Abstract
Background
Slow spaced eating is associated with improved satiety and gut hormone responses in normal-weight participants. This crossover study compared the effect of slow and rapid eating patterns on hunger, fullness, glucose, insulin, and the appetite-related gut hormones peptide YY (PYY), glucagon-like peptide-1 (GLP-1), and ghrelin in overweight and obese participants with type 2 diabetes mellitus (T2DM).
Methods
20 overweight and obese participants with T2DM on metformin were recruited. A test meal of 300 mL ice-cream was consumed in random order in two different sessions by each participant; meal duration was 5 or 30 min. Fullness and hunger as assessed by visual analog scales (VAS), and glucose, insulin, PYY, GLP-1, and ghrelin were measured at baseline and at 30 min intervals after meal termination for 3 h.
Results
Fullness VAS ratings were significantly higher at the 90’, 120’, 150’, and 180’ time points and hunger ratings were lower at 90’, 150’, and 180’ for the 30 min meal. The area under the curve (AUC) for fullness was higher after the 30 min meal than after the 5 min meal (11 943.7±541.2 vs 10 901.0±568.8 mm min, p=0.003) whereas the hunger AUC was lower (4442.9±328 vs 4966.7±347.5 mm min, p=0.012). There were no differences in glucose, insulin, PYY, GLP-1, and ghrelin responses.
Conclusions
Slow spaced eating increased fullness and decreased hunger ratings in overweight and obese participants with T2DM, without the improvement in gut hormone responses found in normal-weight participants. Slow spaced eating may be a useful prevention strategy, but might also help curb food intake in those already suffering from obesity and diabetes.
Keywords: Gut Peptides, Eating Behavior(s), Satiety
Key messages.
Slow spaced eating does not affect glucagon-like peptide-1 and peptide YY responses in participants with type 2 diabetes mellitus (T2DM) as it does in healthy participants.
Slow spaced eating led to substantial increase in ratings of fullness and decrease in ratings of hunger.
This simple behavioral modification could be a useful tool for limiting caloric intake in an overweight/obese population with T2DM, in which weight control is a key issue.
Introduction
Diabetes is considered a contemporary epidemic, and not without reason. More than 300 million people are estimated to suffer from diabetes globally, with type 2 diabetes mellitus (T2DM) making up about 90% of the cases.1 Insulin resistance as a consequence of obesity, along with impaired insulin production, has traditionally been considered the main pathophysiological feature of T2DM.2 The incretin effect, an important link between gut hormones, appetite, and glycemia, has been shown to be impaired in patients with type 2 diabetes.3
A number of gut hormones including incretins have important physiological roles in the regulation of hunger and satiety. The immediate postprandial state is characterized by a decrease in the concentrations of the orexigenic gut hormone ghrelin, coupled with an increase in anorexigenic peptides, such as cholecystokinin, peptide YY (PYY), and glucagon-like peptide-1 (GLP-1).4 These changes act in concert on the brain to induce an eventual decrease in hunger, increase in satiety, and meal termination. Incretin analogs have beneficial effects on glycemic control and body weight in T2DM.5
Eating quickly is associated with reduced satiety, increased body weight and insulin resistance,6–8 while eating slower has been shown to favor weight loss.9 We recently showed that eating slowly increases the postprandial response of the anorexigenic peptides PYY and GLP-1 in healthy participants.10 The effect of eating rate on hunger, satiety, and the gut peptides PYY, GLP-1, and ghrelin in patients with T2DM has not been studied so far to the best of our knowledge. Given the rates of obesity in this patient population, the insulin resistance which characterizes this condition, and the recent introduction of incretin analogs as therapeutic options for diabetes, the present study aimed at exploring whether slow spaced eating would alter the postprandial perception of hunger and satiety, as well as three gut peptides related to appetite regulation in patients with type 2 diabetes.
Participants and methods
All procedures were compliant with the Declaration of Helsinki and the experimental protocol was approved by the local hospital ethics committee. Twenty overweight and obese patients were recruited from our university hospital diabetes outpatient clinic. All patients gave written informed consent. Inclusion criteria were the presence of metformin-treated T2DM with good glycemic control (glycated hemoglobin <7%) and body mass index (BMI) ≥25 kg/m2. Patients requiring any other medication that could interfere with hunger or fullness or their postprandial glycemic and hormonal responses were excluded.
