Abstract
Background
Smoking is associated with poor health-related quality of life (HRQL); however, there are few data regarding effects of smoking cessation treatment on HRQL. The purpose of this study was to describe changes in HRQL after smoking cessation treatment and to elucidate factors influencing this improvement in HRQL.
Setting
Smoking cessation clinic at a 358-bed community teaching hospital in Japan.
Methods
We conducted a prospective cohort study of cigarette smokers who participated in a 3-month smoking cessation programme. HRQL was assessed at baseline and at the end of the programme using the St. George's Respiratory Questionnaire (SGRQ). The abstinence was subjected to verification by an exhaled CO level of ≤10 ppm.
Results
Of 570 participants in the programme, 277 (mean age: 60.9±12.2 y, male/female=180/97) were eligible; excluded were 277 participants who dropped out of the programme and 16 for whom SGRQs were not available or were incomplete. Initial prescribed pharmacotherapy was transdermal nicotine patches in 160 participants and varenicline in 117. At 12 weeks, SGRQ scores improved significantly as follows (mean±SD): Δ symptoms score, −5.7±16.0; Δ activity score, −4.4±18.3; Δ impact score, −5.3±13.5 and Δ total score, −5.1±12.2 (p<0.0001 in all cases). There were no significant differences in changes in SGRQ scores between quitters (n=183) and continuous smokers (n=94). In a multivariate analysis, only the average nicotine addiction level according to the Tobacco Dependence Screener test was associated with a clinically significant improvement in the SGRQ (OR 1.35 (95% CI 1.15 to 1.59)). Marked reduction in number of cigarettes smoked with a corresponding low median exhaled CO level of 7 ppm in continuous smokers following therapy was observed.
Conclusions
Smoking cessation treatment improved HRQL regardless of quit status. Baseline nicotine addiction level was predictive of that improvement.
Keywords: Tobacco and the lung
Key messages.
Smoking is associated with poor health related quality of life.
Smoking cessation treatment improved health-related quality of life regardless of quit status.
Baseline nicotine addiction level was predictive of that improvement.
Introduction
Smoking is a major cause of premature mortality and preventable morbidity worldwide. The WHO Framework Convention on Tobacco Control recognises the substantial harm caused by tobacco use and the critical need to prevent it. Tobacco kills approximately 6 million people and causes more than half a trillion dollars of economic damage each year.1 In the prospective British Doctors Study,2 those who smoked cigarettes throughout their adult life died about 10 years earlier than lifelong non-smokers, while in the US Framingham Heart Study,3 life expectancy in continuing smokers was also nearly 10 years less than in lifelong non-smokers. Japanese smokers born since 1920 and who started to smoke early in adult life had smoking habits similar to those of smokers in the British Doctors Study and the Framingham Heart Study and, as in those studies, continuing smokers lost about 10 years of life compared with lifelong non-smokers.4
Smoking may also be associated with other adverse health characteristics. Recently, the impact of smoking and smoking cessation on quality of life has received increasing attention. Health-related quality of life (HRQL) reflects the patient's evaluation of his/her physical, psychological and social functioning in relation to health. Several studies have found that smoking is related to poor HRQL.5–9 Previous cross-sectional studies of the relationship between smoking status and HRQL found that smokers reported poorer HRQL in general than never and former smokers.5–7 It also has been demonstrated that HRQL is inversely related to the number of cigarettes that people smoke,7 8 and this relationship is even stronger among the more nicotine-dependent smokers.9 However, little is known about HRQL following smoking cessation treatment. HRQL offers a good outcome measure for interventional studies that take into account a patient's physical, psychological, social and spiritual well-being. Furthermore, the positive effects of smoking cessation on HRQL would have a greater influence on smokers’ decisions to quit than avoidance of longer term disease effects, such as lung cancer and heart disease.5 Therefore, we investigated the effect of smoking cessation treatment on HRQL in a sample of participants in a smoking cessation programme. Our hypothesis was that smoking cessation treatment would improve HRQL especially in quitters and our primary objective was to describe changes in HRQL after smoking cessation treatment. A secondary objective was to elucidate factors influencing the improvement in HRQL.
Materials and methods
Study population
The Kobe City Medical Center West Smoking Cessation Registry is a physician-initiated prospective observational study enrolling consecutive patients who participated in the 3-month smoking cessation programme covered by the Japanese medical insurance system. This study was conducted in accordance with the amended Declaration of Helsinki. Written informed consent was obtained from all participants.
From September 2007 to August 2013, a total of 570 persons were enrolled in the registry. Of these, 293 completed the 3-month smoking cessation programme, and the 277 persons who stopped attending the smoking cessation programme and dropped out of the programme were excluded from the study. Of those who completed the programme, HRQL questionnaires were not available or were incomplete at baseline or 12 weeks for 16 persons. Thus the population for this study consisted of 277 participants who completed both HRQL questionnaires at baseline and 12 weeks (see figure 1).
Figure 1.

Flow chart for selection of study population (SGRQ, St. George's Respiratory Questionnaire).
