Abstract
The ethanolic extract of grains of paradise (Aframomum melegueta Schum, Zingiberaceae) has been evaluated for inhibitory activity on cyclooxygenase-2 (COX-2) enzyme, in vivo for the anti-inflammatory activity and expression of several pro-inflammatory genes. Bioactivity-guided fractionation showed that the most active COX-2 inhibitory compound in the extract was [6]-paradol. [6]-Shogaol, another compound from the extract, was the most active inhibitory compound in pro-inflammatory gene expression assays. In a rat paw edema model, the whole extract reduced inflammation by 49% at 1000 mg/kg. Major gingerols from the extract [6]-paradol, [6]-gingerol, and [6]-shogaol reduced inflammation by 20, 25 and 38%. respectively when administered individually at a dose of 150 mg/kg. [6]-Shogaol efficacy was at the level of aspirin, used as a positive control. Grains of paradise extract has demonstrated an anti-inflammatory activity, which is in part due to the inhibition of COX-2 enzyme activity and expression of pro-inflammatory genes.
Keywords: anti-inflammatory, grains of paradise, Aframomum melegueta Schum, Zingiberaceae, gingerols, COX-2, paw edema
Introduction
Inflammation is the localized response of a tissue to injury, caused by a mechanical or biological agent or by an aberrant autoimmune response.1 The arachidonic acid pathway constitutes one of the main cellular mechanisms for mediating inflammation. This pathway includes the cyclooxygenase pathway and the 5-lipoxygenase pathway. Prostaglandins are the end products of the cyclooxygenase pathway. The enzymes involved in prostaglandin synthesis and the receptors to which prostaglandins bind are well-known pharmacological targets. For example, aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs) reduce inflammation by reducing prostaglandin synthesis by inhibiting cyclooxygenase. In humans, cyclooxygenase is present in at least two isoforms, cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2). COX-1 is expressed constitutively in most tissues and performs many “housekeeping” functions, such as maintaining the protective lining of the stomach, regulating blood flow through the kidneys, and promoting platelet aggregation, whereas COX-2 is an inducible isoform that is mainly produced in inflamed tissues.2
Compounds that inhibit COX-2 activity or lower its expression are significant not only for the treatment of inflammatory responses but also for human health and wellness in general.3,4 In our search for botanical cyclooxygenase inhibitors with anti-inflammatory activity, we tested an ethanolic extract of grains of paradise (Aframomum melegueta) in in vitro and in vivo assays. This plant is a member of the ginger family (Zingiberaceae) and grows wild or is cultivated in tropical areas of Africa, notably West Africa. The plant seeds are used to flavor foods and as components of traditional African medicine. In medieval Europe they were a highly prized spice that was eventually replaced by black pepper and other spices after the opening of the Asian trade routes. Ethnobotanically, the seed extract is used as a remedy against stomachache, diarrhea, and snakebite.5,6 Additionally, there are reported studies on antiulcer, cytoprotective, and antimicrobial activities as well as a sexual performance enhancing effects of grains of paradise.7−11 The aqueous seed extract has been shown to reduce the frequency of abdominal constrictions induced by acetic acid in mice and has significant anti-inflammatory activity.12 It was later reported that the same extract has peripheral analgesic activity.12 Additionally, it was suggested that the extract has membrane-stabilizing activity along with antioxidant effects,13 as well as hypotensive and antihypertensive activity in humans.14 Recently, it has been also found that the extract has an effect on the whole-body energy expenditure and visceral fat in humans.15−17
The objectives of this study were to evaluate the anti-inflammatory effects of grains of paradise for the inhibition of COX-2 enzyme activity as well as the inhibition of expression of pro-inflammatory genes (in vitro) and anti-inflammatory activity (in vivo) in a rat paw edema assay and to characterize active compound(s) from the extract that could be potentially beneficial in the treatment of inflammatory conditions.
