Abstract
Background
Hemoglobin A1c (HbA1c) is the standard measure to monitor long-term glucose control in diabetes management and is now used for diagnosis. Fructosamine and glycated albumin are markers of short-term glycemic control that may add complementary information to HbA1c. However, the performance of fructosamine and glycated albumin to identify people at risk of complications is unclear.
Methods
We measured glycated albumin and fructosamine in 11348 adults without diabetes and 958 adults with diagnosed diabetes who attended the second examination of the Atherosclerosis Risk in Communities (ARIC) Study in 1990–1992 (baseline). We evaluated the associations of fructosamine and glycated albumin with retinopathy and risk of incident chronic kidney disease and incident diabetes during two decades of follow-up. We compared these associations to those for HbA1c.
Findings
Fructosamine and glycated albumin were strongly associated with retinopathy and these associations were very similar to that observed for HbA1c. Fructosamine and glycated albumin were also significantly associated with risk of incident chronic kidney disease and incident diabetes. Compared to persons with no diabetes and fructosamine or glycated albumin levels <75th percentile, the multivariable-adjusted hazard ratios (95%CIs) for chronic kidney disease for persons with fructosamine and glycated albumin levels >95th percentile were 1.50 (1.22, 1.85) and 1.48 (1.20, 1.83), respectively. The HRs for incident diabetes were 4.96 (4.36, 5.64) for fructosamine >95th percentile and 6.17 (5.45, 6.99) for glycated albumin >95th percentile. Associations were attenuated but persisted after adjustment for HbA1c. Prediction of incident chronic kidney disease by fructosamine (C-statistic, 0.717) and glycated albumin (C-statistic, 0.717) were nearly as strong as by HbA1c (C-statistic, 0.726) but HbA1c outperformed fructosamine and glycated albumin for prediction of incident diabetes with C-statistics of 0.760, 0.706, and 0.703, respectively.
Interpretation
Fructosamine and glycated albumin were strongly associated with diabetes and its microvascular complications and complemented the prognostic utility of HbA1c.
Hemoglobin A1c (HbA1c) results from the glycation of hemoglobin in erythrocytes and represents long-term (2–3 month) exposure to glucose in the blood. For decades, HbA1c has long been the primary test used to monitor glycemic control and guide treatment of diabetes in clinical practice. In a major change to clinical guidelines in 2010, HbA1c was recommended for use as a diagnostic test for diabetes (1). In addition to its central role in monitoring glycemic control, HbA1c is now widely adopted as the first-line test for screening and diagnosis of diabetes. The epidemiologic evidence supporting current recommendations for use of HbA1c for screening and diagnosis of diabetes comes primarily from studies that have characterized the relationship of HbA1c with microvascular disease, typically prevalent retinopathy (2–6).
Fructosamine and glycated albumin are markers of short-term (2–4 week) glycemic control and may add complementary information to HbA1c for the identification of persons at risk for the development of diabetes and its complications (7–10). Fructosamine and glycated albumin are not affected by erythrocyte or hemoglobin characteristics; they reflect the glycation of serum proteins, which have a faster turnover (~10–14 days) as compared to erythrocytes (~120 days).
Fructosamine is available, but is not routinely used in the U.S. The lack of evidence linking fructosamine to long-term outcomes has been cited as a major barrier to its use and interpretation (11, 12). Glycated albumin is widely used in Japan for monitoring short-term glycemic control in persons with diabetes, but not widely available or used most other countries.
The overarching objective of this study was to characterize the associations of fructosamine and glycated albumin with incident diabetes, retinopathy, and chronic kidney disease in the community-based Atherosclerosis Risk in Communities (ARIC) Study. We evaluated the potential prognostic value of fructosamine and glycated albumin compared to HbA1c for the identification of persons at high risk for diabetes and microvascular conditions.
METHODS
Study Population
The ARIC Study is a community-based prospective cohort of over 15,000 black and white participants sampled from four U.S. communities: Forsyth County, North Carolina (white and black participants), Jackson, Mississippi (black participants), suburban Minneapolis, Minnesota (white participants), and Washington County, Maryland (white participants). The first clinic examinations (visit 1) took place from 1987 to 1989, with three follow-up visits approximately every three years (13). A fifth visit was recently completed (2011–2013). The second clinic examination (visit 2) took place from 1990 to 1992 and is the baseline for the present study. There were 14,348 participants who attended visit 2. Institutional review boards at each clinical site reviewed the study and informed consent was obtained from all participants.
In the present study, we excluded persons who were missing variables of interest and who were fasting less than eight hours. Retinal photographs were not taken at visit 2. Thus, for our analyses of prevalent retinopathy we only included those participants who met our inclusion criteria and who also attended visit 3 at which time the retinal photographs were taken (N=9,445). For analyses of incident chronic kidney disease, we excluded persons with estimated glomerular filtration rate (GFR) <60 ml/min/1.73 m2 at baseline (visit 2), for an analytic study sample of 12,081 persons. In analyses of incident diabetes (new cases occurring after visit 2), we excluded participants with a history of diabetes at baseline, for an analytic sample for this outcome of 10,946 persons. More detailed information regarding response rates and selection of the study participants from the larger ARIC cohort is provided in the Online Supplement (eFigure 1).
Measurement of Fructosamine and Glycated Albumin
Fructosamine (Roche Diagnostics) and glycated albumin (Asashi Kasei Lucica GA-L, Tokyo, Japan) were measured in 2012–2013 from stored serum specimens using a Roche Modular P800 system. The inter-assay coefficients of variation were 3.0% for fructosamine and 1.8% for glycated albumin.
Assessment of Retinopathy
Retinal photographs were taken at visit 3 (1993–1995) following a standardized protocol that has been previously described (14, 15). Briefly, after five minutes of dark adaptation, a nonmydriatic 45-degree retinal photograph centered on the optic disc and macula was taken of one randomly selected eye. Trained graders masked to participant information evaluated each of the photographs. We defined any retinopathy as a severity score ≥20 according to a modification of the Airlie House classification system, as used in the modified Early Treatment Diabetic Retinopathy Study (ETDRS) (14). Level 20 is commonly considered the earliest stage of diabetic retinopathy (4).
Assessment of Incident Chronic Kidney Disease
Among persons with normal kidney function at baseline (visit 2), we defined incident chronic kidney disease as either: 1) GFR < 60 ml/min/1.73m2 estimated using the 2009 CKD-EPI creatinine equation and serum creatinine measured at visit 4 (1996–1998), accompanied by at least a 25% decrease in GFR from baseline (visit 2), or 2) a kidney disease hospitalization or death identified during continuous active surveillance.
Assessment of Incident Diabetes
We identified incident cases of diabetes on the basis of a self-reported diabetes diagnosis or use of diabetes medications during the ARIC visits and subsequent annual telephone calls for a maximum of approximately 20 years of follow-up. In sensitivity analyses, we used two additional definitions of incident diabetes which incorporated glucose measurements from the two subsequent ARIC visits (16).