Smoking cessation programme
To be eligible for the 3-month smoking cessation programme, participants were required to be interested in quitting smoking promptly, to have had a diagnosis of nicotine dependence by the Tobacco Dependence Screener (TDS) test10 (5 points or more) and to have a Brinkman Index,11 which was measured by the number of cigarettes smoked daily×duration (years) of smoking, ≥200. Exclusion criteria included pregnancy, non-adherence to treatment and past participation in the same programme within 1 year. The TDS consists of 10 yes/no questions and is scored according to the number of affirmative answers. Its reliability and validity have been assessed for smokers in Japan. A greater score suggests greater tobacco dependence and a TDS score of 5 or more indicates nicotine/tobacco dependence according to the ICD-10 diagnosis, with a sensitivity of 95% and specificity of 81%.10
A detailed smoking history, medical history and comorbidities, current respiratory symptoms and the TDS test results were ascertained to be used as baseline data. A Micro mobile breath CO monitor (Bedfont Scientific Limited, Kent, UK) was used to determine CO levels in expired air. All participants received either transdermal nicotine patches or varenicline following discussion with the attending physician. Varenicline was first marketed in Japan in May 2008. Next, a pharmacist explained the effect of each drug, its usage and side effects using a leaflet to support the information. A target quit date was set on the day that nicotine patches were applied or on the eighth day after the first dose of varenicline. An educational seminar that introduced the programme and explained the following topics was provided to participants: harmful effects of smoking, possible benefits of quitting smoking, how to handle withdrawal symptoms and how to prevent relapses. A leaflet was used to reinforce the information. The programme consisted of 5 sessions; participants returned 2, 4, 8 and 12 weeks after their baseline visit date for follow-up. At each visit, CO concentration was measured and the attending physician and a nurse with experience in smoking cessation confirmed whether smoking cessation had continued. Brief counselling (≤15 min) was provided and the staff praised those who continued with cessation or expressed appreciation of the efforts of those who had continued to smoke and recommended a rechallenge. No psychosocial support was provided. Those who smoked were not provided with any additional materials or instructions. Patients were considered to be quitters even if they smoked at 8 weeks but had quit completely between the 8-week and 12-week visits. Their reports of abstinence were subjected to verification by an exhaled CO level of ≤10 ppm. Those who smoked between the 8-week and 12-week visits were considered to be continuous smokers and the self-reported maximal numbers of cigarettes smoked daily during the period were recorded.
Pulmonary function tests
Pulmonary function tests (forced vital capacity and forced expiratory volume in 1 s in the absence of recent bronchodilator use) were performed at baseline and at 12 weeks following treatment. Predicted normal values for the Japanese population were derived from reference values of the Japanese Respiratory Society.12 Before each pulmonary function test, height and weight of participants were measured and body mass index (kg/m2) was calculated.
HRQL measurement
To measure HRQL we used the St. George's Respiratory Questionnaire (SGRQ), a respiratory-specific instrument, due to its relative simplicity, wide use in chronic pulmonary disease and the availability of a validated version for the Japanese population. Among our study group, a Brinkman Index ≥200 was considered to indicate smokers at risk of developing chronic obstructive pulmonary disease (COPD) or with early stages of COPD, even if they did not self-report that they had COPD. This questionnaire was translated into Japanese in accordance with standardised methodology, and it was previously validated.13 Permission was obtained for use of the instrument from Dr Koichi Nishimura. The SGRQ contains 50 items in 3 subscales (symptoms, activity and impact). The total score can be calculated from responses to all 50 items. Scores for these components and the total score are on a 100-point scale, with a higher score corresponding to a poorer HRQL14 and a change in score of ≥4 points constituting the minimal clinically important difference.15 Questionnaires were completed at baseline and 12 weeks following treatment.
Statistics
Measurement data were expressed as means±SD. Group differences were compared using the χ2 or Fisher's exact test for categorical variables, or the Student t test or the Mann-Whitney U test for continuous variables. The changes in exhaled CO levels and SGRQ scores at the end of the treatment (12 weeks following treatment) were compared to those of the baseline values using a paired t test or Wilcoxon signed-rank test. p Value <0.05 indicated a significant difference.
We then compared baseline characteristics of ‘SGRQ-improved’ participants with non-improved participants. In addition, we also compared absolute values of changes from baseline to 12 weeks in exhaled CO levels and FEV1 between the two groups. An improvement in SGRQ was defined as achieving a clinically important difference (at least 4 points) for the total score.15 Multiple logistic regression analysis was used to determine independent predictors of clinically significant improvement in SGRQ and to obtain ORs adjusted for possible confounding factors by univariate analysis (p<0.10). The 95% CI for each OR was calculated. Statistical significance was determined from the 95% CI, not including 1.00 for logistic analyses. All analyses were performed using JMP statistical software (JMP, V.9.0.2; SAS Institute Inc, Cary, North Carolina, USA).
Results
Baseline characteristics
The study population consisted of 277 participants; 65% (n=180) were male and 35% (n=97) were female. Average age was 60.9±12.2 years. Baseline characteristics of the study population and excluded participants are compared in table 1. There was a significant difference between groups in age, number of cigarettes smoked daily, duration of smoking (p<0.001, respectively) and frequency of complications such as cardiovascular disease (p=0.002) or COPD (p=0.02). No differences were seen between groups in terms of the Brinkman Index, TDS score, exhaled CO level or baseline SGRQ scores.
Table 1.
