Abstract
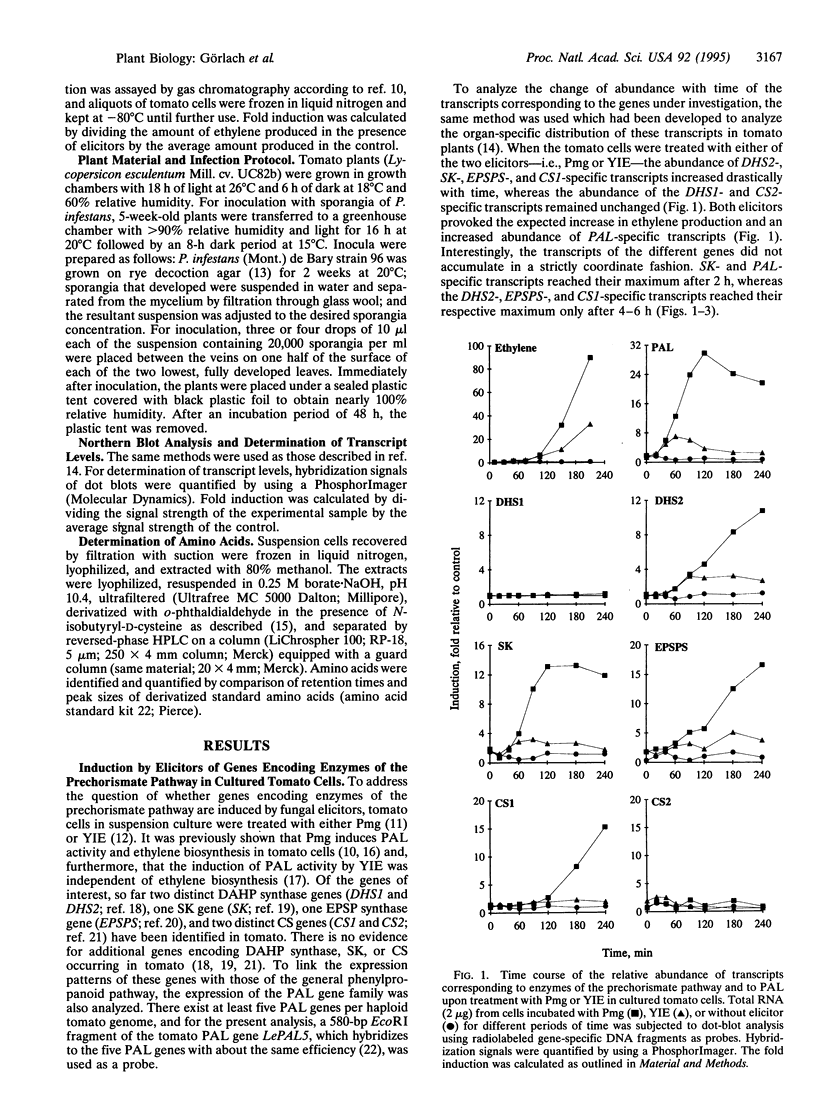
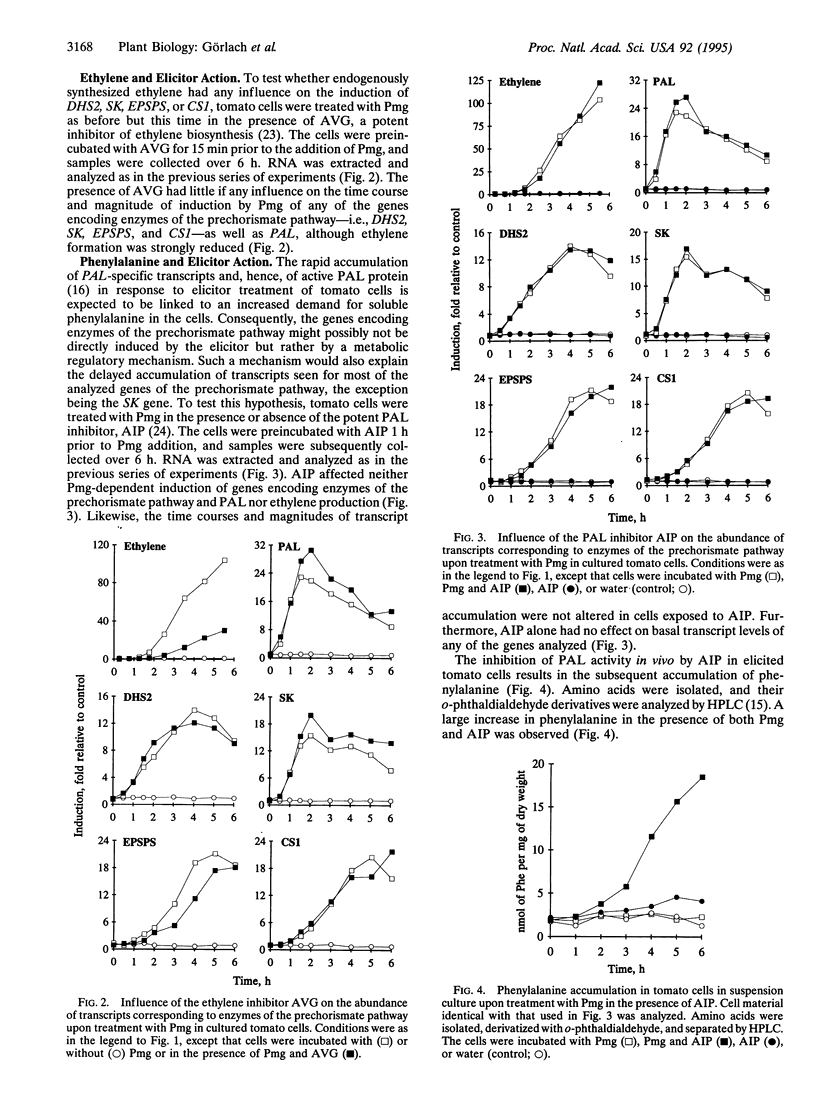
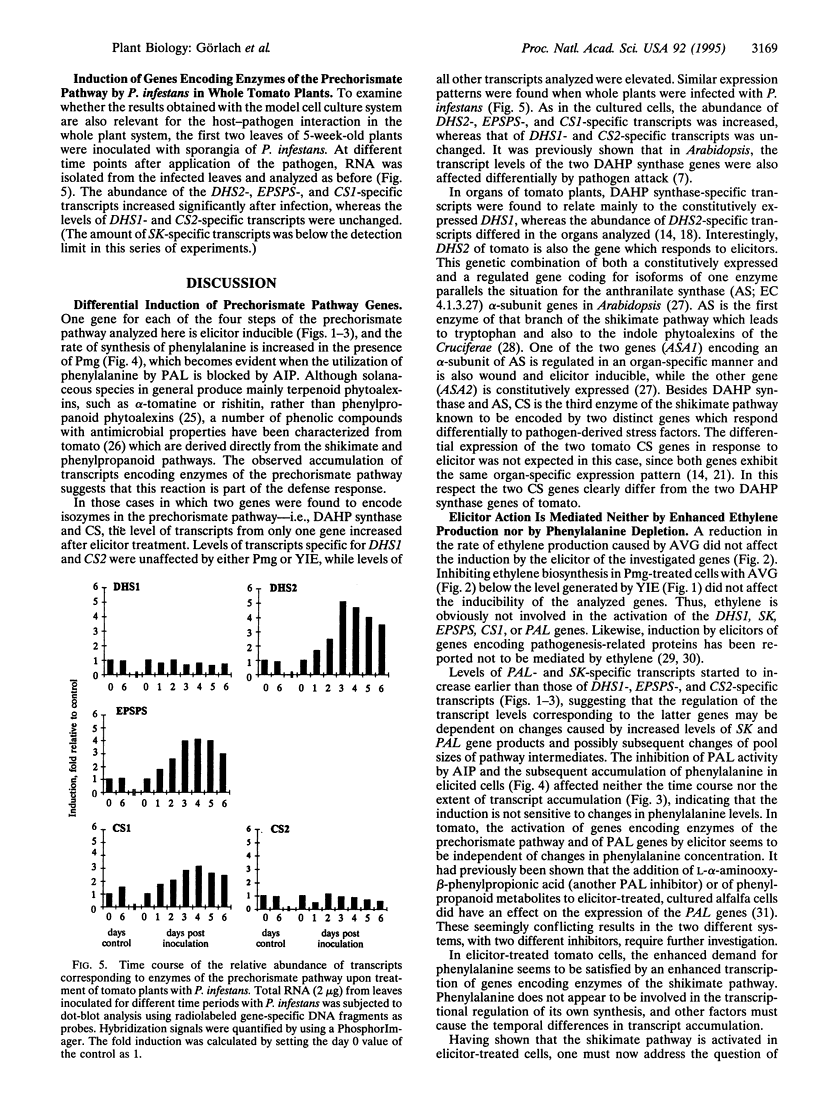
The accumulation of phenylalanine-derived phenolic compounds is a well-known element of a plant's defense in response to pathogen attack. Phenylalanine, as well as the other two aromatic amino acids, tyrosine and tryptophan, is synthesized by way of the shikimate pathway. The first seven steps of the shikimate pathway (the prechorismate pathway) are common for the biosynthesis of all three aromatic amino acids. We have studied transcript levels of six genes--i.e., two 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase genes, one shikimate kinase gene, one 5-enolpyruvylshikimate 3-phosphate synthase gene, and two chorismate synthase genes--corresponding to four steps of the prechorismate pathway, in cultured tomato cells exposed to fungal elicitors. The abundance of transcripts specific for some of these genes increased 10- to 20-fold within 6 h after elicitor treatment, as did the abundance of phenylalanine ammonialyase-specific transcripts and the synthesis of ethylene. Interestingly, transcript accumulation occurred more rapidly for shikimate kinase than for the enzymes preceding or following it in the prechorismate pathway. Neither the inhibition of ethylene biosynthesis by aminoethoxyvinylglycine nor inhibition of phenylalanine ammonia-lyase (EC 4.3.1.5) activity by 2-aminoindan-2-phosphonic acid affected the time course or extent of transcript accumulation. Thus, the increased demand for phenylalanine in the phenylpropanoid pathway required after elicitor treatment appears to be met by increased de novo synthesis of its biosynthetic enzymes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayers A. R., Ebel J., Valent B., Albersheim P. Host-Pathogen Interactions: X. Fractionation and Biological Activity of an Elicitor Isolated from the Mycelial Walls of Phytophthora megasperma var. sojae. Plant Physiol. 