Abstract
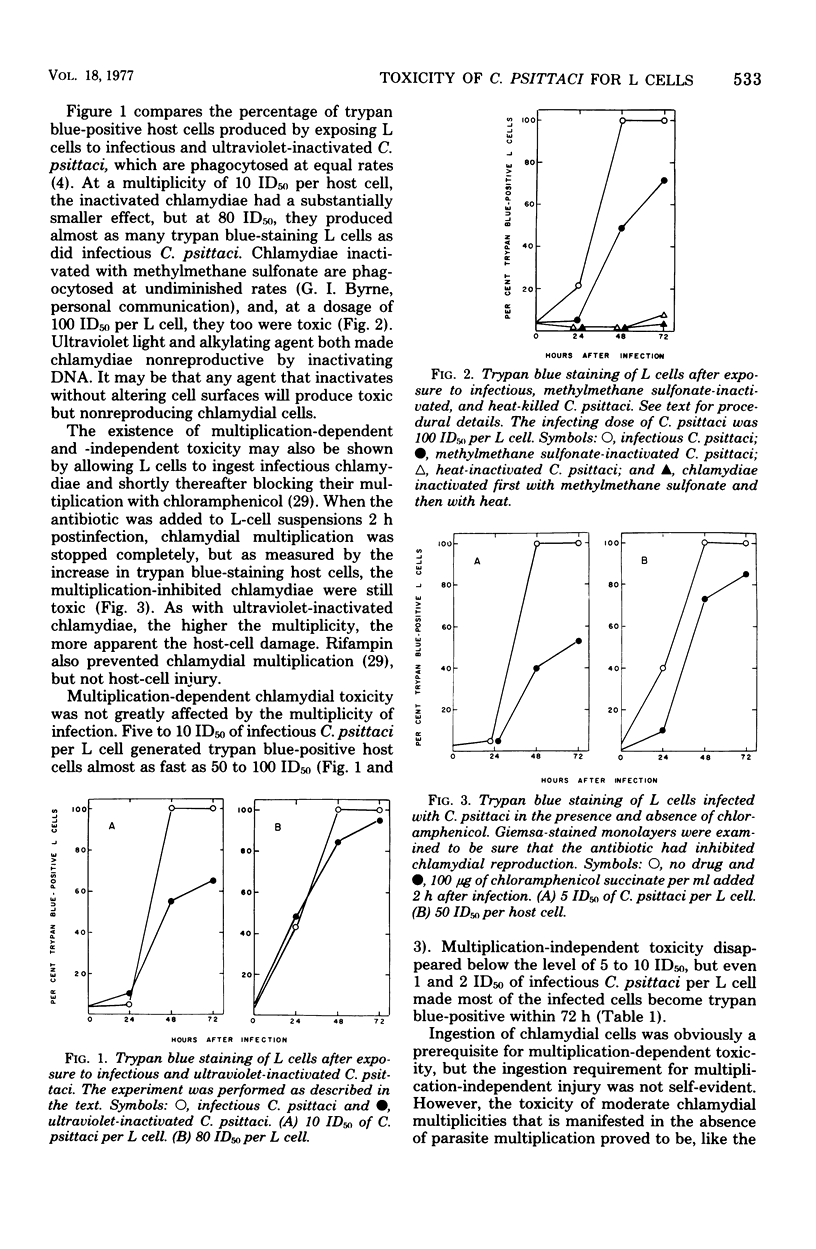
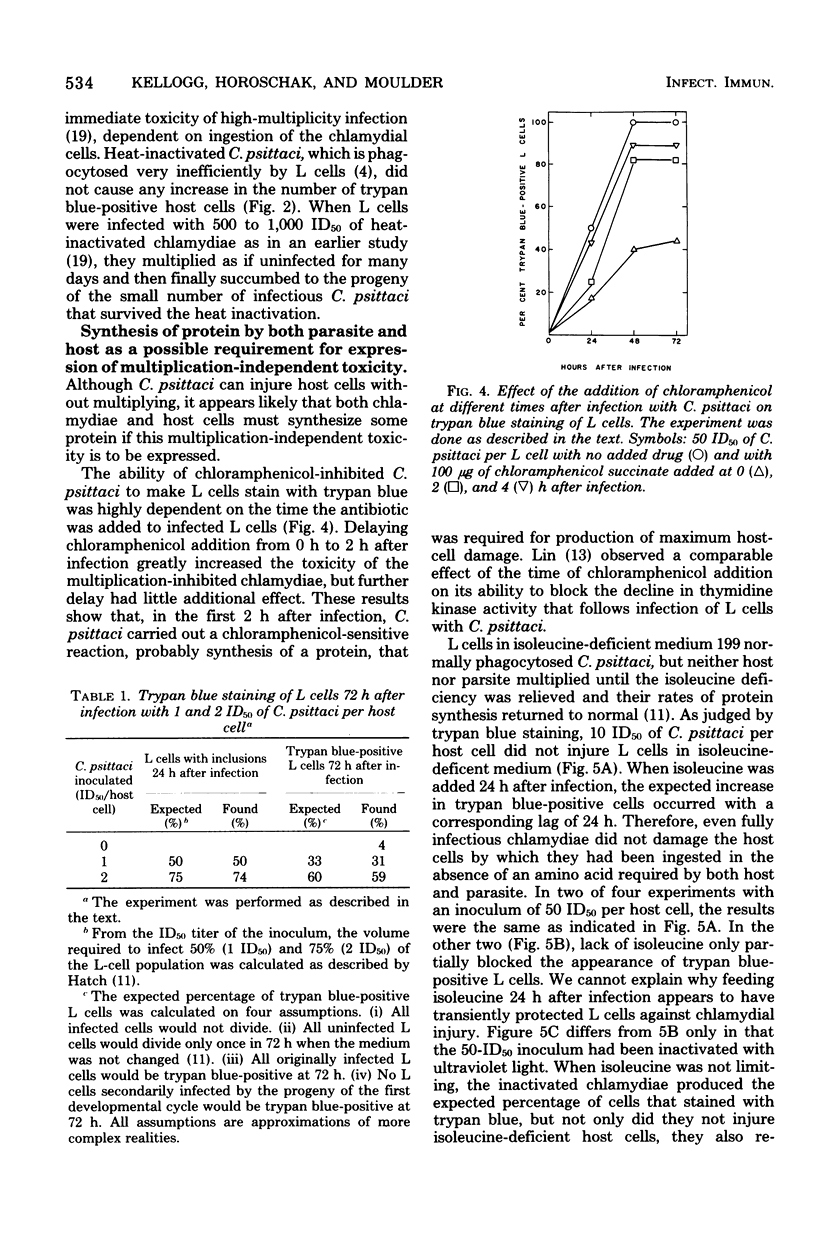
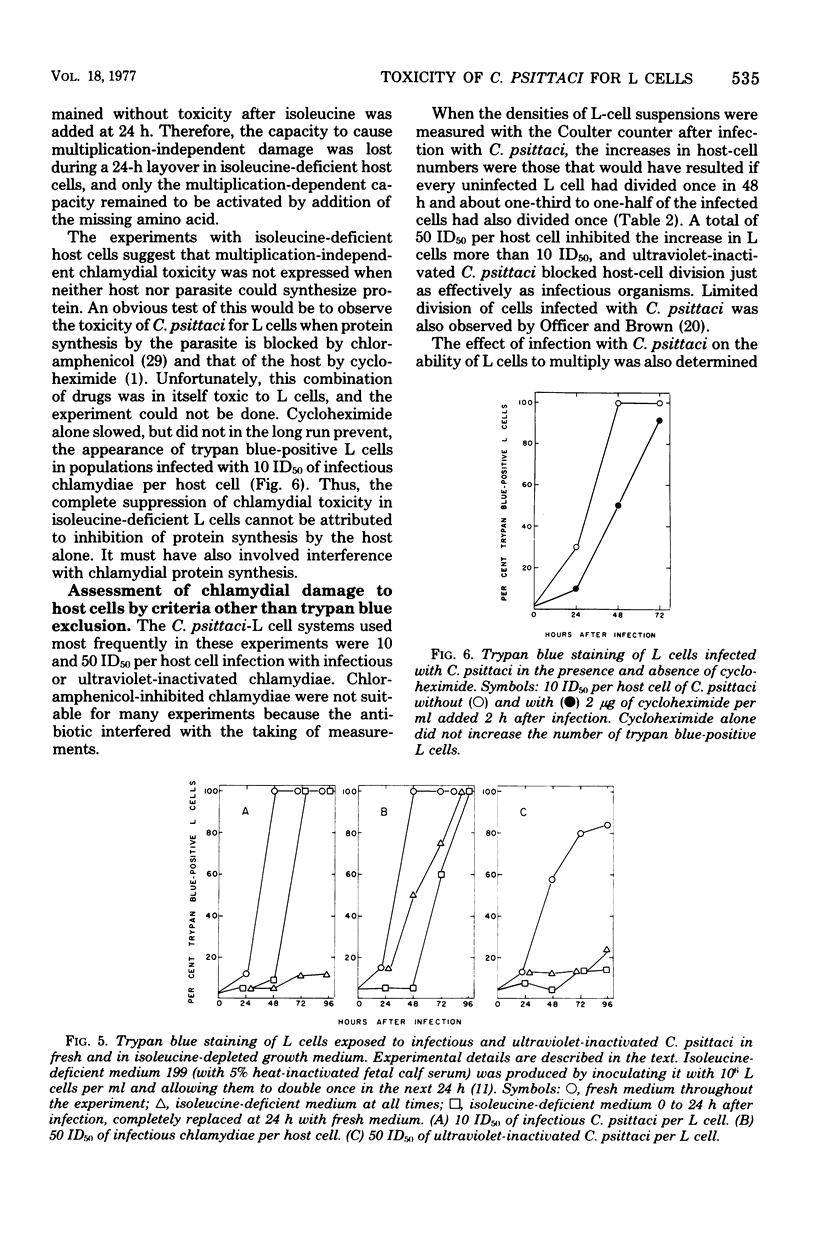
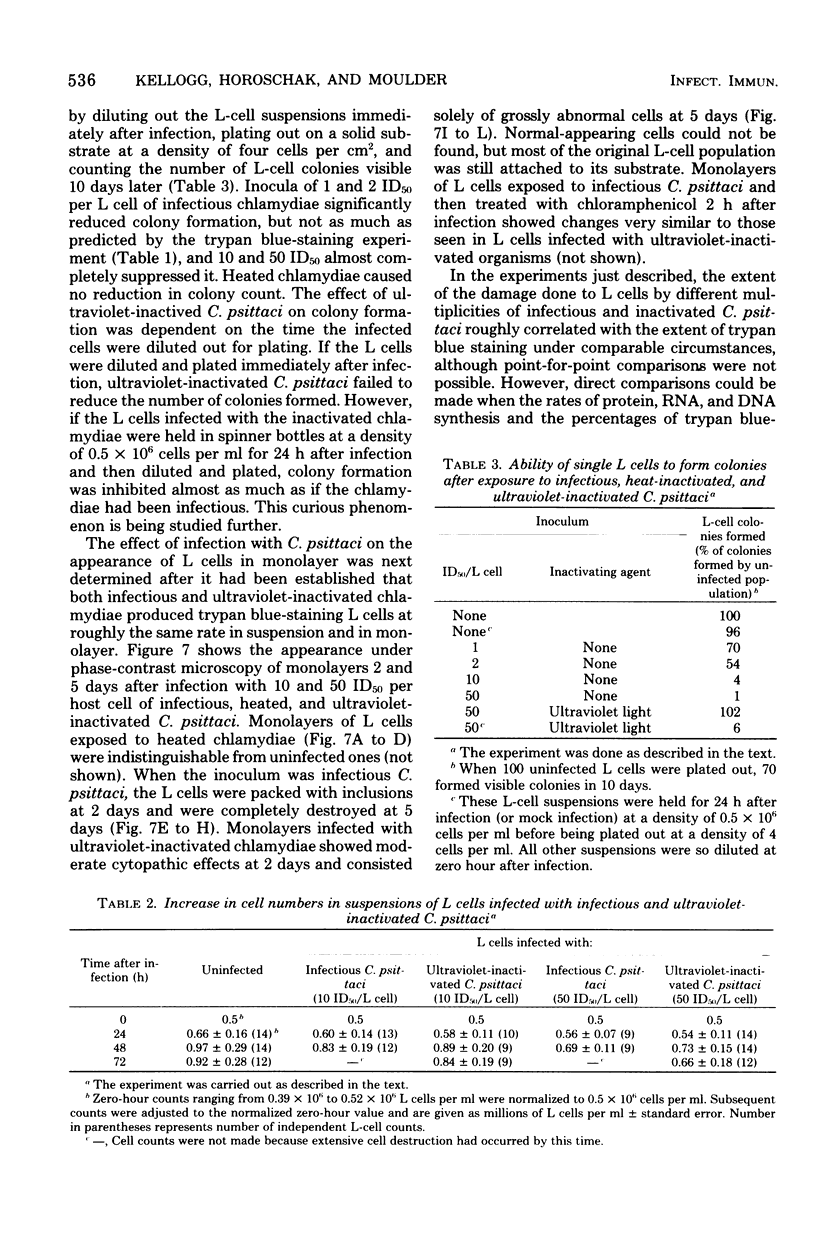
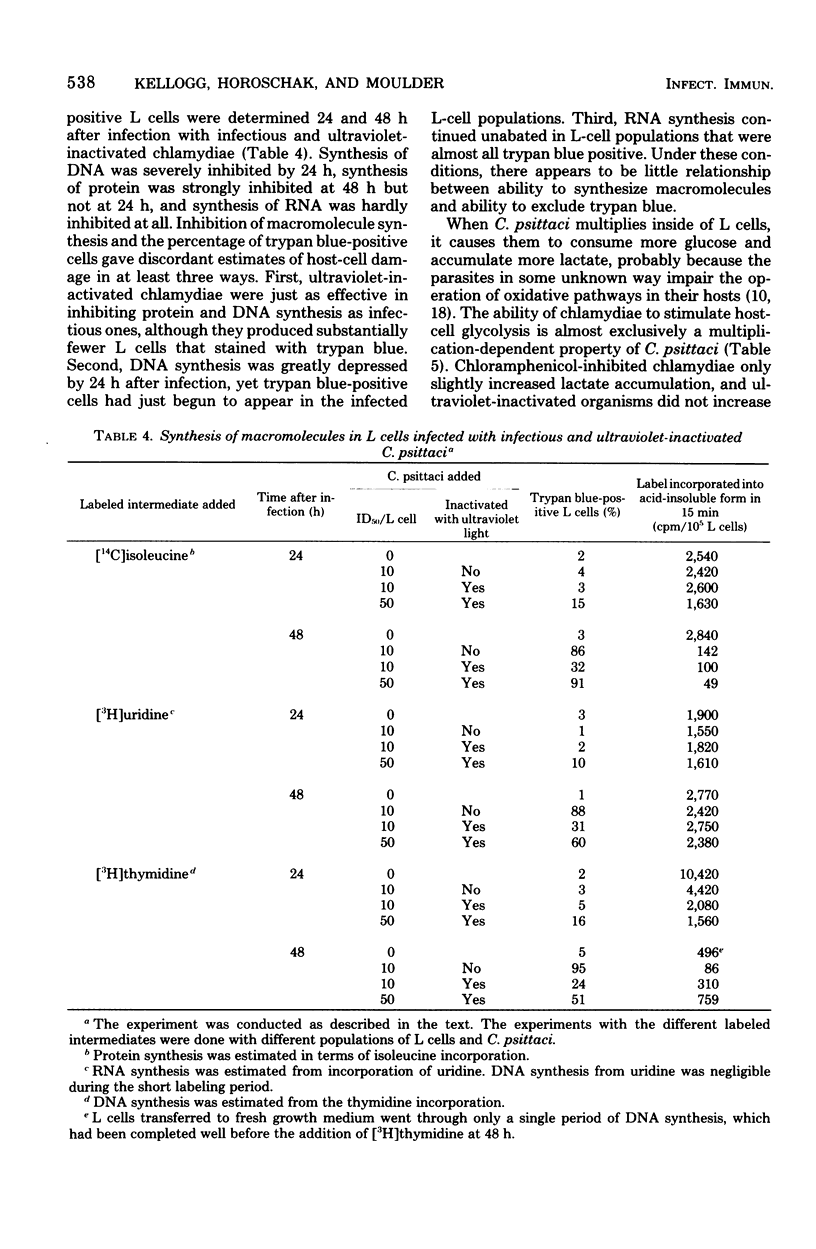
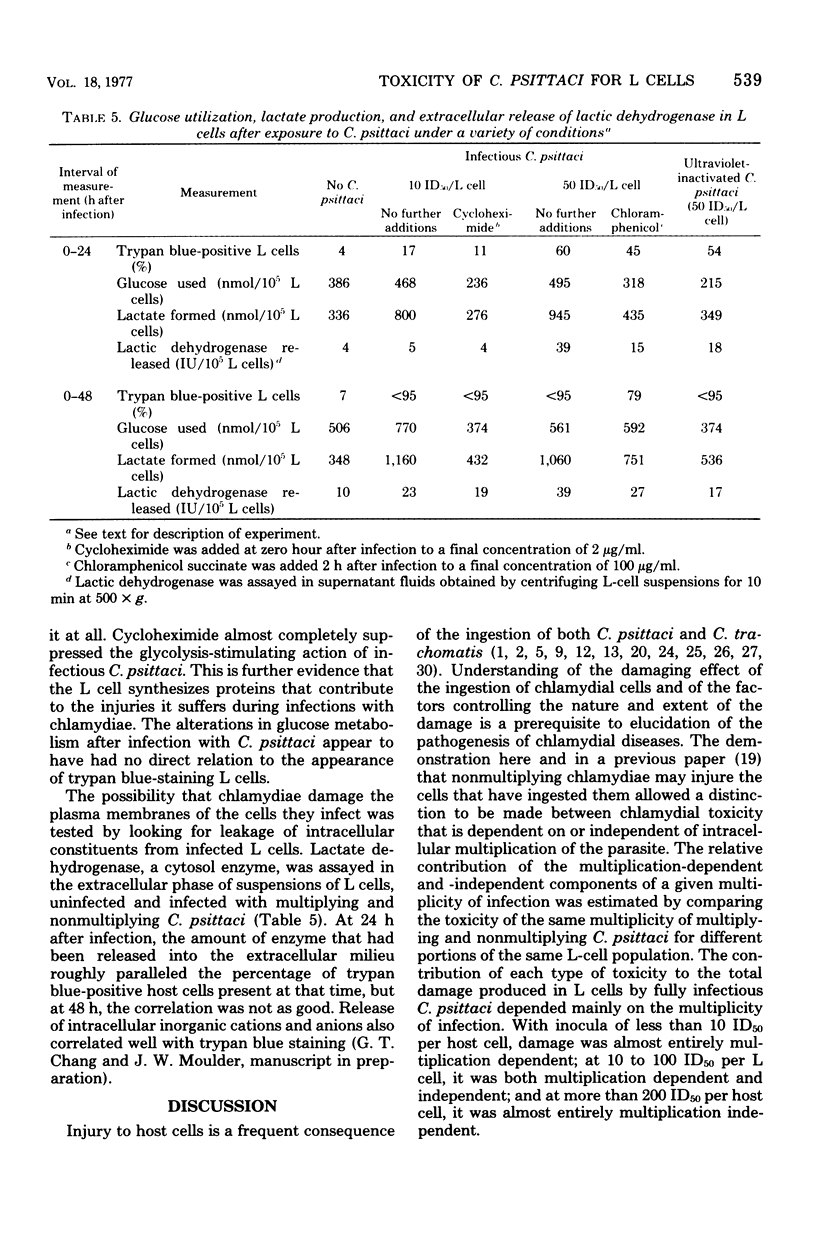
When mouse fibroblasts (L cells) were infected in suspension or in monolayer with 10 to 100 50% infectious doses (ID50) of Chlamydia psittaci (6BC) per host cell, they showed signs of damage 24 to 48 h later. Host-cell injuries were termed multiplication dependent when both the ingestion and subsequent reproduction of C. psittaci were required; when only ingestion but not replication was needed, the injuries were considered to be multiplication independent. The time that the injury was first apparent, as well as its final magnitude, was proportional to the multiplicity of infection. When L cells ingested infectious or ultraviolet-inactivated C. psittaci, damage was manifested by failure to exclude trypan blue, by leakage of lactic dehydrogenase, by inhibition of reproduction as measured by ability to form colonies, by inhibition of protein and deoxyribonucleic acid synthesis, and eventually by cell disintegration. Infectious, but not ultraviolet-killed, chlamydiae stimulated host-cell