Baseline characteristics of study population and dropout/excluded participants
| Variables | Study population n=277 |
Dropout/excluded participants n=293 | p Value |
|---|---|---|---|
| Male sex | 180 (65.0) | 174 (59.4) | 0.168 |
| Age, year | 60.9±12.2 | 55.6±14.1 | <0.001 |
| Cigarettes smoked daily, N | 22.9±12.4 | 26.8±13.5 | <0.001 |
| Duration of smoking, year | 38.5±13.1 | 33.6±13.1 | <0.001 |
| Brinkman Index | 876.3±567.9 | 871.6±534.2 | 0.919 |
| TDS score | 7.7±1.6 | 7.8±1.6 | 0.565 |
| Exhaled CO, ppm | 13.8±9.4 | 15.6±11.9 | 0.053 |
| Body mass index, kg/m2 | 23.2±4.5 | 22.9±4.1 | 0.438 |
| FEV1, % predicted | 75.7±20.2 | 78.4±19.9 | 0.105 |
| FVC, % predicted | 82.8±17.4 | 85.4±16.7 | 0.079 |
| FEV1/FVC, % | 74.9±13.3 | 75.5±11.3 | 0.159 |
| Diseases (self-reported) | |||
| Mental disorder | 79 (28.5) | 88 (30.0) | 0.691 |
| Diabetes mellitus | 49 (17.7) | 48 (16.4) | 0.678 |
| Cardiovascular disease | 60 (21.7) | 35 (12.0) | 0.002 |
| COPD | 49 (17.7) | 32 (10.9) | 0.020 |
| Bronchial asthma | 34 (12.3) | 37 (12.6) | 0.898 |
| Cancer | 22 (7.9) | 28 (9.6) | 0.495 |
| SGRQ score | |||
| Symptoms | 41.8±24.1 | 40.7±21.9* | 0.593 |
| Activity | 35.6±26.3 | 33.7±25.2* | 0.370 |
| Impact | 20.4±18.3 | 19.3±17.9* | 0.505 |
| Total | 28.5±19.3 | 27.2±18.1* | 0.410 |
Data are presented as number (%) or mean±SD.
*Baseline SGRQ was available for 277 participants.
COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; SGRQ, St. George's Respiratory Questionnaire; TDS, Tobacco Dependence Screener test.
Smoking cessation programme
The initial prescriptions were transdermal nicotine patches in 160 participants and varenicline in 117. Six participants in each group were switched to another medication because of adverse events or patient demand. All participants (n=277) smoked at baseline, 115 (41.5%) smoked at 2 weeks, 95 (34.3%) at 4 weeks, 96 (34.8%) at 8 weeks and 94 (33.9%) at 12 weeks. Thus 183 participants were considered to be quitters and 94 were considered to be continuous smokers. Comparisons of baseline characteristics between quitters and continuous smokers are shown in table 2. There was a significant difference between groups in age, duration of smoking, exhaled CO level and frequency of mental disorders (p<0.001, respectively). Changes in median exhaled CO levels from baseline to 12 weeks for quitters and continuous smokers are shown in figure 2. Decreases in exhaled CO levels from baseline to 12 weeks in quitters (−10.1±7.8) as well as continuous smokers (−9.0±11.2) were statistically significant (p<0.0001, respectively). Among the continuous smokers, 64 (68%) had exhaled CO levels of ≤10 ppm at 12 weeks and the maximal number of cigarettes smoked daily between the 8-week and 12-week visits ranged from 1 to 60 (median 5 cigarettes per day). Of quitters, 96 used nicotine patches, 79 used varenicline and 4 in each group were switched to the other medication.
Table 2.
Comparisons of baseline characteristics between quitters and continuous smokers
| Variables | Quitters n=183 |
Continuous smokers n=94 | p Value |
|---|---|---|---|
| Male sex | 122 (66.7) | 58 (61.7) | 0.414 |
| Age, year | 62.9±11.5 | 57.2±12.6 | <0.001 |
| Cigarettes smoked daily, N | 21.9±11.1 | 24.9±14.3 | 0.059 |
| Duration of smoking, year | 40.4±12.8 | 34.9±12.8 | <0.001 |
| Brinkman Index | 889.6±580.1 | 850.3±545.4 | 0.586 |
| TDS score | 7.7±1.6 | 7.8±1.6 | 0.569 |
| Exhaled CO, ppm | 11.6±8.1 | 18.2±10.3 | <0.001 |
| Body mass index, kg/m2 | 23.3±4.2 | 23.0±5.0 | 0.579 |
| FEV1, % predicted | 74.9±19.9 | 77.3±20.9 | 0.360 |
| FVC, % predicted | 82.1±18.2 | 84.4±15.6 | 0.291 |
| FEV1/FVC, % | 73.7±12.9 | 74.6±14.1 | 0.623 |
| Diseases (self-reported) | |||
| Mental disorder | 40 (21.9) | 39 (41.5) | <0.001 |
| Diabetes mellitus | 34 (18.6) | 15 (16.0) | 0.586 |
| Cardiovascular disease | 45 (24.6) | 15 (16.0) | 0.092 |
| COPD | 30 (16.4) | 19 (20.2) | 0.430 |
| Bronchial asthma | 24 (13.1) | 10 (10.6) | 0.548 |
| Cancer | 15 (8.2) | 7 (7.5) | 0.826 |
| SGRQ score | |||
| Symptoms | 41.4±23.9 | 42.4±24.7 | 0.734 |
| Activity | 34.8±25.6 | 37.2±27.6 | 0.478 |
| Impact | 19.3±18.2 | 22.4±18.4 | 0.187 |
| Total | 27.7±19.0 | 30.2±19.8 | 0.304 |
Data are presented as number (%) or mean±SD.
COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; SGRQ, St. George's Respiratory Questionnaire; TDS, Tobacco Dependence Screener test.
Figure 2.
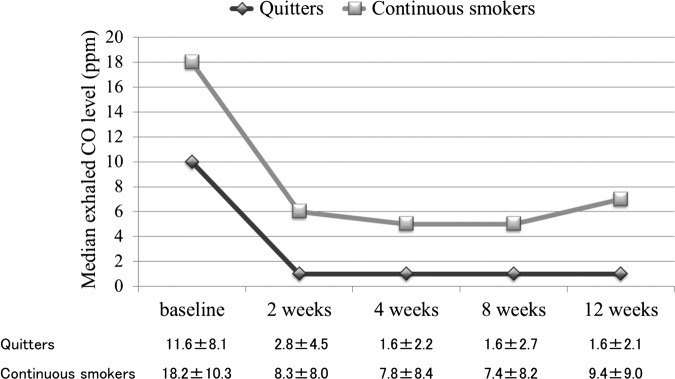
Changes in median exhaled CO levels from baseline to 12 weeks in quitters (n=183) and continuous smokers (n=94). Decreases in exhaled CO levels from baseline to 12 weeks in quitters (−10.1±7.8) as well as continuous smokers (−9.0±11.2) were statistically significant (p<0.0001, respectively). Numerical data are presented as mean±SD.
