Abstract
We report here two cases of vitiligo vulgaris successfully treated with the combination therapy of topical steroid and vitamin D3 compound and currently maintained by vitamin D3 analog without any adverse effects: skin atrophy, striae or telangiectasia on the exposed areas. The best-known mechanism of topical vitamin D3 analog is the enhancement of keratinocytes differentiation and anti-proliferative effects. Vitamin D3 analog is also reported to suppress T-cell mediated immunity, T-cell skin recruitment, and skin infiltration via down-regulating cutaneous lymphocyte antigen expression. Furthermore, vitamin D3 compounds are known to influence melanocyte maturation and differentiation and also to up-regulate melanogenesis. Autoreactive lymphocytes against melanocytes are one of the causes. Topical vitamin D3 analog may control vitiligo itself, however stronger immunosuppressive effects of topical corticosteroid may contribute to rapid re-pigmentation suppressing auto-reactive lymphocytes. The topical combination therapy is a simple, effective and safe option for vitiligo vulgaris in sun-exposed areas.
Key words: vitiligo, topical steroid, vitamin D3 compound.
Introduction
Vitiligo vulgaris is one of the commonest acquired idiopathic hypomelanotic disorders. Involvement of autoimmune mechanism for melanocytes has been suspected,1,2 and systemic and lesional immunosuppressive treatments are indicated. Narrow band UVB as well as psoralen UVA (PUVA) or solar irradiation therapies have been used for vitiligo. Some cases are resistant to therapies, and UV therapies are not recommended for face and scalp areas because of unwanted pigmentation and the risk of carcinogenesis in the future. We report here two cases of vitiligo vulgaris successfully treated with the combination of topical steroid and vitamin D3 analog, and currently maintained by vitamin D3 analog without any adverse effects. This topical combination therapy is simple, effective and safe, and can be considered as one of the options for vitiligo vulgaris in sunexposed areas.
Case Report #1
A 68-year-old Japanese man presented with asymptomatic round white patches on his head (Figure 1A). The skin lesions developed 6 months before his first visit. He was diagnosed having vitiligo vulgaris and treated with daily use of 0.05% Betamethasone butyrate propionate lotion (Antebate® lotion) and vitamin D3 analog: 2 μg/g tacalcitol ointment (Bonalfa® Ointment) once a day. After 3 months therapy, re-pigmentation developed, and the therapy was switched daily topical tacalcitol with once a week topical corticosteroid. Re-pigmentation has been gradually recovered without any adverse effect (Figure 1B).
Figure 1.
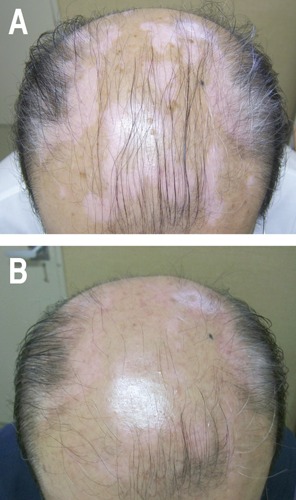
Case Report #1. A) Diffuse round white and pinkish patches on his head; B) the re-pigmented areas expanded gradually. Picture two years after of his first visit is shown.
Case Report #2
A 66-year old Japanese man presented with diffuse white patches on both of his face and forehead. The involved areas expanded gradually (Figure 2A). He has a history of lupus erythematosus and has been treated with daily 5 mg of oral prednisolone. We treated him in the same protocol as described above, and then the skin lesions were improved (Figure 2B). Currently he has been treated with topical tacalcitol.
Figure 2.
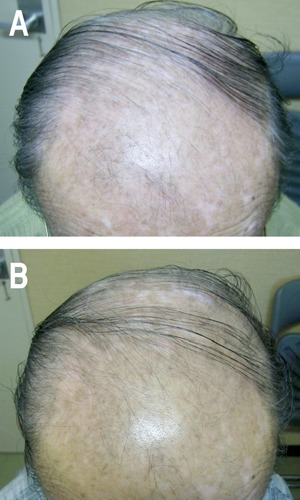
Case Report #2. A) Diffuse irregular white and pinkish patches on his head. B) The vitiliginous area had been improved with pigmentation one year after his first visit.
Discussion
Vitiligo vulgaris is characterized by de-pigmented macules with reduced or absent cutaneous melanocytes. Pathogenesis of vitiligo is still unclear, and the autoimmune,3,4 the neurogenic,5,6 and the redox control abnormalities,7–9 have been suggested. Several medical and surgical options are currently available for the treatment of vitiligo, including calcineurin inhibitors, topical corticosteroids, topical and/or oral PUVA, UVA, nB-UVB therapy, topical tacrolimus, laser therapy, and surgical therapies; however, there are resistant cases. Topical steroid therapy has been widely used to treat vitiligo with efficacy especially in patients with less than 20% of involved body surface area.10
Topical vitamin D3 analog, tacalcitol is used for treatment of psoriasis, and the best-known mechanism of this compound is the enhancement of keratinocytes differentiation and anti-proliferative effects. Vitamin D3 analog is also reported to suppress T cell mediated immunity.11 As we reported previously, vitamin D3 and tacalcitol suppresses T-cell skin recruitment and skin infiltration via down-regulating cutaneous lymphocyte antigen expression and binding to the E- and P-selectin.12,13 Furthermore, vitamin D3 compounds are known to influence melanocyte maturation and differentiation and also to up-regulate melanogenesis through pathways activated by specific ligand receptors, such as endothelin receptor and c-kit.14
There are some reports describing the effect of calcipotriol or calcipotriene for vitiligo.15–17 In this report, we have initially treated vitiligo with the topical corticosteroid and tacalcitol, and then gradually reduced topical corticosteroid to avoid the adverse effects: skin atrophy, striae and telangiectasia on the exposed areas. Topical vitamin D3 analog may control vitiligo itself, however stronger immunosuppressive effects of topical corticosteroid may contribute to rapid re-pigmentation suppressing auto-reactive lymphocytes against melanocytes.
