the authors of this study: Mioko Gomyo (Kobe, Japan). The investigators gratefully acknowledge the commitment and dedication of the following institutions of the study; Otaru City Health Center, Mizusawa Health Center, Funagata Town Health Center, Kasagake Town Health Center, Toyota Kenpo, Rakuwakai Healthcare System, Toyooka City Health Center, Kasai City Health Center, Mitoyo Municipal Eikou Hospital, Kumamoto General Health Center, Kyusyu Health Center, Nakagawa Health Center, Sue Town Health Center, Shime Town Health Center, Kasuya Town Health Center, Sasaguri Town Health Center, Hisayama Health C & C Center, KDD Shinjyuku Health Center, Aichi Health Promotion Center, Ashibetu Health Center, Kanie Town Health Center, Ohara Hospital, Kakogawa City Health Center, Chiba City Health Promotion Center, Inuyamacyuo Hospital, AIR WATER KENPO, Haruhi Town Health Center, OKA KOUKI Health Management Center, Shikatsu Town Health Center, Nisibiwa Town Health Center, Hikami Town Health Center, and Tomari Town Health Center, Japan.
Footnotes
Contributors: HK, the project leader, was involved in all aspects of the study. JS, KTsushita, ST, MT, SK, YS, IK, YK, and SS designed the study and prepared the protocol of intervention. KTsuzaki contributed to the study design and coordination. NS prepared the manuscript. KK and KTakahashi performed the statistical analysis. TU and TY helped to draft the manuscript, and participated in the critical revision of the manuscript and the trial management. All the authors have read and approved the final version of the manuscript.
Funding: The Ministry of Health, Welfare, and Labour of Japan provided funding for the study (H14-41).
Competing interests: None.
Patient consent: Obtained.
Ethics approval: National Hospital Organization Kyoto Medical Center.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Ginter E, Simko V. Type 2 diabetes mellitus, pandemic in 21st century. Adv Exp Med Biol 2012;771:42–50 [DOI] [PubMed] [Google Scholar]
- 2.Johnson M, Jones R, Freeman C, et al. Can diabetes prevention programmes be translated effectively into real-world settings and still deliver improved outcomes? A synthesis of evidence. Diabet Med 2013;30:3–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344: 1343–92 [DOI] [PubMed] [Google Scholar]
- 4.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuroe A, Fukushima M, Usami M, et al. Impaired beta-cell function and insulin sensitivity in Japanese subjects with normal glucose tolerance. Diabetes Res Clin Pract 2003;59: 71–7 [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association. Standards of medical care in diabetes 2010. Diabetes Care 2010;33:S11–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heianza Y, Hara S, Arase Y, et al. HbA1c 5•7–6•4% and impaired fasting plasma glucose for diagnosis of prediabetes and risk of progression to diabetes in Japan (TOPICS 3): a longitudinal cohort study. Lancet 2011;378:147–55 [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association and National Institute of Diabetes, Digestive and Kidney Diseases. The prevention or delay of type 2 diabetes. Diabetes Care 2002;25:742–9 [DOI] [PubMed] [Google Scholar]
- 9.Gomyo M, Sakane N, Kamae I, et al. Effects of sex, age, and BMI on screening tests for impaired glucose tolerance. Diabetes Res Clin Pract 2004;64:129–36 [DOI] [PubMed] [Google Scholar]
- 10.Sakane N, Sato J, Tsushita K, et al. Prevention of type 2 diabetes in a primary healthcare setting: three-year results of lifestyle intervention in Japanese subjects with impaired glucose tolerance. BMC Public Health 2011;11:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alberti KG, Zimmet PZ. Definition diagnosis and classification of diabetes and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539–53 [DOI] [PubMed] [Google Scholar]
- 12.Lin Y, Kikuchi S, Tamakoshi A, et al. Alcohol consumption and mortality among middle-aged and elderly Japanese men and women. Ann Epidemiol 2005;15:590–7 [DOI] [PubMed] [Google Scholar]
- 13.Scott NW, McPherson GC, Ramsay CR, et al. The method of minimization for allocation to clinical trials. Control Clin Trials 2002;23:662–74 [DOI] [PubMed] [Google Scholar]
- 14.Kuzuya T, Nakagawa S, Satoh J, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. Diabetes Res Clin Pract 2002;55:65–85 [DOI] [PubMed] [Google Scholar]
- 15.Ito C. Epidemiological study of diabetes mellitus in the Hiroshima area prevalence of diabetes mellitus and follow-up studies using the glucose tolerance test. Tohoku J Exp Med 1983;141:115–18 [DOI] [PubMed] [Google Scholar]
- 16.Ito C, Maeda R, Nakamura K, et al. Prediction of diabetes mellitus (NIDDM). Diabetes Res Clin Pract 1996;34:S7–11 [PubMed] [Google Scholar]
- 17.Salmela S, Poskiparta M, Kasila K, et al. Transtheoretical model-based dietary interventions in primary care: a review of the evidence in diabetes. Health Educ Res 2009;24:237–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodall I. HbA1c standardisation destination––global IFCC Standardisation. How, why, where and when––a tortuous pathway from kit manufacturers, via inter-laboratory lyophilized and whole blood comparisons to designated national comparison schemes. Clin Biochem Rev 2005;26:5–19 [PMC free article] [PubMed] [Google Scholar]
- 19.Seino Y, Nanjo K, Tajima N, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. Diabetol Int 2010;1:2–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–19 [DOI] [PubMed] [Google Scholar]
- 21.Heianza Y, Arase Y, Fujihara K, et al. High normal HbA(1c) levels were associated with impaired insulin secretion without escalating insulin resistance in Japanese individuals: the Toranomon Hospital Health Management Center Study 8 (TOPICS 8). Diabet Med 2012;29:1285–90 [DOI] [PubMed] [Google Scholar]
- 22.Saito T, Watanabe M, Nishida J, et al. Lifestyle modification and prevention of type 2 diabetes in overweight Japanese with impaired fasting glucose levels: a randomized controlled trial. Arch Intern Med 2011;171:1352–60 [DOI] [PubMed] [Google Scholar]
- 23.Zhuo X, Zhang P, Selvin E, et al. Alternative HbA1c cutoffs to identify high-risk adults for diabetes prevention: a cost-effectiveness perspective. Am J Prev Med 2012;42:374–81 [DOI] [PubMed] [Google Scholar]
- 24.Wang H, Shara NM, Calhoun D, et al. Incidence rates and predictors of diabetes in those with prediabetes: the Strong Heart Study. Diabetes Metab Res Rev 2010;26:378–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waugh N, Scotland G, McNamee P, et al. Screening for type 2 diabetes: literature review and economic modelling. Health Technol Assess 2007;11:1–125 [DOI] [PubMed] [Google Scholar]
- 26.Reaven GM. HOMA-beta in the UKPDS and ADOPT. Is the natural history of type 2 diabetes characterised by a progressive and inexorable loss of insulin secretory function? Maybe? Maybe not? Diab Vasc Dis Res 2009;6:133–8 [DOI] [PubMed] [Google Scholar]