Materials and Methods
Chemicals and Reagents
All chemicals and reagents were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA) unless otherwise indicated. [6]-Gingerol and [6]-shogaol (95% purity) were purchased from Dalton Chemical Laboratories Inc. (Toronto, ON, Canada). Aspirin (purity > 99%) and Vioxx (Merck and Co., Inc., West Point, PA, USA; purity > 98%) were used as positive controls.
Plant Material
Seeds of A. melegueta were commercially purchased from Abidjan, Ivory Coast, and identified by Dr. L. Struwe, Rutgers University. A voucher specimen (Struwe 1424 CHRB) is deposited at the Chrysler Herbarium, Rutgers University, New Brusnwick, NJ, USA.
Preparation of the Extract
Dry seeds were ground into powder, and the seed powder (2 g) was extracted in 95% ethanol (20 mL) for 24 h at room temperature with continuous agitation provided by a platform shaker. After extraction, the sample was filtered and the solvent removed by rotary evaporation, yielding 40.25 mg (2%). The extract was dissolved in 95% ethanol to appropriate concentrations for in vitro assays, chemical characterization, and bioactivity-guided fractionation.
COX-2 in Vitro Assay
Grains of paradise extract was dissolved in 95% ethanol at a concentration of 1 mg/mL. The activity of the extract was tested with a colorimetric COX (ovine) inhibitor screening assay (catalog no. 760111, Cayman Chemical, Ann Arbor, MI, USA). The screening assay measures the peroxidase activity of cyclooxygenase by monitoring the appearance of oxidized N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD) at 590 nm as previously described.12 A relatively higher TMPD concentration corresponds to a greater absorbance at 590 nm, a greater peroxidase activity, and a greater COX-2 activity (i.e., less inhibition of COX-2 activity). The assays were performed according to the manufacturer’s instructions.
Bioassay-Guided Purification of the Extract
Purification and isolation of compounds were carried out using a preparatory HPLC from Waters Corp. (Milford, MA, USA) consisting of a W717 plus autosampler, a W600E multisolvent delivery system, a W600 controller, a W490E multiwavelength detector, and a Waters fraction collector. A Waters liquid chromatography–mass spectrometry (LC-MS) Integrity system consisting of a solvent delivery system with a W616 pump and a W600S controller, a W717 plus autosampler, a W996 PDA detector, and a Waters TMD Thermabeam electron impact (EI) single-quadrupole mass detector with fixed ionization energy of 70 eV was used for analysis. Data were collected and analyzed with the Waters Millennium v. 3.2 software, linked with the sixth edition of the Wiley Registry of Mass Spectral Data, containing 229,119 EI spectra of 200,500 compounds.
Gene Expression: Macrophage Cell Culture
The mouse monocyte/macrophage cell line RAW 264.7 (ATCC TIB-71 obtained from American Type Culture Collection, Manassas, VA, USA) was maintained in Dulbecco’s modified Eagle medium (DMEM; Invitrogen Inc., Carlsbad, CA, USA) supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% heat-inactivated fetal bovine serum (Sigma-Aldrich Co.). The cells were kept in a 37 °C incubator with 5% CO2. Cells were subcultured by scraping when plates reached 90% confluence with a 1:5 ratio in fresh medium.
Cells were seeded at a density of 4 × 105 cells per well (viable cell counts were carried out by trypan blue staining using a hemocytometer) in 24-well plates 12 h prior to treatment. The cells were then treated with test compounds dissolved in dimethyl sulfoxide (DMSO) at predetermined doses (based on MTS assays) for 2 h before elicitation with bacterial endotoxin lipopolysaccharide from Escherichia coli serotype 055:B5 (LPS) (Sigma-Aldrich Co.) at 1 μg/mL for an additional 6 h. For every experiment one positive control (cells treated only with LPS and vehicle) and one negative control (cells treated with vehicle only) were included. Three replicates were made for both treatments and the controls. Final concentration of DMSO in cells was 1%. The same concentration was used as vehicle control. At the end of the treatment period, cells were harvested in Trizol reagent for subsequent cellular RNA extraction.