Health-related quality of life
Changes in SGRQ scores from baseline to 12 weeks for the total sample, quitters and continuous smokers are shown in figure 3. At 12 weeks, the mean changes in the SGRQ scores for the total sample were as follows: symptoms, −5.7±16.0; activity, −4.4±18.3; impact, −5.3±13.5 and total, −5.1±12.2. For each subscale and for the total score, the increments in HRQL scores from baseline were statistically significant (p<0.0001 in all cases). Similarly, the increments in HRQL scores from baseline in quitters and continuous smokers were statistically significant (symptoms, p=0.0002 and p<0.0001; activity, p=0.03 and p=0.0009; impact, p=0.0002 and p<0.0001; and total, p<0.0001 and p<0.0001 for quitters and continuous smokers, respectively). When comparing the change in SGRQ scores between quitters and continuous smokers, no statistically significant difference was found. The proportions of participants achieving a clinically meaningful change (at least 4 points) in the SGRQ total score were 45%, 44% and 48% for the total sample, quitters and continuous smokers, respectively. Thus, 125 participants (45%) were considered SGRQ-improved and 152 participants (55%) were considered non-improved. Baseline SGRQ scores for the SGRQ-improved and non-improved participants are compared in table 3. The baseline scores for each subscale and for the total score were significantly higher in the SGRQ-improved participants than in the non-improved participants (p<0.001, respectively).
Figure 3.
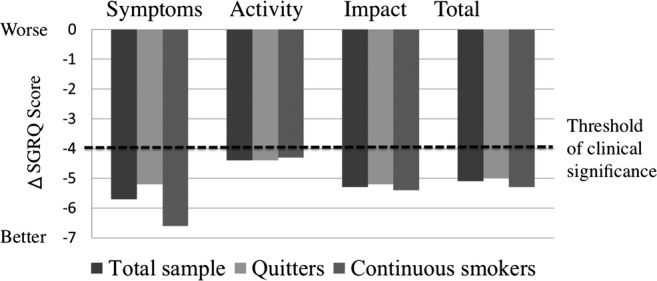
Changes in St. George's Respiratory Questionnaire (SGRQ) scores from baseline to 12 weeks for total samples (n=277), quitters (n=183) and continuous smokers (n=94). No statistically significant differences in changes in SGRQ scores were found between quitters and continuous smokers.
Table 3.
Comparisons of baseline St. George's Respiratory Questionnaire (SGRQ) between SGRQ-improved and non-improved participants
| Variables | Improved n=125 |
Non-improved n=152 |
p Value |
|---|---|---|---|
| Symptoms | 48.4±24.2 | 36.3±22.7 | <0.001 |
| Activity | 45.4±25.0 | 27.6±24.6 | <0.001 |
| Impact | 27.9±19.4 | 14.1±14.6 | <0.001 |
| Total | 36.6±18.9 | 21.9±16.9 | <0.001 |
Data are presented as mean±SD.
Results of univariate analysis of SGRQ-improved and non-improved participants are shown in table 4. SGRQ-improved participants were younger (59.1±12.2 years) than non-improved participants (62.5±12.0 years; p=0.021). The average duration of smoking was significantly shorter in SGRQ-improved participants (36.6±13.3 years) than in non-improved participants (40.1±12.7 years; p=0.028). The average TDS score was significantly higher in SGRQ-improved participants (8.1±1.5) than in non-improved participants (7.4±1.6; p<0.001). No other parameters differed between the two groups, although higher frequencies of mental disorders and bronchial asthma in the SGRQ-improved participants were considered a trend that approached significance (p=0.090 and 0.087, respectively). There was no difference between the two groups with regard to changes from baseline to 12 weeks in exhaled CO levels and FEV1.
Table 4.
Univariate analysis of SGRQ-improved versus non-improved participants
| Improved (n=125) | Non-improved (n=152) | p Value | |
|---|---|---|---|
| Male sex | 76 (60.8) | 104 (68.2) | 0.186 |
| Age, year | 59.1±12.2 | 62.5±12.0 | 0.021 |
| Cigarettes smoked daily, N | 23.9±13.0 | 22.1±11.7 | 0.214 |
| Duration of smoking, year | 36.6±13.3 | 40.1±12.7 | 0.028 |
| Brinkman Index | 874.1±586.9 | 878.1±553.7 | 0.953 |
| TDS score | 8.1±1.5 | 7.4±1.6 | <0.001 |
| Baseline exhaled CO, ppm | 13.8±9.1 | 13.9±9.6 | 0.937 |
| Changes in exhaled CO, ppm | −10.1±9.1 | −9.4±9.1 | 0.535 |
| Body mass index, kg/m2 | 23.1±4.7 | 23.2±4.3 | 0.926 |
| Baseline FEV1, % predicted | 74.1±20.9 | 77.0±19.6 | 0.221 |
| Baseline FVC, % predicted | 81.0±18.1 | 84.4±16.6 | 0.110 |
| FEV1/FVC, % | 74.1±14.8 | 74.0±12.0 | 0.951 |
| Changes in FEV1, L | 0.06±0.28 | 0.04±0.25 | 0.598 |
| Treated by varenicline | 55 (44.0) | 62 (40.8) | 0.590 |
| Quitter | 80 (64.0) | 103 (67.8) | 0.511 |
| Mental disorder | 42 (33.6) | 37 (24.3) | 0.090 |
| Diabetes mellitus | 21 (16.8) | 28 (18.4) | 0.725 |
| Cardiovascular disease | 29 (23.0) | 31 (20.4) | 0.573 |
| COPD | 24 (19.2) | 25 (16.5) | 0.551 |
| Bronchial asthma | 20 (16.0) | 14 (9.2) | 0.087 |
| Cancer | 8 (6.4) | 14 (9.2) | 0.386 |
Data are presented as number (%) or mean±SD.
COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; SGRQ, St. George's Respiratory Questionnaire; TDS, Tobacco Dependence Screener test.
Multivariate analysis is shown in table 5. The TDS score was only associated with clinically significant improvement in SGRQ and had an OR (95% CI) of 1.35 (1.15 to 1.59; p<0.001). In addition, we performed subgroup analysis. First, we restricted the analysis to subgroups with airway obstruction defined by forced expiratory volume in 1 s/forced vital capacity (FEV1/FVC) <0.7 (n=76). Multivariate analysis showed that the TDS score was still associated with a clinically significant improvement in the SGRQ and had an OR (95% CI) of 1.64 (1.16 to 2.44; p=0.005). In this analysis, quitting smoking was rather negatively associated with a clinically significant improvement in the SGRQ scores (tables 6 and 7). Second, we defined ‘early quitters’ as participants who quit smoking at 4 weeks and continued to quit until 12 weeks, and we restricted the analysis to subgroups consisting of ‘early quitters’ (n=156) and continuous smokers (n=94). Multivariate analysis showed that the TDS score was still the only factor associated with a clinically significant improvement in the SGRQ and had an OR (95% CI) of 1.39 (1.17 to 1.66; p<0.001; tables 8 and 9).
Table 5.
Multivariate analysis of SGRQ-improved versus non-improved participants
| Variable | OR | 95% CI | p Value |
|---|---|---|---|
| Age, year | 0.99 | 0.95 to 1.02 | 0.465 |
| Duration of smoking, year | 1.00 | 0.97 to 1.03 | 0.888 |
| TDS score | 1.35 | 1.15 to 1.59 | <0.001 |
| Mental disorder | 1.20 | 0.67 to 2.14 | 0.535 |
| Bronchial asthma | 1.39 | 0.65 to 3.04 | 0.396 |
SGRQ, St. George's Respiratory Questionnaire; TDS, Tobacco Dependence Screener test.
Table 6.
Univariate analysis of SGRQ-improved versus non-improved participants with airway obstruction defined by FEV1/FVC<0.7
| Improved (n=31) | Non-improved (n=45) | p Value | |
|---|---|---|---|
| Male sex | 21 (67.7) | 34 (75.6) | 0.456 |
| Age, year | 66.6±9.7 | 67.8±9.7 | 0.597 |
| Cigarettes smoked daily, N | 23.3±12.4 | 22.4±12.6 | 0.758 |
| Duration of smoking, year | 45.4±12.0 | 46.3±11.5 | 0.733 |
| Brinkman Index | 1072.7±675.9 | 994.0±525.8 | 0.570 |
| TDS score | 8.0±1.5 | 7.2±1.7 | 0.040 |
| Baseline exhaled CO, ppm | 11.6±7.0 | 10.5±8.4 | 0.529 |
| Changes in exhaled CO, ppm | −8.6±7.9 | −5.0±7.0 | 0.041 |
| Body mass index, kg/m2 | 23.4±3.5 | 21.8±3.5 | 0.639 |
| Baseline FEV1, % predicted | 52.0±19.8 | 60.3±17.8 | 0.061 |
| Baseline FVC, % predicted | 73.6±21.5 | 81.5±17.4 | 0.085 |
| FEV1/FVC, % | 56.0±11.6 | 58.9±9.4 | 0.223 |
| Changes in FEV1, L | 0.13±0.28 | 0.08±0.32 | 0.513 |
| Treated by varenicline | 13 (41.9) | 20 (44.4) | 0.828 |
| Quitter | 17 (54.8) | 33 (73.3) | 0.096 |
| Mental disorder | 6 (19.4) | 10 (22.2) | 0.762 |
| Diabetes mellitus | 3 (9.7) | 5 (11.1) | 0.841 |
| Cardiovascular disease | 8 (25.8) | 6 (13.3) | 0.172 |
| COPD | 17 (54.8) | 16 (35.6) | 0.095 |
| Bronchial asthma | 6 (19.4) | 7 (15.6) | 0.667 |
| Cancer | 4 (12.9) | 6 (13.3) | 0.957 |
Data are presented as number (%) or mean±SD.
COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; SGRQ, St. George's Respiratory Questionnaire; TDS, Tobacco Dependence Screener test.
Table 7.
Multivariate analysis of SGRQ-improved versus Non-improved patients with airway obstruction defined by FEV1/FVC<0.7
| Variable | OR | 95% CI | p Value |
|---|---|---|---|
| TDS score | 1.64 | 1.16 to 2.44 | 0.005 |
| Changes in exhaled CO, ppm | 0.90 | 0.82 to 0.99 | 0.012 |
| Baseline FEV1, % predicted | 0.99 | 0.93 to 1.05 | 0.712 |
| Baseline FVC, % predicted | 0.99 | 0.93 to 1.05 | 0.772 |
| Quitter | 0.26 | 0.07 to 0.85 | 0.026 |
| COPD | 1.85 | 0.53 to 6.72 | 0.335 |
COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; SGRQ, St. George's Respiratory Questionnaire; TDS, Tobacco Dependence Screener test.