Other Variables
Serum glucose was measured using the hexokinase method. HbA1c was measured in stored whole blood samples using high-performance liquid chromatography with instruments standardized to the Diabetes Control and Complications Trial assay (Tosoh A1c 2.2 Plus Glycohemoglobin Analyzer and Tosoh G7)(17). Lipid concentrations (18), body mass index (19), and blood pressure (20) were measured using standard methods. Hypertension was defined as the mean of the second and third readings at the visit (with cutoff for systolic blood pressure of 140 mm Hg or higher and a cutoff for diastolic blood pressure of 90 mm Hg or higher) or the use of hypertension medication. Participants reported their education level, alcohol use, and smoking status. The level of physical activity was assessed with Baecke’s questionnaire at visit 1 (21).
Statistical Analyses
Baseline characteristics of the study population were calculated overall and by categories of glycated albumin and fructosamine at baseline. Because there are no established clinical cut-points for fructosamine or glycated albumin, we divided persons without diabetes into three categories defined by the <75th, 75th to 95th, and >95th percentiles. These cut-points were used to approximate the clinical (diagnostic) HbA1c categories of <5.7, 5.7–6.4% and ≥6.5% (22). In persons with diagnosed diabetes at baseline, we categorized the fructosamine and glycated albumin by the <33rd, 33rd to 67th, and >67th percentiles, roughly equivalent to HbA1c categories of <7.0%, 7.0 to 9.0% and >9.0%. In the ARIC population, HbA1c values of 5.7% and 6.5% reflect the 75th and 96th percentiles of the distribution in persons without a diagnosis of diabetes and HbA1c values of 7.0% and 9.0% represent the 37th and 69th percentiles of the distribution of HbA1c in persons with diagnosed diabetes. For comparison, we also conducted all analyses using diabetes-specific quartiles of fructosamine, glycated albumin, and HbA1c.
Adjusted odds ratios (ORs) and their corresponding 95% confidence intervals (CIs) for retinopathy were estimated using multivariable logistic regression models. Model accuracy was assessed with area under the receiver operating characteristic curve (AUC). For consistency with landmark studies of the cross-sectional association of measures of hyperglycemia and prevalent retinopathy (2, 5, 23, 24), we also compared the crude prevalence of retinopathy across deciles of fasting glucose, HbA1c, glycated albumin and fructosamine. For analyses of incident chronic kidney disease and diabetes, adjusted hazard ratios (HRs) and their corresponding 95% CIs were estimated using Cox proportional hazards models. We verified that the proportional hazards assumption was met using log-log plots and by testing for risk factor-by-time interactions. Model discrimination was assessed using Harrell’s C-statistic (25). To evaluate the overall improvement in risk classification for the addition of fructosamine or glycated albumin to adjusted models (including models with HbA1c or fasting glucose), we calculated the net-reclassification improvement (NRI) statistic and the integrated-discrimination-improvement (IDI) statistic (26). To characterize the shape and assess the continuous associations of glycated albumin and fructosamine with each of the clinical outcomes, we fit restricted cubic splines (27).
We constructed three models for each of the outcomes. Model 1 was adjusted for age, sex, race-center (five categories: Minnesota, Maryland, Mississippi, North Carolina white participants, and North Carolina black participants), body mass index, and body mass index squared (to account for the non-linearity in the associations of this variable with the clinical outcomes). Model 2 was adjusted for all variables in Model 1 plus LDL-cholesterol, HDL-cholesterol, triglycerides, waist-to-hip ratio, systolic blood pressure, blood pressure lowering medication use, family history of diabetes, education level, alcohol use, smoking status, and physical activity level. Model 3 was adjusted for all variables in Model 2 plus HbA1c. Model 4 was adjusted for all variables in Model 2 plus fasting glucose. All analyses were conducted using Stata/SE version 13.0 (StataCorp, College Station, Texas).
Role of the Funding Source
The funding source played no role in the design of the study, data collection, analysis, interpretation of data, writing of the report nor in the decision to submit the paper for publication. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
RESULTS
Baseline characteristics of the study population, both overall and according to categories of fructosamine and glycated albumin in persons with and without diagnosed diabetes, are shown in Table 1. Patterns of association with baseline study population characteristics were similar for fructosamine and glycated albumin. Participants with elevated fructosamine or glycated albumin were more likely to be black than white, to have had fewer years of education, and to have hypertension. Body mass index and obesity prevalence were higher at both high and low categories of baseline fructosamine and glycated albumin in persons with and without diagnosed diabetes. The associations with HDL-cholesterol and triglycerides were also non-monotonic. Baseline characteristics of the study population and the risk associations are also shown according to baseline quartiles of glycated albumin and fructosamine (Online Supplement, eTables 1–3). Inferences from these results were similar. Furthermore, in the total population, HbA1c was highly correlated with fructosamine (r=0.82) and glycated albumin (r=0.86). High correlations were also observed for fasting glucose with fructosamine (r=0.81) and glycated albumin (r=0.83) (Online Supplement, eFigure 2).
Table 1.
Study Population Characteristics by Categories of Fructosamine and Glycated Albumin at Baseline in Persons without Diabetes (<75th, 75–95th, >95th percentiles) and in Persons with a History of Diabetes (<33rd, 33rd–67th, >67th percentiles), N=12,306
| No Diagnosed Diabetes
|
Diagnosed Diabetes
|
||||||
|---|---|---|---|---|---|---|---|
| Fructosamine | Total | < 75th percentile | 75th – 95th percentile | > 95th percentile | < 33rd percentile | 33rd – 67th percentile | > 67th percentile |
| Range, [min, max] | [89, 706] | [89, 240] | [241, 264] | [265, 594] | [159, 265] | [266, 355] | [356, 706] |
| Fructosamine, umol/L | 237 | 219 | 249 | 295 | 236 | 305 | 445 |
| Glycated albumin, % | 13.4 | 12.3 | 13.7 | 16.7 | 13.6 | 18.9 | 30.0 |
| Hemoglobin A1c, % | 5.7 | 5.4 | 5.6 | 6.6 | 6.2 | 7.7 | 10.5 |