Gene Expression: Cell Viability Assay and Dose Range Determination
A Cell Titer 96 MTS assay kit (Promega Corp., Madison, WI, USA) was used to determine the relative number of viable cells remaining after incubation with treatments. The assay has been performed according to the manufacturer’s protocol and a previously described method.18. In short, the assay was performed by treating cells with different extract concentrations, followed by adding 20 μL of reagent 3-4,5-(dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium/phenazine methosulfate (MTS/PMS) directly to culture wells and incubating for 3 h at 37 °C. Plates were read using a microplate reader (Molecular Devices, Sunnyvale, CA, USA) at 490 nm. For dose range studies, the highest nontoxic concentrations for respective compound/extract were selected.
Gene Expression: Quantitative Polymerase Chain Reaction (qPCR) and Data Analysis
The synthesized cDNAs were diluted 4-fold. Two microliters of each diluted sample was added to 0.5 μL of gene-specific primers (6 μM, oligos synthesized by IDT Inc., Coralville, IA, USA) and 12.5 μL of Brilliant SYBR green PCR master mix (2×) (Stratagene, La Jolla, CA, USA) containing green jump-start Taq ready mix, and the final volume was brought to 25 μL by adding sterile distilled water. Carboxy-X-rhodamine (ROX) was used as an internal dye. To avoid interference due to genomic DNA contamination, only intron-overlapping primers were selected using Primer Express vers. 2.0 software (Applied Biosystems, Foster City, CA, USA) as shown in Table 1. PCR amplifications were performed on a MX3000p system (Stratagene, La Jolla, CA, USA) as described in ref (19). RNA expressions for genes of interest, normalized with respect to the expression of housekeeping β-actin gene, were analyzed using the ΔΔCt method.19
Table 1. Primer Sequences Used for RT-PCR.
| gene (accession no.) | forward | reverse |
|---|---|---|
| COX-2 (NM_011198) | 5′-TGGTGCCTGGTCTGATGATG-3′ | 5′-GTGGTAACCGCTCAGGTGTTG-3′ |
| iNos2 (XM_147149) | 5′-CCCTCCTGATCTTGTGTTGGA-3 | 5′-TCAACCCGAGCTCCTGGAA-3′ |
| IL1β (NM_008361) | 5′-CAACCA ACAAGTGATATTCTCCATG-3′ | 5′-GATCCACACTCTCCA GCTGCA-3′ |
| TNF-α (NM_013693) | 5′-CATCTTCTCAAAATTCGAGTGACAA-3′ | 5′-TGGGAGTAGACAAGGTACAACCC-3′ |
| IL6 (NM_031168) | 5′-TAGTCCTTCCTACCCCAATTTCC-3′ | 5′-TTGGTCCTTAGCCACTCCTTC-3′ |
| actin (NM_007393) | 5′-AACCGTGAAAAGATGACCCAGAT-3′ | 5′-CACAGCCTGGATGGCTACGT-3′ |
Carrageenan-Induced Rat Paw Edema
Groups of Long Evans-derived male rats with body weights of 150 ± 10 g were housed six animals per cage in a room kept at 22–23 °C with 60–70% humidity and a 12 h light/dark cycle for 1 week prior to the start of the study. Access to standard laboratory chow and water was ad libitum. All aspects of this work including housing, experimentation, and disposal of animals were performed in general accordance with the International Guiding Principles for Biomedical Research Involving Animals (CIOMS Publication ISBN 9290360194, 1985), and the protocol was approved by MDS Pharma (King of Prussia, PA, USA). The animals were fasted overnight prior to use.