glycolysis. Heat-killed chlamydiae were without measurable toxicity. The time of appearance of host-cell injury was always earlier, and its terminal magnitude always greater, with infectious inocula than with ultraviolet-inactivated ones. The multiplication-independent toxicity of ultraviolet-killed C. psittaci disappeared with inocula of less than 10 ID50 per L cell, but an inoculum of only a single ID50 of infectious chlamydiae per host cell injured most of the cells it infected, as evidenced by increased trypan blue staining and decreased efficiency of colony formation. The toxicity of multiplicities of infection between 10 and 100 ID50 of infectious C. psittaci per host cell was the sum of both multiplication-dependent and -independent components. The effects of chloramphenicol and isoleucine deficiency on the ability of C. psittaci to injure L cells suggested that some synthesis of protein by both parasite and host may be essential for expression of multiplication-independent chlamydial toxicity. The failure of infectious chlamydiae to stimulate host-cell glycolysis in the presence of cycloheximide suggested that this multiplication-dependent consequence of chlamydial infection was also dependent on protein synthesis by the host.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. J. Effect of infection with the meningopneumonitis agent on deoxyribonucleic acid and protein synthesis by its L-cell host. J Bacteriol. 1969 Feb;97(2):653–657. doi: 10.1128/jb.97.2.653-657.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J. J. Separation of protein synthesis in meningopneumonitisgent from that in L cells by differential susceptibility to cycloheximide. J Bacteriol. 1968 Feb;95(2):327–332. doi: 10.1128/jb.95.2.327-332.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks J., Eddie B., Schachter J., Meyer K. F. Plaque formation by Chlamydia in L cells. Infect Immun. 1970 Mar;1(3):259–262. doi: 10.1128/iai.1.3.259-262.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I. Requirements for ingestion of Chlamydia psittaci by mouse fibroblasts (L cells). Infect Immun. 1976 Sep;14(3):645–651. doi: 10.1128/iai.14.3.645-651.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROCKER T. T., PELC S. R., NIELSEN B. I., EASTWOOD J. M., BANKS J. POPULATION DYNAMICS AND DEOXYRIBONUCLEIC ACID SYNTHESIS IN HELA CELLS INFECTED WITH AN ORNITHOSIS AGENT. J Infect Dis. 1965 Apr;115:105–122. doi: 10.1093/infdis/115.2.105. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- FETERIS W. A. A SERUM GLUCOSE METHOD WITHOUT PROTEIN PRECIPITATION. Am J Med Technol. 