Conclusions
In conclusion, although further studies are required, the combination therapy of topical steroid and tacalcitol is effective and safety therapeutic candidate for vitiligo vulgaris.
References
- 1.Harris JE, Harris TH, Weninger W, et al. A mouse model of vitiligo with focused epidermal depigmentation requires IFN-gamma for autoreactive CD8(+) t-cell accumulation in the skin. J Invest Dermatol. 2012 Feb 2; doi: 10.1038/jid.2011.463. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oiso N, Suzuki T, Fukai K, et al. Nonsegmental vitiligo and autoimmune mechanism. Dermatol Res Pract. 2011;2011:518090. doi: 10.1155/2011/518090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naughton GK, Eisinger M, Bystryn JC. Antibodies to normal human melanocytes in vitiligo. J Exp Med. 1983;158:246–51. doi: 10.1084/jem.158.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogg GS, Rod Dunbar P, Romero P, et al. High frequency of skin-homing melanocyte-specific cytotoxic T lymphocytes in autoimmune vitiligo. J Exp Med. 1998;188:1203–8. doi: 10.1084/jem.188.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lerner AB. Vitiligo. J Invest Dermatol. 1959;32:285–310. [PubMed] [Google Scholar]
- 6.Al'Abadie MS, Senior HJ, Bleehen SS, Gawkrodger DJ. Neuropeptide and neuronal marker studies in vitiligo. Br J Dermatol. 1994;131:160–5. doi: 10.1111/j.1365-2133.1994.tb08486.x. [DOI] [PubMed] [Google Scholar]
- 7.Schallreuter KU, Wood JM, Ziegler I, et al. Defective tetrahydrobiopterin and catecholamine biosynthesis in the depigmentation disorder vitiligo. Biochim Biophys Acta. 1994;1226:181–92. doi: 10.1016/0925-4439(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 8.Schallreuter KU, Moore J, Wood JM, et al. In vivo and in vitro evidence for hydrogen peroxide (H2O2) accumulation in the epidermis of patients with vitiligo and its successful removal by a UVB-activated pseudocatalase. J Investig Dermatol Symp Proc. 1999;4:91–6. doi: 10.1038/sj.jidsp.5640189. [DOI] [PubMed] [Google Scholar]
- 9.Schallreuter KU, Wood JM. Thioredoxin reductase - its role in epidermal redox status. J Photochem Photobiol B. 2001;64:179–84. doi: 10.1016/s1011-1344(01)00235-4. [DOI] [PubMed] [Google Scholar]
- 10.Njoo MD, Spuls PI, Bos JD, et al. Nonsurgical repigmentation therapies in vitiligo. Meta-analysis of the literature. Arch Dermatol. 1998;134:1532–40. doi: 10.1001/archderm.134.12.1532. [DOI] [PubMed] [Google Scholar]
- 11.Lemire JM, Adams JS, Kermani-Arab V, et al. 1,25-dihydroxyvitamin D3 suppresses human T helper/inducer lymphocyte activity in vitro. J Immunol. 1985;134:3032–5. [PubMed] [Google Scholar]
- 12.Yamanaka K, Dimitroff CJ, Fuhlbrigge RC, et al. Vitamins A and D are potent inhibitors of cutaneous lymphocyte-associated antigen expression. J Allergy Clin Immunol. 2008;121:148–57. doi: 10.1016/j.jaci.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamanaka KI, Kakeda M, Kitagawa H, et al. 1,24-Dihydroxyvitamin D (tacalcitol) prevents skin T-cell infiltration. Br J Dermatol. 2010;162:1206–15. doi: 10.1111/j.1365-2133.2010.09692.x. [DOI] [PubMed] [Google Scholar]
- 14.Birlea SA, Costin GE, Norris DA. Cellular and molecular mechanisms involved in the action of vitamin D analogs targeting vitiligo depigmentation. Curr Drug Targets. 2008;9:345–59. doi: 10.2174/138945008783954970. [DOI] [PubMed] [Google Scholar]
- 15.Travis LB, Silverberg NB. Calcipotriene and corticosteroid combination therapy for vitiligo. Pediatr Dermatol. 2004;21:495–8. doi: 10.1111/j.0736-8046.2004.21418.x. [DOI] [PubMed] [Google Scholar]
- 16.Kumaran MS, Kaur I, Kumar B. Effect of topical calcipotriol, betamethasone dipropionate and their combination in the treatment of localized vitiligo. J Eur Acad Dermatol Venereol. 2006;20:269–73. doi: 10.1111/j.1468-3083.2006.01420.x. [DOI] [PubMed] [Google Scholar]
- 17.Newman MD, Silverberg NB. Once-daily application of calcipotriene 0.005%-betamethasone dipropionate 0.064% ointment for repigmentation of facial vitiligo. Cutis. 2011;88:256–9. [PubMed] [Google Scholar]