Grains of paradise extract and pure compounds ([6]-paradol, [6]-shogaol, [6]-gingerol) were administered per os (extract at 1000 and 500 mg/kg and pure compounds at 150 mg/kg) suspended in 0.2% Tween-80/10% ethanol 1 h before right hind paw injection of carrageenan (0.1 mL of 1% suspension intraplantar, TCI, Tokyo, Japan). Aspirin (150 mg/kg, Sigma-Aldrich Co.) was used as a reference compound (positive control). The severity of the hind paw edema as a measure of inflammation was recorded 3 h after carrageenan administration using a plethysmometer (Ugo Basile, Comerio, VA, Italy) with water cell (25 mm diameter). Reduction of edema was calculated by comparison with vehicle control. The anti-inflammatory activity is expressed as a percentage of edema reduction.
Statistical Analysis
Statistical comparisons were performed between the vehicle control and treated groups. For the in vitro and in vivo assays, the results are presented as the mean ± standard deviation (SD) The statistical analysis was calculated by using the analysis of variance (ANOVA) test and followed by post hoc analysis using Tukey’s multiple-comparison test.
Results and Discussion
In a search for novel anti-inflammatory compounds we evaluated ethanolic extract of grains of paradise (A. melegueta) for inhibition of COX-2 enzyme activity, inhibition of pro-inflammatory genes expression (in vitro), and anti-inflammatory activity in rat paw edema assay (in vivo), and the active compounds from the extract were characterized.
COX-2 Enzyme Inhibitory Activity and Active Compound Characterization
At an initial concentration of 1 mg/mL, the ethanolic extract demonstrated inhibitory activity against COX-2, similar to the drug Vioxx that was used as a positive control. The level of inhibition for the extract was 76% compared to 87% for Vioxx (Table 2). Biochemical analytical data indicated that the extract is a complex mixture of compounds. In order to identify compounds responsible for the observed activity, the extract was subjected to bioactivity guided fractionation with concurrent LC-MS analysis. Bioactivity guided fractionation was performed in the following manner:
Table 2. In Vitro COX-2 Enzyme Inhibition by the Ethanolic Extract of Grains of Paradise and Its Anti-inflammatory Componentsa.
| treatment | inhibition (%) ± SD |
|---|---|
| controlb | 2 ± 2 |
| A. melegueta extract | 76 ± 6.7*** |
| [6]-gingerol | 7 ± 11.7 |
| [6]-shogaol | 68 ± 6.4*** |
| [6]-paradol | 91 ± 3.6*** |
| Vioxx | 87 ± 1.5*** |
Data are shown as mean inhibition ± SD in %. Significance: ***, 0.1%.
All samples were tested at the concentration of 1 mg/mL. Control, celery extract at 1 mg/mL; whole extract, grains of paradise (A. melegueta) extract at 1 mg/mL; Vioxx (MW 314.36) at 1 mg/mL, 3.18 mM; [6]-gingerol (MW 294.38) at 1 mg/mL, 3.39 mM; [6]-shogaol (MW 276.37) at 1 mg/mL, 3. 62 mM; [6]-paradol (MW 278) at 1 mg/mL, 3.60 mM.