Table 8.
Univariate analysis of SGRQ-improved versus non-improved participants among early quitters and continuous smokers
| Improved (n=109) | Non-improved (n=141) | p Value | |
|---|---|---|---|
| Male sex | 66 (60.6) | 99 (70.2) | 0.110 |
| Age, year | 59.2±12.0 | 62.2±12.2 | 0.055 |
| Cigarettes smoked daily, N | 24.1±13.2 | 22.8±11.9 | 0.407 |
| Duration of smoking, year | 36.9±13.1 | 40.0±12.7 | 0.060 |
| Brinkman Index | 885.7±591.5 | 905.0±564.3 | 0.792 |
| TDS score | 8.1±1.5 | 7.3±1.6 | <0.001 |
| Baseline exhaled CO, ppm | 13.6±9.3 | 14.3±9.7 | 0.542 |
| Changes in exhaled CO, ppm | −9.6±9.2 | −9.6±9.3 | 0.985 |
| Body mass index, kg/m2 | 23.0±4.8 | 23.2±4.2 | 0.729 |
| Baseline FEV1, % predicted | 73.2±21.8 | 77.9±18.4 | 0.068 |
| Baseline FVC, % predicted | 81.0±18.6 | 84.8±14.9 | 0.078 |
| FEV1/FVC, % | 73.5±13.7 | 74.6±11.9 | 0.529 |
| Changes in FEV1, L | 0.05±0.28 | 0.03±0.21 | 0.580 |
| Treated by varenicline | 44 (40.4) | 53 (37.6) | 0.655 |
| Quitter | 64 (58.2) | 92 (65.3) | 0.291 |
| Mental disorder | 36 (33.0) | 35 (24.8) | 0.155 |
| Diabetes mellitus | 19 (17.4) | 27 (19.2) | 0.728 |
| Cardiovascular disease | 23 (21.1) | 27 (19.2) | 0.702 |
| COPD | 23 (21.0) | 23 (16.3) | 0.334 |
| Bronchial asthma | 16 (14.7) | 11 (7.8) | 0.084 |
| Cancer | 6 (5.5) | 12 (8.5) | 0.356 |
Data are presented as number (%) or mean±SD.
COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; SGRQ, St. George's Respiratory Questionnaire; TDS, Tobacco Dependence Screener test.
Table 9.
Multivariate analysis of SGRQ-improved versus non-improved among early quitters and continuous smokers
| Variable | OR | 95% CI | p Value |
|---|---|---|---|
| Age, year | 0.98 | 0.94 to 1.02 | 0.330 |
| Duration of smoking, year | 0.99 | 0.96 to 1.03 | 0.603 |
| TDS score | 1.39 | 1.17 to 1.66 | <0.001 |
| Baseline FEV1, % predicted | 0.98 | 0.96 to 1.01 | 0.134 |
| Baseline FVC, % predicted | 1.00 | 0.97 to 1.03 | 0.988 |
| Bronchial asthma | 1.35 | 0.55 to 3.39 | 0.508 |
FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; SGRQ, St. George's Respiratory Questionnaire; TDS, Tobacco Dependence Screener test.
Discussion
In the present study, HRQL improved significantly during the 3-month follow-up period among smokers who participated in a smoking cessation programme. Our smoking cessation programme improved HRQL not only in quitters but also in continuous smokers; no significant differences existed between quitters and continuous smokers with regard to HRQL improvement. Furthermore, we found that the average nicotine addiction level was associated with a clinically significant improvement in HRQL. These findings highlight an additional effect of smoking cessation treatment not previously discussed.
There is currently little information about potential changes in HRQL that can be provided to smokers who are trying to quit. Several previous studies6 8 16 showed that smoking cessation leads to improvement in HRQL. Our results support such findings. Therefore, when promoting smoking cessation to smokers, the impact on HRQL is highlighted as well as on longer term disease effects. What then about the quality of life of patients who fail to quit smoking?
There are conflicting findings about HRQL in patients who failed to quit smoking. McClave et al17 found that HRQL outcomes among current smokers who had unsuccessfully attempted to quit were worse than outcomes among former smokers. Croghan et al18 also found that continuous smokers treated for nicotine dependence reported less improvement in HRQL compared with those who stopped smoking. These studies suggested that HRQL in unsuccessful quitters may have been negatively affected by their failure to actually quit smoking. On the other hand, Wiggers et al19 examined the extent to which smoking cessation leads to changes in HRQL in cardiovascular patients. Multilevel modelling showed that generic and disease-specific HRQL in atherosclerotic patients improved significantly. No main differences were found between quitters and smokers in terms of improvement in HRQL. In fact, some subgroups reported a poorer HRQL after smoking cessation. Thus, atherosclerotic patients who quit smoking did not experience more improvement in HRQL compared with those who continued smoking. Quist-Paulsen et al16 reported that quitters and sustained smokers with coronary artery disease had similar improvements in HRQL from baseline to the 12-month follow-up. Similar to these reports (although they relate to patients with specific diseases), our results showed that improvements in HRQL were not significantly different in quitters compared to continuous smokers. Furthermore, after adjustment for comorbidities, the quit status after smoking cessation therapy was not associated with a clinically significant improvement in HRQL. Hays et al20 suggested that the positive impact on HRQL was mediated primarily by abstinence from smoking, but there also appeared to be direct effects of pharmacological treatment (eg, amelioration of withdrawal symptoms) that directly contributed to improved self-control and health transition.