The study visits were performed in random order and at least 1 week apart from each other. On the day of each visit, all participants arrived at the clinical research facility after a 10 h overnight fast, without having taken their morning doses of metformin. Their weight, height, waist and hip circumference were measured and their BMI and waist-to-hip ratio calculated.
The patients consumed the same standard test meal of 300 mL of ice-cream (675 kcal, 59% of kcal fat, 33% carbohydrates, 8% protein) at different rates. In one session, the meal was weighed and divided into two equal portions of 150 mL, which were consumed 5 min apart, whereas in the other it was divided into seven equal portions of ∼43 mL, given to the participant every 5 min and consumed over 30 min. Participants were specifically instructed to consume each portion in less than 1 min, in order to maintain a uniform rate of meal ingestion. Blood samples for the measurement of glucose, insulin, triglycerides, ghrelin, PYY, and GLP-1 were collected before the meal and at 30 min intervals after the beginning of meal consumption until the end of the session 180 min later. At the same time points, patients were asked to answer the questions “how hungry do you feel?” and “how full do you feel?” using 10 cm horizontal visual analog scales (VAS) with the anchors ‘not at all’ and ‘extremely’.
Analytical methods
Serum glucose was measured with the oxidase–peroxidase method (Zafiropoulos, Athens, Greece). Triglycerides were measured enzymatically on an RA-XT analyzer (Technicon, Dublin, Ireland). Serum insulin was assayed with a commercially available RIA kit (Biosure, Brussels, Belgium; coefficient of variation (CV) 3.3±1.2%). GLP-1 and PYY were measured using established in-house RIAs,10 while plasma total ghrelin was measured using a commercially available ELISA kit (Millipore, Billerica, Massachusetts, USA). The GLP-1 assay detected changes of 7.5 pmol/L, with an intra-assay CV of 6.1%. The PYY assay measured total PYY-like immunoreactivity and detected changes of 2 pmol/L, with an intra-assay CV of 5.8%. The ghrelin assay measured octanoyl and des-octanoyl ghrelin with an intra-assay CV of 5.1%.
Statistical analysis
Results are reported as mean±SEM. All parameters, including hormonal responses and appetite ratings, were compared between the two sessions for each individual time point using two-tailed, paired Student t tests (SPSS V.21, Chicago, Illinois, USA). The overall postprandial responses were assessed for each parameter as area under the curve (AUC) using the trapezoid rule. Postprandial AUC for appetite sensations has been shown to be a strong predictor of ad libitum energy intake.11 Values were compared using two-tailed, paired Student t tests (SPSS V.21, Chicago, Illinois, USA).
Results
Baseline characteristics of the study participants are displayed in table 1 and postprandial responses in table 2. The 20 patients who were recruited had T2DM for approximately 5 years. Sixty-five percent were overweight and 35% obese. There were no significant differences between the two study visits for each specific time point or for the overall postprandial response in glucose, insulin, triglycerides, ghrelin, PYY, or GLP-1, as expressed in terms of AUC (table 2).
Table 1.
Baseline characteristics of the study participants (mean±SEM)
| Overweight/obese participants (n) | 13/7 |
|---|---|
| Age (years) | 62.6 ±1.8 |
| Weight (kg) | 87.0±4.4 |
| Height (cm) | 167.9±2.1 |
| BMI (kg/m2) | 30.6±1.1 |
| Waist circumference (cm) | 106.2±3.6 |
| Hip circumference (cm) | 104.4±3.7 |
| Waist-to-hip ratio | 1.02±0.01 |
| Diabetes duration (years) | 4.7±0.6 |
| HbA1c (%) | 6.75±0.06 |
| Overweight/obese BMI (kg/m2) | 27.5±0.3/36.2 ± 1.5 |
BMI, body mass index; HbA1c, glycated hemoglobin.
Table 2.