Grains of paradise extract (1 g, obtained from a large-scale extraction) was dissolved in 95% ethanol (20 mL) and fractionated using a preparatory HPLC. For the initial purification, a Waters 19 × 300 mm symmetry preparative C8 reverse phase column was used. The mobile phases consisted of 100% acetonitrile (CH3CN) and 0.5% of acetic acid in water (v/v). For the initial separation, a gradient from 5% CH3CN to 95% CH3CN over 35 min was used with a flow rate of 8 mL/min. Ten fractions (volume of 40 mL each) at 5 min intervals were collected and tested for COX-2 inhibitory activity. Fraction 8 (F8; yield = 360 mg) had the highest activity and was further purified using similar conditions as before except the gradient was run over 70 min. Four fractions were collected from F8, and they were subjected to the COX-2 assay. The most active fraction, F8-2 (4 mL volume, eluted at 59.2 min at 282 nm; yield = 90 mg), was then purified using a 10 × 250 mm Curacil-PFP column and an isocratic run with a mobile phase consisting of 0.5% acetic acid in water/methanol/CH3CN (2:3:5) at a flow rate 3 mL/min. Four fractions were collected from F8-2, and F8-2-3 (1.45 mL volume, eluted at 8.67 min, at 282 nm; yield = 49.5 mg) was found to have the highest inhibitory activity on COX-2 enzyme. This fraction was refractionated using the conditions described previously, with the exception of the flow rate, which was reduced to 1 mL/min. Three fractions were collected from F8-2-3, and F8-2-3-2 (1.48 mL volume, eluted at 25.29 min, at 282 nm; yield = 32.2 mg) had the highest inhibitory activity. This fraction was identified as [6]-paradol (95% purity) using LC-MS and 1H, 13C, and 2D NMR data.20,21 The purification procedure was repeated multiple times to obtain sufficient amounts of [6]-paradol for in vitro and in vivo experiments.
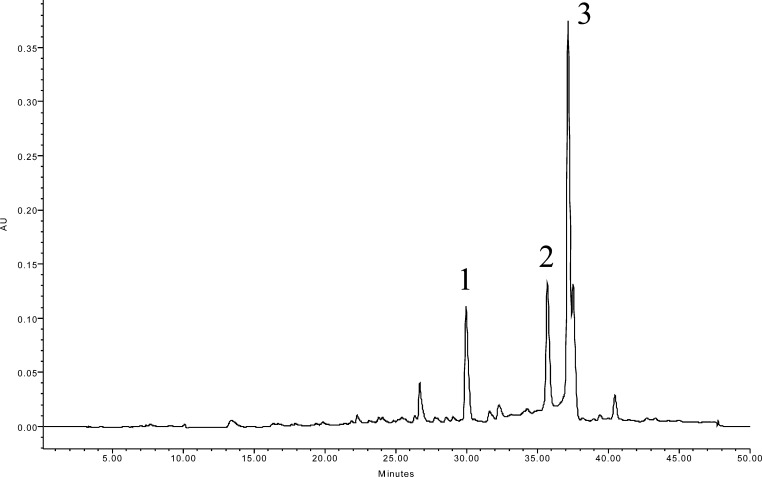
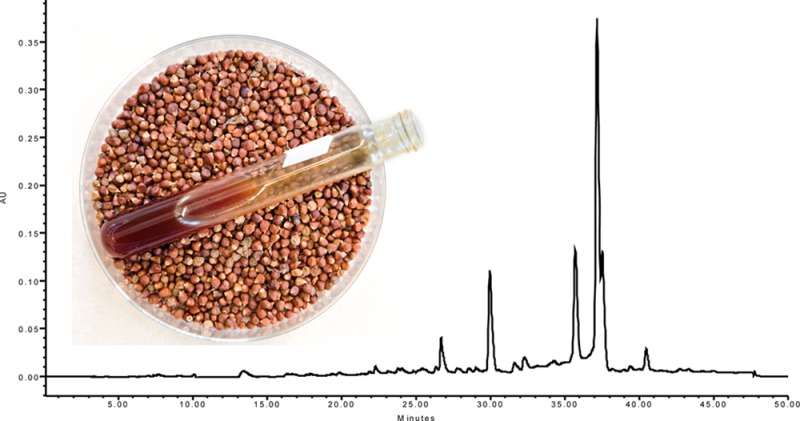
Chemical analysis of the extract revealed three major peaks (Figure 1). The UV spectra and mass fragmentation patterns of these peaks matched the compounds of the gingerol family previously identified in A. melegueta(22) and in other members of Zingiberaceae.23 The most abundant were putatively identified as [6]-gingerol, [6]-shogaol, and [6]-paradol at 1.0 mg/g [6]-gingerol, 0.53 mg/g [6]-shogaol, and 1.2 mg/g [6]-paradol per gram of the extract. All three compounds were assayed for COX-2 enzyme inhibitory activity at the same concentration (1 mg/mL) as the whole extract and Vioxx. [6]-Paradol was the most active with 91% inhibition, followed by [6]-shogaol (68%), and [6]-gingerol (7%) (Table 2). Paradol inhibitory activity was higher than those of Vioxx (87%) and the whole extract (76%), confirming it as the most active compound as found in the bioactivity-guided fractionation assay. [6]-Gingerol and [6]-shogaol were commercially purchased, and [6]-paradol was isolated by bioactivity-guided fractionation (as described above).