1976 May;57(5):760–765. doi: 10.1104/pp.57.5.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basse C. W., Bock K., Boller T. Elicitors and suppressors of the defense response in tomato cells. Purification and characterization of glycopeptide elicitors and glycan suppressors generated by enzymatic cleavage of yeast invertase. J Biol Chem. 1992 May 25;267(15):10258–10265. [PubMed] [Google Scholar]
- Basse C. W., Boller T. Glycopeptide elicitors of stress responses in tomato cells: N-linked glycans are essential for activity but act as suppressors of the same activity when released from the glycopeptides. Plant Physiol. 1992 Apr;98(4):1239–1247. doi: 10.1104/pp.98.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G., Grosskopf D. G., Regenass M., Basse C. W., Boller T. Elicitor-induced ethylene biosynthesis in tomato cells: characterization and use as a bioassay for elicitor action. Plant Physiol. 1991 Sep;97(1):19–25. doi: 10.1104/pp.97.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser C. S., Winter J. A., Hironaka C. M., Shah D. M. Structure, expression, and evolution of the 5-enolpyruvylshikimate-3-phosphate synthase genes of petunia and tomato. J Biol Chem. 1988 Mar 25;263(9):4280–4287. [PubMed] [Google Scholar]
- Görlach J., Beck A., Henstrand J. M., Handa A. K., Herrmann K. M., Schmid J., Amrhein N. Differential expression of tomato (Lycopersicon esculentum L.) genes encoding shikimate pathway isoenzymes. I. 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase. Plant Mol Biol. 1993 Nov;23(4):697–706. doi: 10.1007/BF00021525. [DOI] [PubMed] [Google Scholar]