1965 Jan-Feb;31:17–21. [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S. D., Stewart R. B. Glucose requirements of L cells infected with Chlamydia psittaci. Can J Microbiol. 1970 Oct;16(10):997–1001. doi: 10.1139/m70-169. [DOI] [PubMed] [Google Scholar]
- Hatch T. P. Competition between Chlamydia psittaci and L cells for host isoleucine pools: a limiting factor in chlamydial multiplication. Infect Immun. 1975 Jul;12(1):211–220. doi: 10.1128/iai.12.1.211-220.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordová N., Wilt J. C. Lysosomes and the "toxicity" of Rickettsiales. I. Cytochemical studies of macrophages inoculated in vitro with C. psittaci 6BC. Can J Microbiol. 1972 Apr;18(4):457–464. doi: 10.1139/m72-071. [DOI] [PubMed] [Google Scholar]
- Lin H. S. Inhibition of thymidine kinase activity and deoxyribonucleic acid synthesis in L cells infected with the meningopneumonitis agent. J Bacteriol. 1968 Dec;96(6):2054–2065. doi: 10.1128/jb.96.6.2054-2065.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIKA L. A., PIRSCH J. B. Replication of variola virus in suspended cultures of mammalian cells. Appl Microbiol. 1961 Nov;9:545–548. doi: 10.1128/am.9.6.545-548.