| Fasting glucose, mg/dL | 112 | 102 | 107 | 139 | 126 | 179 | 269 |
| Age, years | 56.9 | 56.5 | 57.5 | 57.8 | 58.0 | 58.8 | 58.1 |
| Female, % | 56.7 | 56.5 | 56.9 | 57.2 | 60.8 | 49.5 | 60.6 |
| Black, % | 23.1 | 17.4 | 31.3 | 48.1 | 29.1 | 40.1 | 51.4 |
| LDL Cholesterol, mg/dL | 134 | 132 | 135 | 142 | 130 | 136 | 145 |
| HDL Cholesterol, mg/dL | 50.0 | 49.8 | 53.4 | 49.3 | 45.1 | 42.0 | 44.7 |
| Triglycerides, mg/dL | 129 | 128 | 119 | 139 | 153 | 163 | 163 |
| Body mass index, kg/m2 | 27.9 | 27.9 | 26.6 | 28.4 | 30.7 | 31.3 | 30.4 |
| Obese, % | 28.5 | 27.8 | 20.1 | 36.2 | 46.8 | 54.4 | 51.4 |
| Hypertension, % | 34.9 | 30.7 | 35.8 | 54.5 | 51.4 | 63.8 | 60.4 |
| Family history of diabetes, % | 24.2 | 22.0 | 23.7 | 29.5 | 40.5 | 42.8 | 43.2 |
| Education, % | |||||||
| Less than high school | 20.7 | 18.6 | 21.1 | 27.9 | 32.0 | 37.0 | 34.3 |
| High school or equivalent | 42.0 | 43.0 | 40.4 | 36.8 | 39.2 | 37.0 | 42.5 |
| College or above | 37.3 | 38.4 | 38.5 | 35.3 | 28.8 | 26.0 | 23.2 |
| Current alcohol use, % | 57.1 | 62.1 | 50.6 | 43.9 | 45.6 | 31.2 | 29.5 |
| Current smoking status, % | 21.7 | 24.7 | 12.4 | 16.2 | 26.9 | 19.3 | 13.3 |
| Physical activity index | 2.4 | 2.5 | 2.5 | 2.4 | 2.3 | 2.3 | 2.2 |
| No Diagnosed Diabetes
|
Diagnosed Diabetes
|
||||||
|---|---|---|---|---|---|---|---|
| Glycated Albumin | Total | < 75th percentile | 75th – 95th percentile | > 95th percentile | < 33rd percentile | 33rd – 67th percentile | > 67th percentile |
| Range, [min, max] | [5.6, 51.5] | [5.6, 13.5] | [13.6, 15.2] | [15.3, 42.4] | [7.9, 15.6] | [15.7, 23.0] | [23.1, 51.5] |
| Fructosamine, umol/L | 237 | 222 | 242 | 281 | 238 | 306 | 441 |
| Glycated albumin, % | 13.4 | 12.2 | 14.1 | 17.6 | 13.3 | 18.9 | 30.3 |
| Hemoglobin A1c, % | 5.7 | 5.4 | 5.6 | 6.7 | 6.1 | 7.7 | 10.5 |
| Fasting glucose, mg/dL | 112 | 102 | 106 | 141 | 124 | 180 | 269 |
| Age, years | 56.9 | 56.5 | 57.5 | 58.0 | 58.0 | 58.7 | 58.1 |
| Female, % | 56.7 | 54.8 | 62.5 | 60.9 | 58.5 | 51.2 | 61.1 |
| Black, % | 23.1 | 16.0 | 35.6 | 51.3 | 27.2 | 38.7 | 54.7 |
| LDL Cholesterol, mg/dL | 134 | 134 | 131 | 135 | 132 | 134 | 145 |
| HDL Cholesterol, mg/dL | 50.0 | 49.5 | 54.2 | 50.7 | 44.5 | 42.3 | 45.0 |
| Triglycerides, mg/dL | 129 | 131 | 110 | 129 | 153 | 166 | 159 |
| Body mass index, kg/m2 | 27.9 | 27.9 | 26.7 | 29.0 | 30.4 | 31.3 | 30.6 |
| Obese, % | 28.5 | 27.3 | 21.3 | 38.2 | 44.0 | 56.1 | 52.5 |
| Hypertension, % | 34.9 | 31.7 | 33.5 | 47.6 | 50.0 | 65.6 | 59.9 |
| Family history of diabetes, % | 24.2 | 21.5 | 25.5 | 28.3 | 39.9 | 44.2 | 42.4 |
| Education, % | |||||||
| Less than high school | 20.7 | 18.2 | 22.8 | 26.4 | 31.6 | 36.5 | 35.1 |
| High school or equivalent | 42.0 | 43.4 | 39.0 | 36.1 | 39.2 | 37.4 | 42.1 |
| College or above | 37.3 | 38.3 | 38.2 | 37.5 | 29.1 | 26.1 | 22.8 |
| Current alcohol use, % | 57.1 | 62.4 | 50.3 | 40.7 | 44.0 | 33.4 | 28.8 |
| Current smoking status, % | 21.7 | 23.9 | 15.2 | 17.5 | 27.2 | 18.1 | 14.2 |
| Physical activity index | 2.4 | 2.5 | 2.5 | 2.3 | 2.3 | 2.3 | 2.2 |
To convert glucose to mmol/L, multiply by 0.0555.
To convert HDL- and LDL-cholesterol to mmol/L, multiply by 0.0259.
In the 9,445 persons with valid retinal photographs there were 332 persons with retinopathy (ETDRS level 20 or higher). In our multivariable models, fructosamine and glycated albumin were strongly associated with prevalent retinopathy, with ORs >20.0 at high values of each glycemic marker among persons with diagnosed diabetes (Table 2). The patterns of association of fructosamine and glycated albumin with retinopathy were very similar to those observed for baseline HbA1c (Table 2 and eFigure 3). Further adjustment for HbA1c (Model 3) or fasting glucose (Model 4) attenuated, but did not eliminate the observed associations of fructosamine or glycated albumin with retinopathy. Baseline deciles of fructosamine, glycated albumin, and HbA1c were also associated with prevalent retinopathy in a similar manner (eFigure 4).
Table 2.
Associations of Baseline Categories* of Fructosamine and Glycated Albumin with Prevalent Retinopathy (ETDRS Level 20 or Higher) in Persons without Diabetes and in Persons with a History of Diabetes, N=9,445†
| Model 1 OR (95% CI) |
Model 2 OR (95% CI) |
Model 3 OR (95% CI) |
Model 4 OR (95% CI) |
|
|---|---|---|---|---|
| Fructosamine, umol/L | ||||
| No Diabetes | ||||
| < 241 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 241 – 264 | 1.30 (0.90, 1.87) | 1.32 (0.91, 1.91) | 1.25 (0.86, 1.81) | 1.27 (0.87, 1.83) |
| > 264 | 2.00 (1.18, 3.39) | 1.84 (1.08, 3.14) | 1.40 (0.78, 2.49) | 1.51 (0.86, 2.66) |
| History of Diabetes | ||||
| < 266 | 1.42 (0.65, 3.10) | 1.31 (0.60, 2.88) | 1.17 (0.53, 2.58) | 1.20 (0.54, 2.65) |
| 266 – 355 | 13.27 (9.15, 19.23) | 11.51 (7.82, 16.96) | 7.51 (4.63, 12.18) | 8.32 (5.20, 13.31) |
| > 355 | 46.64 (32.58, 66.76) | 41.77 (28.41, 61.41) | 16.70 (8.17, 34.13) | 20.52 (10.37, 40.63) |
| Overall p-for-trend | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
|
| ||||
| Glycated albumin, % | ||||
| No Diabetes | ||||
| < 13.6 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 13.6 – 15.2 | 1.06 (0.72, 1.55) | 1.11 (0.75, 1.63) | 1.04 (0.71, 1.54) | 1.07 (0.73, 1.58) |
| > 15.2 | 1.73 (1.01, 2.99) | 1.67 (0.96, 2.88) | 1.24 (0.68, 2.26) | 1.36 (0.76, 2.43) |
| History of Diabetes | ||||
| < 15.7 | 0.93 (0.37, 2.31) | 0.86 (0.34, 2.14) | 0.76 (0.30, 1.91) | 0.79 (0.31, 1.97) |
| 15.7 – 23.0 | 14.03 (9.72, 20.25) | 12.54 (8.54, 18.41) | 8.19 (5.01, 13.38) | 9.03 (5.63, 14.47) |
| > 23.0 | 43.33 (30.26, 62.03) | 38.64 (26.33, 56.72) | 15.42 (7.26, 32.71) | 19.22 (9.66, 38.24) |
| Overall p-for-trend | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
|
| ||||
| Hemoglobin A1c, % | ||||
| No Diabetes | ||||
| < 5.7 | 1.00 (reference) | 1.00 (reference) | – | 1.00 (reference) |
| 5.7 – 6.4 | 1.29 (0.89, 1.86) | 1.16 (0.80, 1.69) | – | 1.10 (0.76, 1.60) |
| > 6.4 | 1.58 (0.86, 2.91) | 1.32 (0.71, 2.46) | – | 0.89 (0.46, 1.73) |
| History of Diabetes | ||||
| < 7.0 | 1.70 (0.84, 3.41) | 1.48 (0.73, 3.00) | – | 1.20 (0.59, 2.47) |
| 7.0 – 8.9 | 17.76 (12.17, 25.93) | 15.48 (10.33, 23.19) | – | 8.65 (5.23, 14.31) |
| > 8.9 | 39.61 (27.37, 57.32) | 33.83 (22.70, 50.41) | – | 10.76 (5.39, 21.48) |
| Overall p-for-trend | < 0.0001 | < 0.0001 | – | < 0.0001 |
Fructosamine and glycated albumin were divided into categories defined by the <75th, 75–95th, >95th percentiles in persons without diabetes and by the <33rd, 33rd–67th, >67th percentiles in persons with a history of diabetes. The overall p-for-trend was obtained by modeling the category median (for all six categories) as a continuous variable.