- Görlach J., Schmid J., Amrhein N. Abundance of transcripts specific for genes encoding enzymes of the prechorismate pathway in different organs of tomato (Lycopersicon esculentum L.) plants. Planta. 1994;193(2):216–223. doi: 10.1007/BF00192533. [DOI] [PubMed] [Google Scholar]
- Görlach J., Schmid J., Amrhein N. Differential expression of tomato (Lycopersicon esculentum L.) genes encoding shikimate pathway isoenzymes. II. Chorismate synthase. Plant Mol Biol. 1993 Nov;23(4):707–716. doi: 10.1007/BF00021526. [DOI] [PubMed] [Google Scholar]
- Henstrand J. M., McCue K. F., Brink K., Handa A. K., Herrmann K. M., Conn E. E. Light and Fungal Elicitor Induce 3-Deoxy-d-arabino-Heptulosonate 7-Phosphate Synthase mRNA in Suspension Cultured Cells of Parsley (Petroselinum crispum L.). Plant Physiol. 1992 Feb;98(2):761–763. doi: 10.1104/pp.98.2.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith B., Dong X. N., Ausubel F. M., Fink G. R. Differential induction of 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase genes in Arabidopsis thaliana by wounding and pathogenic attack. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8821–8825. doi: 10.1073/pnas.88.19.8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb C. J., Lawton M. A., Dron M., Dixon R. A. Signals and transduction mechanisms for activation of plant defenses against microbial attack. Cell. 1989 Jan 27;56(2):215–224. doi: 10.1016/0092-8674(89)90894-5. [DOI] [PubMed] [Google Scholar]
- Lee S. W., Nazar R. N., Powell D. A., Robb J. Reduced PAL gene suppression in Verticillium-infected resistant tomatoes. Plant Mol Biol. 1992 Jan;18(2):345–352. doi: 10.1007/BF00034961. [DOI] [PubMed] [Google Scholar]