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN J. F., MORTON H. J., PARKER R. C. Nutrition of animal cells in tissue culture; initial studies on a synthetic medium. Proc Soc Exp Biol Med. 1950 Jan;73(1):1–8. doi: 10.3181/00379727-73-17557. [DOI] [PubMed] [Google Scholar]
- Marbach E. P., Weil M. H. Rapid enzymatic measurement of blood lactate and pyruvate. Use and significance of metaphosphoric acid as a common precipitant. Clin Chem. 1967 Apr;13(4):314–325. [PubMed] [Google Scholar]
- McLIMANS W. F., DAVIS E. V., GLOVER F. L., RAKE G. W. The submerged culture of mammalian cells; the spinner culture. J Immunol. 1957 Nov;79(5):428–433. [PubMed] [Google Scholar]
- Moulder J. W. Glucose Metabolism of L Cells Before and After Infection with Chlamydia psittaci. J Bacteriol. 1970 Dec;104(3):1189–1196. doi: 10.1128/jb.104.3.1189-1196.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W., Hatch T. P., Byrne G. I., Kellogg K. R. Immediate toxicity of high multiplicities of Chlamydia psittaci for mouse fibroblasts (L cells). Infect Immun. 1976 Jul;14(1):277–289. doi: 10.1128/iai.14.1.277-289.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OFFICER J. E., BROWN A. Serial changes in virus and cells in cultures chronically infected with psittacosis virus. Virology. 1961 May;14:88–99. doi: 10.1016/0042-6822(61)90136-2. [DOI] [PubMed] [Google Scholar]