Baseline study population limited to persons with valid retinal photographs.
Model 1: age (years), race-center, sex (male, female), body mass index (kg/m2), (body mass index)2
Model 2: Variables in Model 1 + LDL-cholesterol (mg/dL), HDL-cholesterol (mg/dL), triglycerides (mg/dL), waist-to-hip ratio, systolic blood pressure (mmHg), blood pressure-lowering medication use (yes, no), family history of diabetes (yes, no), education (less than high school, high school or equivalent, more than high school), drinking status (current, former, never), smoking status (current, former, never), physical activity index
Model 3: Variables in Model 2 + hemoglobin A1c (%)
Model 4: Variables in Model 2 + fasting glucose (mg/dL)
Abbreviations: AUC, area under curve; CI, confidence interval; OR, odds ratio.
In the 12,081 participants with normal kidney function at baseline, there were 1534 incident cases of chronic kidney disease during a median of 18.7 years of follow-up. Baseline categories of both fructosamine and glycated albumin were associated with a significantly increased risk of chronic kidney disease (Table 3). Indeed, persons without a history of diagnosed diabetes but with fructosamine or glycated albumin values above the 95th percentile (fructosamine of 264 umol/L and glycated albumin of 15.2%) had elevated risks of developing chronic kidney disease, even after multivariable adjustment (Model 2): HR 1.50 (95% CI, 1.22, 1.85) for fructosamine and HR 1.48 (95%CI 1.20, 1.83) for glycated albumin compared to persons without diabetes and fructosamine and glycated albumin values <75th percentile. Among persons with diagnosed diabetes, the associations were graded and remained statistically significant even after further adjustment for HbA1c (Model 3) or fasting glucose (Model 4). In general, the associations of fructosamine and glycated albumin with chronic kidney disease were similar to those observed for HbA1c (Figure 1, Panels A-C).
Table 3.
Adjusted Hazard Ratios (95% Confidence Intervals) of Baseline Categories* of Fructosamine and Glycated Albumin with Incident Chronic Kidney Disease† and Incident Diabetes‡
| Model 1 HR (95% CI) |
Model 2 HR (95% CI) |
Model 3 HR (95% CI) |
Model 4 HR (95% CI) |
|
|---|---|---|---|---|
|
Incident Chronic Kidney Disease
|
||||
| Fructosamine, umol/L | ||||
| No Diabetes | ||||
| < 241 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 241 – 264 | 0.94 (0.81, 1.09) | 0.98 (0.85, 1.14) | 0.95 (0.82, 1.10) | 0.98 (0.85, 1.14) |
| > 264 | 1.73 (1.41, 2.13) | 1.50 (1.22, 1.85) | 1.22 (0.97, 1.53) | 1.47 (1.17, 1.83) |
| History of Diabetes | ||||
| < 266 | 1.85 (1.43, 2.39) | 1.54 (1.18, 2.00) | 1.40 (1.08, 1.82) | 1.52 (1.17, 1.98) |
| 266 – 355 | 2.89 (2.35, 3.56) | 2.40 (1.94, 2.97) | 1.74 (1.36, 2.23) | 2.30 (1.79, 2.96) |
| > 355 | 5.73 (4.75, 6.93) | 4.89 (4.02, 5.94) | 2.33 (1.64, 3.32) | 4.45 (3.09, 6.41) |
| Overall p-for-trend | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
|
| ||||
| Glycated albumin, % | ||||
| No Diabetes | ||||
| < 13.6 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 13.6 – 15.2 | 0.92 (0.79, 1.06) | 1.01 (0.87, 1.17) | 0.96 (0.83, 1.12) | 1.01 (0.87, 1.17) |
| > 15.2 | 1.54 (1.25, 1.90) | 1.48 (1.20, 1.83) | 1.20 (0.95, 1.52) | 1.49 (1.19, 1.86) |
| History of Diabetes | ||||
| < 15.7 | 1.74 (1.35, 2.26) | 1.48 (1.14, 1.92) | 1.36 (1.04, 1.77) | 1.48 (1.13, 1.92) |
| 15.7 – 23.0 | 2.88 (2.33, 3.56) | 2.40 (1.93, 2.97) | 1.77 (1.37, 2.29) | 2.40 (1.86, 3.11) |
| > 23.0 | 5.82 (4.82, 7.02) | 5.12 (4.22, 6.23) | 2.59 (1.79, 3.75) | 5.16 (3.58, 7.44) |
| Overall p-for-trend | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
|
| ||||
| Hemoglobin A1c, % | ||||
| No Diabetes | ||||
| < 5.7 | 1.00 (reference) | 1.00 (reference) | – | 1.00 (reference) |
| 5.7 – 6.4 | 1.63 (1.42, 1.86) | 1.37 (1.19, 1.56) | – | 1.36 (1.19, 1.56) |
| > 6.4 | 2.05 (1.64, 2.57) | 1.65 (1.31, 2.08) | – | 1.63 (1.27, 2.09) |
| History of Diabetes | ||||
| < 7.0 | 2.07 (1.62, 2.66) | 1.65 (1.28, 2.12) | – | 1.64 (1.27, 2.11) |
| 7.0 – 8.9 | 3.99 (3.21, 4.96) | 3.15 (2.52, 3.94) | – | 3.08 (2.35, 4.04) |
| > 8.9 | 6.92 (5.69, 8.42) | 5.42 (4.42, 6.64) | – | 5.18 (3.56, 7.54) |
| Overall p-for-trend | < 0.0001 | < 0.0001 | – | < 0.0001 |
|
| ||||
|
Incident Diagnosed Diabetes
|
||||
| Fructosamine, umol/L | ||||
| < 241 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 241 – 264 | 1.81 (1.65, 1.98) | 1.97 (1.80, 2.16) | 1.78 (1.62, 1.95) | 1.74 (1.59, 1.91) |
| > 264 | 5.21 (4.59, 5.92) | 4.96 (4.36, 5.64) | 2.61 (2.24, 3.04) | 2.26 (1.91, 2.67) |
| p-for-trend | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
|
| ||||
| Glycated albumin, % | ||||
| < 13.6 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 13.6 – 15.2 | 1.69 (1.54, 1.86) | 1.92 (1.74, 2.11) | 1.66 (1.51, 1.83) | 1.73 (1.57, 1.90) |
| > 15.2 | 5.59 (4.94, 6.33) | 6.17 (5.45, 6.99) | 3.27 (2.82, 3.79) | 2.97 (2.53, 3.48) |
| Overall p-for-trend | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
|
| ||||
| Hemoglobin A1c, % | ||||
| < 5.7 | 1.00 (reference) | 1.00 (reference) | – | 1.00 (reference) |
| 5.7 – 6.4 | 3.47 (3.18, 3.79) | 3.07 (2.81, 3.35) | – | 2.81 (2.57, 3.07) |
| > 6.4 | 18.30 (16.09, 20.82) | 15.00 (13.16, 17.10) | – | 8.53 (7.32, 9.95) |
| Overall p-for-trend | < 0.0001 | < 0.0001 | – | < 0.0001 |
Fructosamine and glycated albumin were divided into categories defined by the <75th, 75–95th, >95th percentiles in persons without diabetes and by the <33rd, 33rd–67th, >67th percentiles in persons with a history of diabetes. The overall p-for-trend was obtained by modeling the category median (for all six categories) as a continuous variable.