Postprandial responses of the study participants (mean±SEM)
| 5 min Meal | 30 min Meal | Difference* | df | t | p Value | |
|---|---|---|---|---|---|---|
| Glucose AUC (mg/dL × min) | 28 687.7±1490.9 | 28 566.8±1557.9 | 120.9±496.4 | 19 | 0.24 | 0.81 |
| Insulin AUC (IU/L × min) | 6793.7±940.1 | 6752.4±1015.2 | 41.3±321.1 | 19 | 0.13 | 0.90 |
| Triglycerides AUC (mg/dL × min) | 24 203.8±2392.2 | 24 468.7±2085.1 | −264.9±860.0 | 19 | −0.31 | 0.76 |
| Ghrelin AUC (pg/mL × min) | 39 275.9±4359.1 | 40 733.8±5191.7 | −1457.9±1395.0 | 19 | −1.05 | 0.31 |
| PYY AUC (pmol/L × min) | 10 570.9±1550.2 | 9740.0±974.8 | 830.9±831.4 | 19 | 0.99 | 0.34 |
| GLP-1 AUC (pmol/L × min) | 7414.0±948.9 | 7159.4±827.6 | 254.6±356.7 | 19 | 0.71 | 0.49 |
| Fullness AUC (mm × min) | 10 901.0±568.8 | 11 943.7±541.2 | −1042.7±301.9 | 19 | −3.45 | 0.003 |
| Hunger AUC (mm × min) | 4966.7±347.5 | 4442.9±328.0 | 523.8±188.2 | 19 | 2.78 | 0.012 |
*Difference between the 5 and 30 min meal.
AUC, area under the curve; df, degrees of freedom; GLP-1, glucagon-like peptide-1; PYY, peptide YY.
Fullness VAS ratings were higher for the 30 min meal at the 90 min time point and remained so until the end of the study session (figure 1A). Differences between the 30 and 5 min meal for each time point were 0.3±2.3 (p=0.9) at 0 min, 0.4±2.9 (p=0.9) at 30 min, 3.6±2.6 (p=0.18) at 60 min, 7±1.9 (p=0.001) at 90 min, 6.8±2.5 (p=0.01) at 120 min, 8.8±2.6 (p=0.003) at 150 min, and 10.3±2.7 mm (p=0.001) at 180 min.
Figure 1.
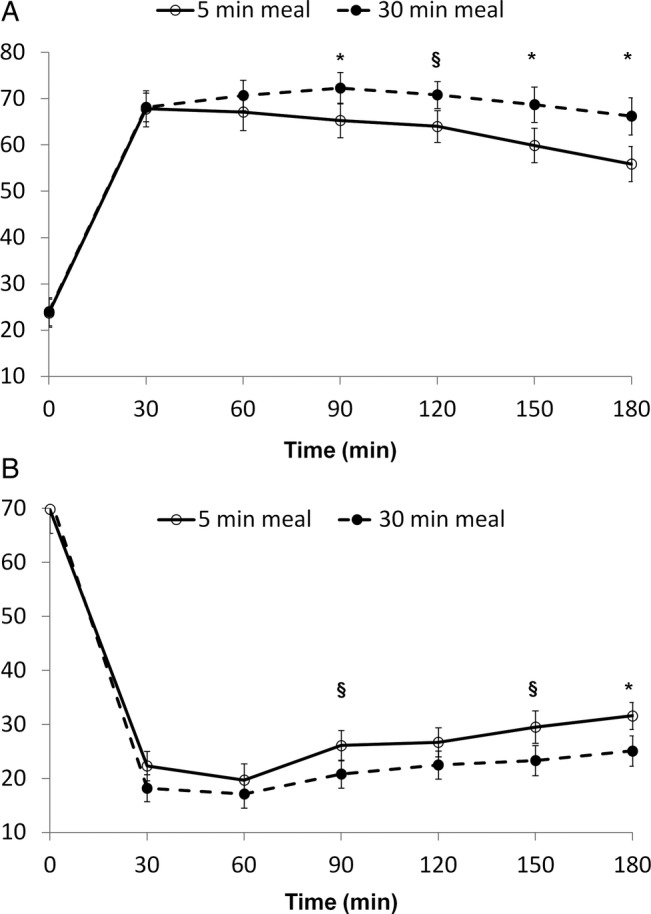
Visual analog scale (VAS) ratings for fullness (A) and hunger (B) after a 5 and 30 min meal (*p<0.01, §p<0.05).