- Roper P. R., Drewinko B. Comparison of in vitro methods to determine drug-induced cell lethality. Cancer Res. 1976 Jul;36(7 Pt 1):2182–2188. [PubMed] [Google Scholar]
- Schechter E. M. Synthesis of nucleic acid and protein in L cells infected with the agent of meningopneumonitis. J Bacteriol. 1966 May;91(5):2069–2080. doi: 10.1128/jb.91.5.2069-2080.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes G. V. Formation and destruction of internal membranes in L cells infected with Chlamydia psittaci. Infect Immun. 1973 Feb;7(2):173–177. doi: 10.1128/iai.7.2.173-177.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAMURA A., IWANAGA M. RNA SYNTHESIS IN CELLS INFECTED WITH THE MENINGOPNEUMONITIS AGENT. J Mol Biol. 1965 Jan;11:97–108. doi: 10.1016/s0022-2836(65)80175-9. [DOI] [PubMed] [Google Scholar]
- Taverne J., Blyth W. A., Ballard R. C. Interactions of TRIC agents with macrophages: effects on lysosomal enzymes of the cell. J Hyg (Lond) 1974 Apr;72(2):297–309. doi: 10.1017/s0022172400023512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd W. J., Storz J. Ultrastructural cytochemical evidence for the activation of lysosomes in the cytocidal effect of Chlamydia psittaci. Infect Immun. 