Baseline study population was limited to persons with normal kidney function (estimated glomerular filtration rate ≥60 ml/min/1.73 m2), N=12,081.
Baseline study population was limited to persons without a history of diabetes, N=10,946.
Model 1: age (years), race-center, sex (male, female), body mass index (kg/m2), (body mass index)2
Model 2: Variables in Model 1 + LDL-cholesterol (mg/dL), HDL-cholesterol (mg/dL), triglycerides (mg/dL), waist-to-hip ratio, systolic blood pressure (mmHg), blood pressure-lowering medication use (yes, no), family history of diabetes (yes, no), education (less than high school, high school or equivalent, more than high school), drinking status (current, former, never), smoking status (current, former, never), physical activity index
Model 3: Variables in Model 2 + hemoglobin A1c (%)
Model 4: Variables in Model 2 + fasting glucose (mg/dL)
Abbreviations: CI, confidence interval; HR, hazard ratio
Figure 1. Adjusted Hazard Ratios (95% Confidence Intervals) for baseline Fructosamine, Glycated Albumin, and Hemoglobin A1c with incident Chronic Kidney Disease and Incident Diabetes.
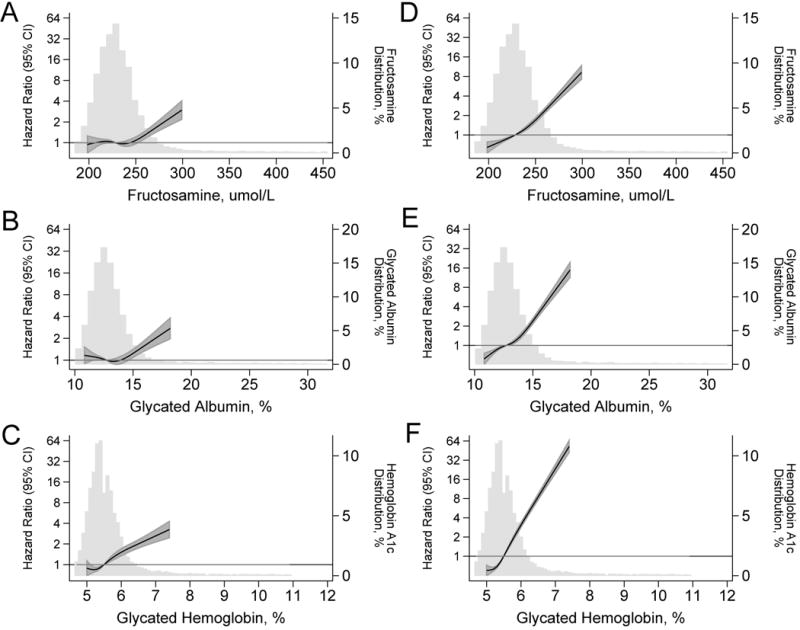
Legend: Adjusted hazard ratios are from Cox proportional hazards models with adjustment for age (years), race-center, sex (male, female), body mass index (kg/m2), (body mass index)2. Baseline fructosamine, glycated albumin, and hemoglobin A1c were modeled using restricted cubic splines (solid lines) with knots at the 5th, 35th, 65th, and 95th percentiles. All models are centered at the 50th percentile (fructosamine: 228 umol/L, glycated albumin: 12.7%, hemoglobin A1c: 5.4%). The shaded areas are the confidence intervals for the restricted cubic spline models. Models are truncated at the 5th and 95th percentile of each marker, histograms are truncated at the 1st and 99th percentile. For incident chronic kidney disease, baseline study population was limited to persons with normal kidney function (estimated glomerular filtration rate ≥60 ml/min/1.73 m2), N=11,932. For incident diabetes, baseline study population was limited to persons without a history of diabetes, N=10,946.
In the 10,946 participants with no history of diagnosed diabetes at baseline, there were 2882 incident cases of diagnosed diabetes during a median of 17.7 years of follow-up. Baseline categories of fructosamine and glycated albumin were strongly associated with subsequent risk of diabetes, even after multivariable adjustment (Table 3, Model 2). These associations remained significant, even after further adjustment for HbA1c (Model 3) or fasting glucose (Model 4). For example, among persons with fructosamine values above the 95th percentile (>264 umol/L), there was a significant association with incident diabetes, even after adjustment for HbA1c at baseline (HR 2.61, 95%CI 2.24, 3.04; Model 3). Among persons with glycated albumin values above the 95th percentile (>15.2%), there was a similarly robust and significant association with diabetes risk, even after adjustment for baseline HbA1c (HR 3.27, 95%CI 2.82, 3.79; Model 3). Nonetheless, the relative associations of HbA1c with incident diabetes were stronger than those observed for fructosamine or glycated albumin (Figure 1, D–F). Results were similar in sensitivity analyses using different definitions of incident diabetes (Online Supplement, eTable 4).
The AUC and C-statistics for model discrimination are provided in Table 4. The overall performances of HbA1c (0.789), fructosamine (0.796), and glycated albumin (0.793) for prediction of prevalent retinopathy were excellent and similar across the different biomarkers. Prediction of incident chronic kidney disease by fructosamine (0.717) and glycated albumin (0.717) were nearly as strong as by HbA1c (0.726) but, as might be expected, HbA1c outperformed fructosamine and glycated albumin for prediction of incident diabetes with C-statistics of 0.760, 0.706, and 0.703, respectively. The NRI and IDI statistics were largely consistent with the AUC and C-statistic results (eTable 5). The NRI results further show that improvements seen by the addition of fructosamine or glycated albumin to the models were primarily driven by the correct reclassification of non-events (downward reclassification).