Figure 1.
HPLC chromatogram of grains of paradise (A. melegueta) extract showing putative compounds. Peaks: 1, [6]-gingerol; 2, [6]-shogaol; 3, [6]-paradol.
Inhibition of Expression of Pro-inflammatory Genes (in Vitro)
In addition to inhibiting the pro-inflammatory COX-2 enzyme, the extract of grains of paradise was also tested for inhibition of pro-inflammatory genes. The genes tested were tumor necrosis factor alpha (TNFα), interleukin-1beta (IL-1β), interleukin-6 (IL-6), COX-2, and inducible nitric oxide synthase (iNOS).
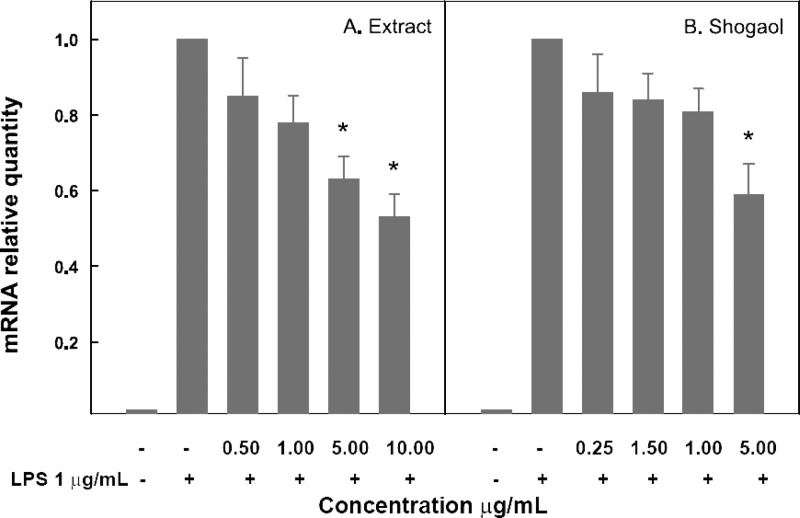
The in vitro experiments were designed to quantify the relative amount of transcripts for target genes (COX2, iNOS, TNFα, IL6, and IL1β) within the total RNA in individual cell batches following treatments with the extract or its three major gingerols. All experiments were started with an equal number of cells for each treatment. For each assay, two control sets were monitored (Figure 2). The in vitro experiments showed that both ethanolic extract of grains of paradise extract and [6]-shogaol were inhibitory in a dose-dependent manner (p < 0.05 by ANOVA) on IL1β (Figure 2) but not on other genes tested. The extract was significantly cytotoxic to macrophages at concentrations >20 μg/mL and hence could not be tested for gene expression inhibition beyond that concentration. [6]-Shogaol did not affect the transcription of other genes at 5 μg/mL, its highest nontoxic concentration. [6]-Paradol and [6]-gingerol did not show any significant inhibitory activities at the noncytotoxic concentration range up to 30 and 50 μg/mL, respectively (data not shown).
Figure 2.
Effect of grains of paradise extract (A) and [6]-shogaol (B) on IL1β gene expression in LPS-activated RAW macrophages measured by the mRNA quantity relative to the response to LPS activation only (positive control) that is normalized to a value of 1.00. Lower values represent greater inhibitory effects with 0.00 corresponding to a complete inhibition of the induced gene expression. Values are the mean ± SD. (∗) p < 0.05 (compared with positive control).