1975 Sep;12(3):638–646. doi: 10.1128/iai.12.3.638-646.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribby I. I. Cell Wall Synthesis by Chlamydia psittaci Growing in L Cells. J Bacteriol. 1970 Dec;104(3):1176–1188. doi: 10.1128/jb.104.3.1176-1188.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribby I. I., Friis R. R., Moulder J. W. Effect of chloramphenicol, rifampicin, and nalidixic acid on Chlamydia psittaci growing in L cells. J Infect Dis. 1973 Feb;127(2):155–163. doi: 10.1093/infdis/127.2.155. [DOI] [PubMed] [Google Scholar]
- Tribby I. I., Moulder J. W. Inhibition of Deoxyribonucleic Acid Synthesis in Synchronized Populations of L Cells Infected with Chlamydia psittaci. Infect Immun. 1971 Feb;3(2):363–364. doi: 10.1128/iai.3.2.363-364.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WROBLEWSKI F., LADUE J. S. Lactic dehydrogenase activity in blood. Proc Soc Exp Biol Med. 1955 Oct;90(1):210–213. doi: 10.3181/00379727-90-21985. [DOI] [PubMed] [Google Scholar]
- Warters R. L., Hofer K. G. The in vivo reproductive potential of density separated cells. Exp Cell Res. 1974 Jul;87(1):143–151. doi: 10.1016/0014-4827(74)90536-9. [DOI] [PubMed] [Google Scholar]