Table 4.
Prediction Statistics and Differences Between Models
| Prevalent Retinopathy | AUC | Difference in AUC from reference model | p-value for difference | Difference in AUC from model with HbA1c | p-value for difference |
|---|---|---|---|---|---|
| Model 1 | 0.6947 | (ref) | – | – | – |
| Model 1 + HbA1c | 0.7885 | 0.0948 | < 0.0001 | (ref) | – |
| Model 1 + FA | 0.7958 | 0.1021 | < 0.0001 | – | – |
| Model 1 + GA | 0.7931 | 0.0994 | < 0.0001 | – | – |
| Model 1 + HbA1c + FA | 0.7959 | 0.1022 | < 0.0001 | 0.0074 | 0.1755 |
| Model 1 + HbA1c + GA | 0.7938 | 0.1001 | < 0.0001 | 0.0053 | 0.3491 |
| Model 1 + HbA1c + FA + GA | 0.7953 | 0.1016 | < 0.0001 | 0.0068 | 0.2439 |
| Model 2 | 0.7435 | (ref) | – | – | – |
| Model 2 + HbA1c | 0.8048 | 0.0641 | < 0.0001 | (ref) | – |
| Model 2 + FA | 0.8121 | 0.0714 | < 0.0001 | – | – |
| Model 2 + GA | 0.8105 | 0.0698 | < 0.0001 | – | – |
| Model 2 + HbA1c + FA | 0.8116 | 0.0709 | < 0.0001 | 0.0068 | 0.1158 |
| Model 2 + HbA1c + GA | 0.8108 | 0.0701 | < 0.0001 | 0.006 | 0.2041 |
| Model 2 + HbA1c + FA + GA | 0.8118 | 0.0711 | < 0.0001 | 0.007 | 0.1476 |
| Incident Chronic Kidney Disease | C-statistic | Difference in C-statistic from reference model | p-value for difference | Difference in C-statistic from model with HbA1c | p-value for difference |
|---|---|---|---|---|---|
| Model 1 | 0.6937 | (ref) | – | – | – |
| Model 1 + HbA1c | 0.7261 | 0.0324 | < 0.001 | (ref) | – |
| Model 1 + FA | 0.7172 | 0.0235 | < 0.001 | – | – |
| Model 1 + GA | 0.7165 | 0.0228 | < 0.001 | – | – |
| Model 1 + HbA1c + FA | 0.7284 | 0.0347 | < 0.001 | 0.0022 | 0.0504 |
| Model 1 + HbA1c + GA | 0.729 | 0.0353 | < 0.001 | 0.0029 | 0.0098 |
| Model 1 + HbA1c + FA + GA | 0.73 | 0.0363 | < 0.001 | 0.0039 | 0.0057 |
| Model 2 | 0.7407 | (ref) | – | – | – |
| Model 2 + HbA1c | 0.7575 | 0.0168 | < 0.001 | (ref) | – |
| Model 2 + FA | 0.7559 | 0.0152 | < 0.001 | – | – |
| Model 2 + GA | 0.755 | 0.0143 | < 0.001 | – | – |
| Model 2 + HbA1c + FA | 0.7589 | 0.0182 | < 0.001 | 0.0014 | 0.0617 |
| Model 2 + HbA1c + GA | 0.7581 | 0.0174 | < 0.001 | 0.0006 | 0.1428 |
| Model 2 + HbA1c + FA + GA | 0.7589 | 0.0182 | < 0.001 | 0.0014 | 0.0791 |
|
Incident Diabetes
|
|||||
| Model 1 | 0.6751 | (ref) | – | – | – |
| Model 1 + HbA1c | 0.7589 | 0.0838 | < 0.0001 | (ref) | – |
| Model 1 + FA | 0.7064 | 0.0313 | < 0.0001 | – | – |
| Model 1 + GA | 0.703 | 0.0279 | < 0.0001 | – | – |
| Model 1 + HbA1c + FA | 0.7616 | 0.0865 | < 0.0001 | 0.0027 | 0.0216 |
| Model 1 + HbA1c + GA | 0.7589 | 0.0838 | < 0.0001 | 0.0000 | 0.9939 |
| Model 1 + HbA1c + FA + GA | 0.7613 | 0.0862 | < 0.0001 | 0.0024 | 0.0406 |
| Model 2 | 0.7231 | (ref) | – | – | – |
| Model 2 + HbA1c | 0.7793 | 0.0562 | < 0.0001 | (ref) | – |
| Model 2 + FA | 0.7504 | 0.0273 | < 0.0001 | – | – |
| Model 2 + GA | 0.7518 | 0.0287 | < 0.0001 | – | – |
| Model 2 + HbA1c + FA | 0.7829 | 0.0598 | < 0.0001 | 0.0036 | 0.0011 |
| Model 2 + HbA1c + GA | 0.782 | 0.0589 | < 0.0001 | 0.0027 | 0.0075 |
| Model 2 + HbA1c + FA + GA | 0.7834 | 0.0603 | < 0.0001 | 0.0041 | 0.0006 |
Model 1: age (years), race-center, sex (male, female), body mass index (kg/m2), (body mass index)2
Model 2: Variables in Model 1 + LDL-cholesterol (mg/dL), HDL-cholesterol (mg/dL), triglycerides (mg/dL), waist-to-hip ratio, systolic blood pressure (mmHg), blood pressure-lowering medication use (yes, no), family history of diabetes (yes, no), education (less than high school, high school or equivalent, more than high school), drinking status (current, former, never), smoking status (current, former, never), physical activity index.
Abbreviations: FA, fructosamine; GA, glycated albumin; HbA1c, Hemoglobin A1c;
CONCLUSIONS
Our study demonstrated that fructosamine and glycated albumin were independently associated with prevalent retinopathy and the risk of incident chronic kidney disease and diabetes. These associations persisted after adjustment for traditional risk factors and added information after further adjustment for HbA1c or fasting glucose. Our findings suggest that fructosamine and glycated albumin have prognostic utility for the prediction of diabetes, chronic kidney disease, and retinopathy in the community. In general, the associations of glycated albumin and fructosamine with retinopathy and chronic kidney disease were similar to those observed for HbA1c in this study population. These results suggest that these nontraditional markers of hyperglycemia might be useful in clinical practice.