Hunger VAS ratings were lower for the 30 min meal at the 90', 150', and 180' time points (figure 1B). Differences between the 30 and 5 min meal for each time point were 2.5±3.1 (p=0.4) at 0 min, −4.1±2.1 (p=0.07) at 30 min, −2.7±2.6 (p=0.3) at 60 min, −5.3±2.1 (p=0.02) at 90 min, −4.2±2.7 (p=0.14) at 120 min, −6.2±2.5 (p=0.02) at 150 min, and −6.5±2 mm (p=0.004) at 180 min. Furthermore, fullness AUC was significantly higher after the 30 min meal than after the 5 min meal, whereas hunger AUC was significantly lower (table 2).
Discussion
The aim of the present study was to assess the effect of slow spaced eating on hunger and satiety ratings and hormonal mediators in patients with T2DM treated with metformin. The overall postprandial response for insulin, glucose, triglycerides, the orexigenic hormone ghrelin, and the anorexigenic peptides GLP-1 and PYY was not significantly different when patients ate a fixed-portion meal at a fast or slow spaced rate.
Ghrelin, a gastric peptide hormone, increases hunger through its action on the arcuate hypothalamic nucleus. Ghrelin concentrations rise in the fasting state and decrease rapidly after food ingestion.12 Young healthy Chinese men showed lower postprandial ghrelin concentrations and lower energy intake when food was chewed 40 instead of 10 times.13 In our study, a similar, but not identical, manipulation of food ingestion did not have the same results. However, in the present study, food was ingested in a semiliquid state without being chewed, and the participants were overweight and obese middle-aged patients with T2DM. These differences could account for the discrepant results.
PYY and GLP-1 are secreted by endocrine L cells in the small bowel. Their plasma concentrations increase postprandially, enhancing the feeling of satiety.4 We showed that eating slower increased postprandial levels of PYY and GLP-1 in healthy volunteers.10 This has been supported by recent findings, especially in lean adults and obese adolescents.9 13 14 We were not able to replicate these findings in the present study of overweight and obese patients with T2DM.
Our results did not come as a surprise, as it has been well established that the incretin effect is blunted in overweight and obese patients with T2DM.3 A recent study also found that obese adults were not able to increase postprandial PYY and GLP-1 responses when they ate slower.14 Our patients were not only overweight and obese but also had diabetes, and this could explain the blunted gut peptide response we observed, confirming results from Rigamonti et al14 in patients with obesity but no diabetes. The different PYY and GLP-1 responses between obese adolescents and adults in the latter study is intriguing; it could be interpreted as a progressive impairment of the effect of slow eating on gut peptides as obesity becomes a chronic condition. On the other hand, Rigamonti et al found no differences in VAS measures between the two eating conditions in their obese adult group, whereas in the present study there was a distinct effect of slow spaced eating, favoring less hunger and more fullness. This discrepancy may be due to the fact that in the study by Rigamonti et al, adult participants were markedly more obese than in the present study (mean BMI 44.1 vs 30.6 kg/m2) and did not have T2DM. Nonetheless, it must be acknowledged that the current study design did not allow us to distinguish between the effects of overweight/obesity and T2DM on hunger and fullness perception or gut peptide responses.
Postprandial levels of insulin, glucose, and triglycerides were not affected by eating rate.10 14 Despite this, a recent study in healthy individuals demonstrated that an increased number of masticatory cycles reduced appetite and increased postprandial responses of gut hormones, glucose, and insulin.6 This was not evident by the AUC for insulin, glucose, and triglycerides in the present study, possibly owing to the diabetic state itself.