- Lotan T., Fluhr R. Xylanase, a novel elicitor of pathogenesis-related proteins in tobacco, uses a non-ethylene pathway for induction. Plant Physiol. 1990 Jun;93(2):811–817. doi: 10.1104/pp.93.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch F., Hadwiger L. A., Boller T. Ethylene: Symptom, Not Signal for the Induction of Chitinase and beta-1,3-Glucanase in Pea Pods by Pathogens and Elicitors. Plant Physiol. 1984 Nov;76(3):607–611. doi: 10.1104/pp.76.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCue K. F., Conn E. E. Induction of 3-deoxy-D-arabino-heptulosonate-7-phosphate synthase activity by fungal elicitor in cultures of Petroselinum crispum. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7374–7377. doi: 10.1073/pnas.86.19.7374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi K. K., Fink G. R. Two anthranilate synthase genes in Arabidopsis: defense-related regulation of the tryptophan pathway. Plant Cell. 1992 Jun;4(6):721–733. doi: 10.1105/tpc.4.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr J. D., Edwards R., Dixon R. A. Stress Responses in Alfalfa (Medicago sativa L.) (XIV. Changes in the Levels of Phenylpropanoid Pathway Intermediates in Relation to Regulation of L-Phenylalanine Ammonia-Lyase in Elicitor-Treated Cell-Suspension Cultures). Plant Physiol. 1993 Mar;101(3):847–856. doi: 10.1104/pp.101.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid J., Schaller A., Leibinger U., Boll W., Amrhein N. The in-vitro synthesized tomato shikimate kinase precursor is enzymatically active and is imported and processed to the mature enzyme by chloroplasts. Plant J. 1992 May;2(3):375–383. [PubMed] [Google Scholar]