The limitations of HbA1c are widely acknowledged (28) and include its cost, assay interferences such as hemoglobin variants (29), other conditions that affect the validity of test results (e.g. hemolytic anemia, recent transfusion, pregnancy, or blood loss), and that it does not change rapidly in response to changes in treatment (2–3 month measure of glycemic control). The acceptance of new measures of hyperglycemia is dependent on establishing their association with clinical events and prognostic value (30), especially relative to standard biomarkers used to identify persons at risk for diabetes and its complications. Our results suggest that fructosamine and/or glycated albumin testing may add complementary information to HbA1c and may be particularly useful in settings where HbA1c testing is problematic or in research settings where whole blood samples are not available. Fructosamine and glycated albumin may also be useful as adjuncts to standard measures and in situations where short-term (2–4 week) glycemic control is specifically of interest. For example, short-term measures may be important in evaluating the effects of diabetes treatment or changes to a treatment regime shortly after implementation or in resource-intensive trials (such as feeding studies) that are only able to follow participants for a few weeks. Because HbA1c has been shown to underestimate glycemic control in dialysis patients, fructosamine and glycated albumin have been of particular interest and have been shown to be associated with mortality and other important outcomes in this patient population (31, 32). Nonetheless, future studies including randomized clinical trials will be needed to evaluate whether fructosamine or glycated albumin testing in the management of diabetes can improve clinical outcomes.
Fructosamine is formed by the covalent attachment of glucose to serum proteins, primarily albumin. In the present study, we also specifically assayed glycated albumin using a novel enzymatic method (33). Both assays are rapid and not technically challenging. Nonetheless, despite its availability from most major clinical laboratories, fructosamine testing is not widely used in research or clinical practice. The limited adoption of fructosamine may reflect the lack of evidence regarding its associations with long-term outcomes and perhaps, also, that earlier generations of fructosamine assays demonstrated poor laboratory performance (34). The glycated albumin and fructosamine assays examined here demonstrated excellent laboratory performance with method CVs of 3% or less, similar to or lower than many other commonly used clinical tests (35). The fructosamine and glycated albumin assays also have their own limitations. Alterations in serum protein turnover will affect the values of these assays and it remains unclear whether fructosamine should be corrected for total serum protein concentration. Conditions that may affect the interpretation of fructosamine and glycated albumin values include liver disease, hyperuricemia, acute illness or infection, and thyroid dysfunction (36).
Important limitations of our study include the reliance on single measurements of fructosamine and glycated albumin at baseline, a limited number of fasting glucose and serum creatinine measurements during the follow-up period, that retinal photographs were taken in one eye only (4), and that the retinal exam was conducted at visit 3 and that measurements of fructosamine, glycated albumin, HbA1c, were only available at visit 2 (3 years earlier). The reliance on self-report to identify diabetes cases is also a limitation; but self-reported diabetes in the ARIC Study has been shown to be highly reliable and specific (37). The consistency of our results across different cases definitions of diabetes including those incorporating glucose measures is reassuring. Strengths of this study include the large, community-based sample, the availability of both HbA1c and fasting glucose measurements for comparison to fructosamine and glycated albumin, the rigorous measurement of potential confounding variables, and the long-term prospective follow-up of participants with high retention (>90% contact rate).
In conclusion, these results suggest the complementary nature of fructosamine and glycated albumin to HbA1c for risk stratification for diabetes and its microvascular complications in a community-based population.
Supplementary Material
Panel. Research in context.
Review
Non-traditional biomarkers of short-term (2–4 week) hyperglycemia such as fructosamine and glycated albumin are of increasing clinical interest as adjuncts to hemoglobin A1c (HbA1c) or as alternatives when HbA1c is unreliable or unavailable. However, the prognostic value of these short-term markers is largely uncharacterized. There are scant data linking modern fructosamine and glycated albumin assays to long-term outcomes, particularly in the general population. There is evidence from studies of dialysis populations that fructosamine and glycated albumin are associated with mortality and other important clinical outcomes (31, 38) and may perform similarly or better than HbA1c in this patient population (32). Furthermore, a recent study in persons with type 1 diabetes reported that HbA1c and glycated albumin had similar associations with retinopathy and kidney disease (39). We have previously shown in small studies that glycated albumin and fructosamine are cross-sectionally associated with prevalent microvascular conditions (8) and prospectively associated with incident diabetes (9). We undertook this study to evaluate the performance of fructosamine and glycated albumin to identify people at risk for clinical complications in the general population.
Interpretation
In a community-based population of over 10,000 persons with 20 years of follow-up, we found that fructosamine and glycated albumin were independently associated with prevalent retinopathy and risk of incident chronic kidney disease and incident diabetes. The associations of fructosamine and glycated albumin with retinopathy and incident chronic kidney disease were similar to those observed for HbA1c. Fructosamine and glycated albumin were also useful for identifying people at risk for diabetes, but these associations were more modest compared to that for HbA1c. Our study suggests that fructosamine and glycated albumin might be useful complements to HbA1c in clinical practice, particularly in situations where HbA1c testing is problematic or unavailable.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions. This paper is dedicated to our brilliant and beloved friend and colleague Frederick L. Brancati, MD, MHS (1959–2013).
Funding: This research was supported by NIH/NIDDK grant R01 DK089174 to Dr. Selvin. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The Asahi Kasei Corporation and Roche Diagnostics provided material support (kits only) for the glycated albumin (Asahi Kasei) and fructosamine (Roche) assays used in this research
References
- 1.American Diabetes A. Standards of medical care in diabetes–2010. Diabetes Care. 2010;33(Suppl 1):S11–61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colagiuri S, Lee CM, Wong TY, Balkau B, Shaw JE, Borch-Johnsen K, et al. Glycemic thresholds for diabetes-specific retinopathy: implications for diagnostic criteria for diabetes. Diabetes Care. 2011;34(1):145–50. doi: 10.2337/dc10-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng YJ, Gregg EW, Geiss LS, Imperatore G, Williams DE, Zhang X, et al. Association of A1C and fasting plasma glucose levels with diabetic retinopathy prevalence in the U.S. population: Implications for diabetes diagnostic thresholds. Diabetes Care. 2009;32(11):2027–32. doi: 10.2337/dc09-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong TY, Liew G, Tapp RJ, Schmidt MI, Wang JJ, Mitchell P, et al. Relation between fasting glucose and retinopathy for diagnosis of diabetes: three population-based cross-sectional studies. Lancet. 2008;371(9614):736–43. doi: 10.1016/S0140-6736(08)60343-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davidson MB, Schriger DL, Peters AL, Lorber B. Relationship Between Fasting Plasma Glucose and Glycosylated Hemoglobin: Potential for False-Positive Diagnoses of Type 2 Diabetes Using New Diagnostic Criteria. JAMA: The Journal of the American Medical Association. 1999;281(13):1203–10. doi: 10.1001/jama.281.13.1203. [DOI] [PubMed] [Google Scholar]