Although the main focus in our study was on smoking cessation, the reduction in smoking in our study was notable. Even continuous smokers reported that the maximal numbers of cigarettes smoked daily during the previous 4 weeks ranged from 1 to 60 (median 5 cigarettes), with a corresponding low median exhaled CO level of 7 ppm, which was comparable to the ‘abstinent range’. This suggested that even though the participants in this group did not quit smoking completely they might have markedly reduced the number of cigarettes smoked. This might have led to an improvement in HRQL similar to the quitters, although changes in exhaled CO levels from baseline to 12 weeks were not related to an improvement in HRQL. Therefore, knowledge of the impact of smoking cessation therapy on HRQL may be important in encouraging smokers to participate in a smoking cessation programme. Improvement in health status or HRQL has been identified as an outcome criterion for the effectiveness of treatment for addictions.21
Furthermore, we found that after adjustment for baseline characteristics and comorbidities, the average nicotine addiction level was associated with a clinically significant improvement in HRQL. To the best of our knowledge, this is the first study to elucidate factors influencing the improvement in HRQL after smoking cessation therapy. In addition, the relationship between the nicotine addiction level and HRQL has not been elucidated to date. Although we made a diagnosis of nicotine dependence using the TDS test, this test was initially developed to screen for cases with nicotine dependence according to the DSM-III-R (Diagnostic and Statistical Manual of Mental Disorders), DSM-IV and ICD-10 (International Classification of Diseases).10 Thus, the TDS is regarded as a measure of the psychological and behavioural aspects of nicotine dependence.10 Such aspects of dependence may be important for prediction of changes in HRQL. Furthermore, nicotine dependence is a significant factor preventing smoking cessation. Ota et al22 reported high predictability of the TDS concerning smoking cessation among patients with coronary heart disease. Thus, our results encourage smokers, even if they are highly nicotine dependent and likely to quit smoking with difficulty, to participate in the smoking cessation programme to improve their health status.
When evaluating HRQL, both generic questionnaires and disease-specific questionnaires are available. Disease-specific questionnaires are likely to be more sensitive to particular symptoms and to slight responses to therapeutic interventions than are generic measures.23 Previous studies on the association between smoking cessation and HRQL used generic questionnaires such as the SF-36,6 8 9 18 20 the most popular generic instrument, EuroQOL6 or CDC HRQOL-4.17 In the present study, we used the SGRQ to measure HRQL in our cohort, which consisted of healthy smokers or smokers with various underlying diseases. The SGRQ, a respiratory-specific instrument, is especially designed to measure HRQL in patients with COPD.14 The reason we used the SGRQ was described in Methods section because our cohort consisted of smokers at risk of developing COPD or with early stages of COPD with a Brinkman Index ≥200. As we expected, previous studies using the SGRQ to measure HRQL in smoking cessation have been limited to those for patients with COPD. Tønnesen et al24 described changes in the SGRQ score in a smoking cessation study by nicotine replacement therapy for 370 smokers with COPD. A characteristic of their study is that the outcome of the smoking cessation therapy was divided into three groups: sustained abstainers, continuous smokers with no reduction and reducers. They found that reducers and sustained abstainers had both clinically and statistically significant improvements in all SGRQ scores and, with the exception of the activity score, improvements were greater in sustained abstainers than in reducers. No improvements in any of the SGRQ scores were shown in continuous smokers with no reduction. From these observations our results can be interpreted as follows. That is, most continuous smokers in our study might be considered as reducers as shown by the low exhaled CO levels at 12 weeks and the improvement in their SGRQ scores as well as those of quitters. Chen et al25 also reported changes in SGRQ scores by individual smoking cessation counselling for 85 smokers with COPD. They found that SGRQ scores were significantly improved in patients who abstained from smoking compared with those who failed to stop smoking. In that study, they did not mention the reduction in the number of cigarettes smoked or the exhaled CO level among patients with COPD who failed to stop smoking.
This study has some limitations. First, it was limited to one medical centre; therefore, the small sample size weakens the power of the study. Second, diagnoses of complications were self-reported and may underestimate the true population of participants with these complications. Third, we did not evaluate the follow-up HRQL in the dropout group, which might have biased the results. However, our study has highlighted the importance of completion of the smoking cessation treatment in improving HRQL regardless of quit status. An effort is needed to increase the completion rate of the smoking cessation programme in the future. Fourth, although we showed a short-term effect of smoking cessation therapy on improvements in HRQL, information on the long-term effects is not yet available. Continued improvement in HRQL with longer continuous abstinence has been confirmed.18 20 Therefore, long-term changes in HRQL, in particular in patients who failed to quit smoking, should be elucidated in the future. Finally, as we used a disease-specific HRQL measure, rather than a global measure designed to capture multiple important life domains such as the Quality of Life Inventory,26 our results should apply to only disease-specific HRQL.
In conclusion, HRQL of participants in the smoking cessation programme, being those who successfully quit as well as those who failed, improved significantly after the treatment. Baseline nicotine addiction level measured by the TDS was a predictor of that improvement. Further studies are needed to clarify the long-term effect of smoking cessation therapy on HRQL.
Acknowledgments
Special thanks to Dr Koichi Nishimura and Dr Paul W Jones for permitting use of the Japanese version of the SGRQ. Also, thanks to Dr Toshihiko Kaneda, Dr Yoko Kida, Dr Masahiro Kaneko, Dr Yukiko Odake and Dr Takehiro Nakamura who aided in data collection. In addition, the authors wish to thank the exceptional staff at the smoking cessation clinic for their assistance.
Footnotes
Contributors: HT takes responsibility for the study as a whole; undertook the statistical analyses and drafted the manuscript. All authors agreed on the study methodology. CN and RS obtained the data. All authors agreed on the interpretation of the results. GI revised the draft.
Funding : This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None.