The most important finding of the present study is that slow spaced eating, but still at a physiologically moderate pace, resulted in a decrease in hunger and an increase in fullness, as quantified through VAS ratings in patients with T2DM. This could suggest that advising patients with diabetes to pace themselves when eating could be beneficial as far as caloric intake is concerned. Consistent with this is the association between eating slower and lower BMI in patients with T2DM,15 while two other studies have reported an association of thorough mastication with improved glycemic response, decreased postprandial appetite, and even prevention of T2DM occurrence.16 17 It is not clear whether the effect slow spaced eating has on perceived appetite translates into reduced food consumption. A study in healthy lean women showed lower ad libitum energy intake and increased satiety when a meal was completed in 29 vs 8.6 min.18 In a recent study, eating slowly decreased ad libitum food intake in normal weight but not overweight and obese participants, but led to lower hunger ratings in both.19 Whether our findings would translate into less ad libitum food consumption cannot be gleaned from that study, since it assessed hunger for only 60 min and did not include diabetic individuals. Eating quickly has also been associated with obesity and cardiovascular risk factors in healthy and diabetic individuals.20
Appetite is regulated by a close interplay between the digestive tract, adipose tissue, and the brain, in which peripheral signals and numerous neuronal circuits, among them serotoninergic, dopaminergic, endocannabinoid and opioid-related, as well as cognitive and hedonic perceptions, interact.21 The effect of slow eating on perceived hunger is thus more complex than what can be distilled from only measuring three gut hormone responses. The emerging pattern would suggest that the normal physiological responses of gut hormones and appetite control as observed in normal-weight participants can be disturbed by weight gain and is possibly further deranged by T2DM. Thus advising patients to pace themselves when eating could be potentially more helpful in the prevention of obesity in participants with intact physiological responses, rather than for patients with established pathological responses, such as those with obesity and T2DM. Still, eating slower may be a good way of reducing caloric intake during a meal in obese or overweight patients with T2DM, and potentially translate to weight loss and glycemic improvements. Although we found no differences in the postprandial responses for the three hormones we measured, the intervention was not necessarily ineffective, since participants felt fuller and less hungry. Slow spaced eating may have affected other hormonal or neuronal mechanisms that we did not measure, and this could be an interesting area for future study.
To the best of our knowledge, this is the first study aimed to assess the effect of eating rate on hunger and fullness as well as appetite-related gut hormones in patients with T2DM. We chose patients on metformin only in order to eliminate the possible confounding effect of drugs interfering with insulin secretion or incretin metabolism.
A limitation of our study is the fact that meal consumption was not continuous, but instead consisted of discreet, differently sized portions consumed at different intervals. Thus, the possibility remains that the differences in fullness and hunger could in fact be the result of a spacing and portion size effect. Still, the method used was designed to maintain a uniform rate of consumption, and has been used for this purpose previously by our group and others.10 14 On the other hand, a self-selected rate would be subject to increased variability and make results more difficult to interpret. Furthermore, the 30 min meal duration almost exactly corresponds to what has been previously described in ad libitum meal studies,18 19 and is also physiologically relevant.
The fact that we only recruited overweight and obese participants could be regarded as another limitation of the study. However, this was a conscious decision, since this group is quite representative of patients in diabetes clinics worldwide.
In conclusion, slow spaced eating increased satiety and suppressed hunger more effectively than rapid eating in overweight and obese patients with T2DM. This was not accompanied by statistically significant differences in GLP-1, PYY, or ghrelin responses. Prevention is certainly better than cure and maybe more emphasis should be placed on eating at a slower pace in normal-weight non-diabetic participants as they potentially stand to gain most. Still, eating slower could possibly help overweight and obese patients with diabetes attenuate their appetite, thus promoting weight loss and glycemic control.
Footnotes
Contributors: TA, AK, CL, NT, and CWlR were involved in study design, data collection, data analysis, data interpretation, and manuscript preparation. KA, ADM, IM, DP, and CP were involved in data collection, sample processing and assay, manuscript editing. NK and SRB were involved in study design and supervision, manuscript editing.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None.
Ethics approval: Laiko General Hospital Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Specific values for the parameters not presented in detail in the manuscript (hormonal and biochemical measurements) are freely available to any interested party on contact with the corresponding author.