- 6.The International Expert C. International Expert Committee Report on the Role of the A1C Assay in the Diagnosis of Diabetes. Diabetes Care. 2009 doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selvin E, Steffes MW, Ballantyne CM, Hoogeveen RC, Coresh J, Brancati FL. Racial differences in glycemic markers: a cross-sectional analysis of community-based data. Ann Intern Med. 2011;154(5):303–9. doi: 10.1059/0003-4819-154-5-201103010-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selvin E, Francis LM, Ballantyne CM, Hoogeveen RC, Coresh J, Brancati FL, et al. Nontraditional markers of glycemia: associations with microvascular conditions. Diabetes Care. 2011;34(4):960–7. doi: 10.2337/dc10-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Juraschek SP, Steffes MW, Miller ER, 3rd, Selvin E. Alternative markers of hyperglycemia and risk of diabetes. Diabetes Care. 2012;35(11):2265–70. doi: 10.2337/dc12-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Juraschek SP, Steffes MW, Selvin E. Associations of alternative markers of glycemia with hemoglobin a1c and fasting glucose. Clin Chem. 2012;58(12):1648–55. doi: 10.1373/clinchem.2012.188367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sacks DB. Hemoglobin A1c in diabetes: panacea or pointless? Diabetes. 2013;62(1):41–3. doi: 10.2337/db12-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan DM, Peterson CM. Tests of glycemia in diabetes. Diabetes Care. 2003;26(Suppl 1):S106–S8. doi: 10.2337/diacare.26.2007.s106. [DOI] [PubMed] [Google Scholar]
- 13.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 14.Hubbard LD, Brothers RJ, King WN, Clegg LX, Klein R, Cooper LS, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the atherosclerosis risk in communities study. Ophthalmology. 1999;106(12):2269–80. doi: 10.1016/s0161-6420(99)90525-0. [DOI] [PubMed] [Google Scholar]
- 15.Couper DJ, Klein R, Hubbard LD, Wong TY, Sorlie PD, Cooper LS, et al. Reliability of retinal photography in the assessment of retinal microvascular characteristics: the Atherosclerosis Risk in Communities Study. Am J Ophthalmol. 2002;133(1):78–88. doi: 10.1016/s0002-9394(01)01315-0. [DOI] [PubMed] [Google Scholar]
- 16.Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A, et al. Low-Grade Systemic Inflammation and the Development of Type 2 Diabetes: The Atherosclerosis Risk in Communities Study. Diabetes. 2003;52(7):1799–805. doi: 10.2337/diabetes.52.7.1799. [DOI] [PubMed] [Google Scholar]
- 17.SELVIN E, CORESH J, ZHU H, FOLSOM A, STEFFES MW. Measurement of HbA1c from stored whole blood samples in the Atherosclerosis Risk in Communities study. Journal of Diabetes. 2010;2(2):118–24. doi: 10.1111/j.1753-0407.2010.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Operations manual no. 10: clinical chemistry determinations, version 1.0. Chapel Hill, NC: ARIC Coordinating Center, School of Public Health, University of North Carolina; 1987. [Google Scholar]
- 19.Operations manual no. 2: cohort component procedures, version 1.0. Chapel Hill, NC: ARIC Coordinating Center, School of Public Health, University of North Carolina; 1987. [Google Scholar]
- 20.Operations manual no. 11: sitting blood pressure, version 1.0. Chapel Hill, NC: ARIC Coordinating Center, School of Public Health, University of North Carolina; 1987. [Google Scholar]
- 21.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. American Journal of Clinical Nutrition. 1982;36(5):936–42. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes A. Standards of medical care in diabetes–2013. Diabetes Care. 2013;36(Suppl 1):S11–66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCance DR, Hanson RL, Charles MA, Jacobsson LT, Pettitt DJ, Bennett PH, et al. Comparison of tests for glycated haemoglobin and fasting and two hour plasma glucose concentrations as diagnostic methods for diabetes. BMJ. 1994;308(6940):1323–8. doi: 10.1136/bmj.308.6940.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engelgau MM, Thompson TJ, Herman WH, Boyle JP, Aubert RE, Kenny SJ, et al. Comparison of fasting and 2-hour glucose and HbA1c levels for diagnosing diabetes. Diagnostic criteria and performance revisited. Diabetes Care. 1997;20(5):785–91. doi: 10.2337/diacare.20.5.785. [DOI] [PubMed] [Google Scholar]
- 25.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 26.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–72. doi: 10.1002/sim.2929. discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 27.Harrell FE. Regression modeling strategies with applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001. [Google Scholar]
- 28.Sacks DB. A1C versus glucose testing: a comparison. Diabetes Care. 2011;34(2):518–23. doi: 10.2337/dc10-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Little RR, Roberts WL. A review of variant hemoglobins interfering with hemoglobin A1c measurement. J Diabetes Sci Technol. 2009;3(3):446–51. doi: 10.1177/193229680900300307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nathan DM, Singer DE, Hurxthal K, Goodson JD. The clinical information value of the glycosylated hemoglobin assay. N Engl J Med. 1984;310(6):341–6. doi: 10.1056/NEJM198402093100602. [DOI] [PubMed] [Google Scholar]
- 31.Shafi T, Sozio SM, Plantinga LC, Jaar BG, Kim ET, Parekh RS, et al. Serum fructosamine and glycated albumin and risk of mortality and clinical outcomes in hemodialysis patients. Diabetes Care. 2013;36(6):1522–33. doi: 10.2337/dc12-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freedman BI, Shenoy RN, Planer JA, Clay KD, Shihabi ZK, Burkart JM, et al. Comparison of glycated albumin and hemoglobin A1c concentrations in diabetic subjects on peritoneal and hemodialysis. Perit Dial Int. 2010;30(1):72–9. doi: 10.3747/pdi.2008.00243. [DOI] [PubMed] [Google Scholar]
- 33.Kohzuma T, Yamamoto T, Uematsu Y, Shihabi ZK, Freedman BI. Basic performance of an enzymatic method for glycated albumin and reference range determination. J Diabetes Sci Technol. 2011;5(6):1455–62. doi: 10.1177/193229681100500619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Upton J, Lever M, Sadler WA, George PM, Boswell DR. The quality of performance of the fructosamine test. N Z Med J. 1988;101(854):606–8. [PubMed] [Google Scholar]
- 35.Lacher DA, Hughes JP, Carroll MD. Estimate of Biological Variation of Laboratory Analytes Based on the Third National Health and Nutrition Examination Survey. Clinical Chemistry. 2005;51(2):450–2. doi: 10.1373/clinchem.2004.039354. [DOI] [PubMed] [Google Scholar]
- 36.Cohen RM, Sacks DB. Comparing multiple measures of glycemia: how to transition from biomarker to diagnostic test? Clin Chem. 2012;58(12):1615–7. doi: 10.1373/clinchem.2012.196139. [DOI] [PubMed] [Google Scholar]
- 37.Schneider AL, Pankow JS, Heiss G, Selvin E. Validity and reliability of self-reported diabetes in the atherosclerosis risk in communities study. Am J Epidemiol. 2012;176(8):738–43. doi: 10.1093/aje/kws156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fukuoka K, Nakao K, Morimoto H, Nakao A, Takatori Y, Arimoto K, et al. Glycated albumin levels predict long-term survival in diabetic patients undergoing haemodialysis. Nephrology (Carlton) 2008;13(4):278–83. doi: 10.1111/j.1440-1797.2007.00864.x. [DOI] [PubMed] [Google Scholar]
- 39.Nathan DM, McGee P, Steffes MW, Lachin JM, the DERG Relationship of Glycated Albumin to Blood Glucose and Glycated Hemoglobin (HbA1C) Values and to Retinopathy, Nephropathy and Cardiovascular Outcomes in the DCCT/EDIC Study. Diabetes. 2013 doi: 10.2337/db13-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