Ethics approval: The Institutional Review Board (Clinical Research) Kobe City Medical Center West Hospital (project approval number 12-10).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.WHO. Report on the global tobacco epidemic, 2013: Enforcing bans on tobacco advertising, promotion and sponsorship. Geneva, Switzerland: World Health Organization, 2013 [Google Scholar]
- 2.Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observation on male British doctors. BMJ 2004;328:1519–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mamun AA, Peeters A, Barendregt J, et al. Smoking decreases the duration of life lived with and without cardiovascular disease: a life course analysis of the Framingham Heart Study. Eur Heart J 2004;25:409–15 [DOI] [PubMed] [Google Scholar]
- 4.Sakata R, McGale P, Grant EJ, et al. Impact of smoking on mortality and life expectancy in Japanese smokers: a prospective cohort study. BMJ 2012;345:e7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyons RA, Lo SV, Littlepage BNC. Perception of health amongst ever-smokers and never-smokers: a comparison using the SF-36 Health Survey Questionnaire. Tob Control 1994;3:213–15 [Google Scholar]
- 6.Tillmann M, Silcock J. A comparison of smokers’ and ex-smokers’ health-related quality of life. J Public Health Med 1997;19:268–73 [DOI] [PubMed] [Google Scholar]
- 7.Mulder I, Tijhuis M, Smit HA, et al. Smoking cessation and quality of life: the effect of amount of smoking and time since quitting. Prev Med 2001;33:653–60 [DOI] [PubMed] [Google Scholar]
- 8.Wilson D, Parsons J, Wakefield M. The health-related quality-of-life of never smokers, ex-smokers, and light, moderate, and heavy smokers. Prev Med 1999;29:139–44 [DOI] [PubMed] [Google Scholar]
- 9.Schmitz N, Kruse J, Kugler J. Disabilities, quality of life, and mental disorders associated with smoking and nicotine dependence. Am J Psychiatry 2003;160:1670–6 [DOI] [PubMed] [Google Scholar]
- 10.Kawakami N, Takatsuka N, Inaba S, et al. Development of a screening questionnaire for tobacco/nicotine dependence according to ICD-10, DSM-III-R, DSM-IV. Addict Behav 1999;24:155–66 [DOI] [PubMed] [Google Scholar]
- 11.Brinkman GL, Coates EO, Jr. The effect of bronchitis, smoking and occupation on ventilation. Am Rev Respir Dis 1963;87:684–93 [DOI] [PubMed] [Google Scholar]
- 12.The Japanese Respiratory Society. Special Committee of Pulmonary Physiology. Standard values of spirogram and arterial blood gas in normal Japanese subjects. J Jpn Respir Soc 2001;39:s1–17 (in Japanese). [Google Scholar]
- 13.Hajiro T, Nishimura K, Tsukino M, et al. Comparison of discriminative properties among disease-specific questionnaires for measuring health-related quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:785–90 [DOI] [PubMed] [Google Scholar]
- 14.Jones PW, Quirk FH, Baveystock CM, et al. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis 1992;145:1321–7 [DOI] [PubMed] [Google Scholar]
- 15.Jones PW, Quirk FH, Baveystock CM. The St. George's Respiratory Questionnaire. Respir Med 1991;85(Suppl B):25–31 [DOI] [PubMed] [Google Scholar]
- 16.Quist-Paulsen P, Bakke PS, Gallefoss F. Does smoking cessation improve quality of life in patients with coronary heart disease? Scand Cardiovasc J 2006;40:11–16 [DOI] [PubMed] [Google Scholar]
- 17.McClave AK, Dube SR, Strine TW, et al. Association between health-related quality of life and smoking status among a large sample of U.S. adults. Prev Med 2009;48:173–9 [DOI] [PubMed] [Google Scholar]
- 18.Croghan IT, Schroeder DR, Hays JT, et al. Nicotine dependence treatment: perceived health status improvement with 1-year continuous smoking abstinence. Eur J Pub Health 2005;15:251–5 [DOI] [PubMed] [Google Scholar]
- 19.Wiggers LC, Oort FJ, Peters RJ, et al. Smoking cessation may not improve quality of life in atherosclerotic patients. Nicotine Tob Res 2006;8:581–9 [DOI] [PubMed] [Google Scholar]
- 20.Hays JT, Croghan IT, Baker CL, et al. Changes in health-related quality of life with smoking cessation treatment. Eur J Public Health 2012;22:224–9 [DOI] [PubMed] [Google Scholar]
- 21.McLellan AT, Woody GE, Metzger D. Evaluating the effectiveness of addiction treatment: reasonable expectation, approaches, and comparisons. Milbank Q 1996;74:51–85 [PubMed] [Google Scholar]
- 22.Ota A, Mino Y, Mikouchi H, et al. Nicotine dependence and smoking cessation after hospital discharge among inpatients with coronary heart attacks. Environ Health Prev Med 2002;7:74–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marcia AT, Simonson DC. Assessment of quality-of-life. N Engl J Med 1996;334:835–40 [DOI] [PubMed] [Google Scholar]
- 24.Tønnesen P, Mikkelsen K, Bremann L. Nurse-conducted smoking cessation in patients with COPD using nicotine sublingual tablets and behavioral support. Chest 2006;130:334–42 [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Chen Y, Chen P, et al. Effectiveness of individual counseling for smoking cessation in smokers with chronic obstructive pulmonary disease and asymptomatic smokers. Exp Ther Med 2014;7:716–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frisch MB, Cornell J, Villanueva M, et al. Clinical validation of the Quality of Life Inventory. A measure of life satisfaction for use in treatment planning and outcome assessment. Psychol Assess 1992;4:92–101 [Google Scholar]