References
- 1.Ginter E, Simko V. Type 2 diabetes mellitus, pandemic in 21st century. Adv Exp Med Biol 2012;771:42–50 [DOI] [PubMed] [Google Scholar]
- 2.Sherwin R, Jastreboff AM. Year in diabetes 2012: the diabetes tsunami. J Clin Endocrinol Metab 2012;97:4293–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahrén B. Incretin dysfunction in type 2 diabetes: clinical impact and future perspectives. Diabetes Metab 2013;39:195–201 [DOI] [PubMed] [Google Scholar]
- 4.Field BC, Chaudhri OB, Bloom SR. Bowels control brain: gut hormones and obesity. Nat Rev Endocrinol 2010;6:444–53 [DOI] [PubMed] [Google Scholar]
- 5.Grigoropoulou P, Eleftheriadou I, Zoupas C, et al. Incretin-based therapies for type 2 diabetes mellitus: effects on insulin resistance. Curr Diabetes Rev 2013;9:412–17 [DOI] [PubMed] [Google Scholar]
- 6.Zhu Y, Hsu WH, Hollis JH. Increasing the number of masticatory cycles is associated with reduced appetite and altered postprandial plasma concentrations of gut hormones, insulin and glucose. Br J Nutr 2013;110:384–90 [DOI] [PubMed] [Google Scholar]
- 7.Maruyama K, Sato S, Ohira T, et al. The joint impact on being overweight of self reported behaviours of eating quickly and eating until full: cross sectional survey. BMJ 2008;337:a2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otsuka R, Tamakoshi K, Yatsuya H, et al. Eating fast leads to insulin resistance: findings in middle-aged Japanese men and women. Prev Med 2008;46:154–9 [DOI] [PubMed] [Google Scholar]
- 9.Galhardo J, Hunt LP, Lightman SL, et al. Normalizing eating behavior reduces body weight and improves gastrointestinal hormonal secretion in obese adolescents. J Clin Endocrinol Metab 2012;97:E193–201 [DOI] [PubMed] [Google Scholar]
- 10.Kokkinos A, le Roux CW, Alexiadou K, et al. Eating slowly increases the postprandial response of the anorexigenic gut hormones, peptide YY and glucagon-like peptide-1. J Clin Endocrinol Metab 2010;95:333–7 [DOI] [PubMed] [Google Scholar]
- 11.Drapeau V, Blundell J, Therrien F, et al. Appetite sensations as a marker of overall intake. Br J Nutr 2005;93:273–80 [DOI] [PubMed] [Google Scholar]
- 12.Schellekens H, Dinan TG, Cryan JF. Ghrelin at the interface of obesity and reward. Vitam Horm 2013;91:285–323 [DOI] [PubMed] [Google Scholar]
- 13.Li J, Zhang N, Hu L, et al. Improvement in chewing activity reduces energy intake in one meal and modulates plasma gut hormone concentrations in obese and lean young Chinese men. Am J Clin Nutr 2011;94:709–16 [DOI] [PubMed] [Google Scholar]
- 14.Rigamonti AE, Agosti F, Compri E, et al. Anorexigenic postprandial responses of PYY and GLP1 to slow ice cream consumption: preservation in obese adolescents, but not in obese adults. Eur J Endocrinol 2013;168:429–36 [DOI] [PubMed] [Google Scholar]
- 15.Saito A, Kawai K, Yanagisawa M, et al. Self-reported rate of eating is significantly associated with body mass index in Japanese patients with type 2 diabetes. Japan Diabetes Clinical Data Management Study Group (JDDM26). Appetite 2012;59:252–5 [DOI] [PubMed] [Google Scholar]
- 16.Sonoki K, Iwase M, Takata Y, et al. Effects of thirty-times chewing per bite on secretion of glucagon-like peptide-1 in healthy volunteers and type 2 diabetic patients. Endocr J 2013;60:311–19 [DOI] [PubMed] [Google Scholar]
- 17.Yamazaki T, Yamori M, Asai K, et al. Mastication and risk for diabetes in a Japanese population: a cross-sectional study. PLoS ONE 2013;8:e64113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrade AM, Greene GW, Melanson KJ. Eating slowly led to decreases in energy intake within meals in healthy women. J Am Diet Assoc 2008;108:1186–91 [DOI] [PubMed] [Google Scholar]
- 19.Shah M, Copeland J, Dart L, et al. Slower eating speed lowers energy intake in normal-weight but not overweight/obese subjects. J Acad Nutr Diet 2014;114:393–402 [DOI] [PubMed] [Google Scholar]
- 20.Ohkuma T, Fujii H, Iwase M, et al. Impact of eating rate on obesity and cardiovascular risk factors according to glucose tolerance status: the Fukuoka Diabetes Registry and the Hisayama Study. Diabetologia 2013;56:70–7 [DOI] [PubMed] [Google Scholar]
- 21.Yu JH, Kim MS. Molecular mechanisms of appetite regulation. Diabetes Metab J 2012;36:391–8 [DOI] [PMC free article] [PubMed] [Google Scholar]