Anti-inflammatory Activity in Rat Paw Edema Assay (in Vivo)
To investigate whether the observed in vitro COX-2 enzyme and gene expression inhibitory properties translate into in vivo anti-inflammatory activity, we tested the extract in a rat paw carrageenan edema assay, which is commonly used for testing anti-inflammatory agents. Initially, two doses of the whole extract were given per os (orally) to the animals, 1000 and 500 mg/kg. Aspirin was used as a positive control at a 150 mg/kg dose. At 1000 mg/kg, the extract demonstrated anti-inflammatory effect, reducing edema by 49% (Table 3). At the lower dose (500 mg/kg) the extract exhibited a reduced anti-inflammatory effect (11% edema reduction). At the same time, aspirin reduced edema by 43% at 150 mg/kg. Therefore, grains of paradise extract does have in vivo anti-inflammatory activity, but it was not as effective as aspirin.
Table 3. Inhibition of Carrageenan-Induced Rat Paw Edema by Grains of Paradise Ethanolic Extract and Its Main Components, [6]-Gingerol, [6]-Shogaol, and [6]-Paradol, with Aspirin as a Positive Controla.
| treatment | dose | average paw difference ± SD | inhibition (%) |
|---|---|---|---|
| I | |||
| vehicle | 10 mL/kg | 90 ± 3.5 | |
| A. melegueta extract | 500 mg/kg | 80 ± 13.7 | 11 |
| 1000 mg/kg | 46 ± 4.2** | 49 | |
| aspirin | 150 mg/kg | 51 ± 5.1** | 43 |
| II | |||
| vehicle | 10 mL/kg | 85 ± 3.3 | |
| [6]-paradol | 150 mg/kg | 68 ± 6.7* | 20 |
| aspirin | 150 mg/kg | 54 ± 8.5*** | 36 |
| III | |||
| vehicle | 10 mL/kg | 79 ± 3.4 | |
| [6]-shogaol | 150 mg/kg | 49 ± 5.4*** | 38 |
| [6]-gingerol | 150 mg/kg | 59 ± 8.5** | 25 |
| aspirin | 150 mg/kg | 50 ± 8.6*** | 37 |
Test substances were administered orally 60 min before the right hind paw received an injection of carrageenan (0.1 mL of 1% suspension intraplantar). Hind paw edema was measured 3 h later; paw sizes were compared, and average paw difference (expressed in mL) established and inhibition were calculated relative to the vehicle-treated animals in the control group. Data are shown as the mean ± SD and percentages of inhibition. Significance levels: *, 5%; **, 1%; ***, 0.1%.
To analyze the anti-inflammatory activity of single compounds, the most active compound in the COX-2 enzymatic assay, [6]-paradol, has been isolated from the extract and tested in in vivo assay at the same dose level as aspirin (150 mg/kg), which was used as a positive control. At this dose it inhibited the rat paw edema by 20% and aspirin by 36%, respectively. Two other compounds from the extract, [6]-gingerol and [6]-shogaol, given at 150 mg/kg, inhibited edema by 25 and 38%, respectively. In the same assay aspirin given at 150 mg/kg inhibited the edema by 37%. Therefore, [6]-shogaol, the most active compound in the gene expression assays, was also the most active compound in vivo and demonstrated anti-inflammatory activity similar to that of aspirin (Table 3).
Results of our study demonstrated the inhibitory activity of grains of paradise ethanolic extract in COX-2 in vitro enzymatic assay. The bioactivity-guided fractionation revealed that the [6]-paradol present in the extract is the most effective inhibitor of COX-2 enzymatic activity. [6]-Paradol and two other major compounds present in the extract, [6]-gingerol and [6]-shogaol, belong to the gingerol family of compounds. The results for the enzymatic inhibition support previous reports on gingerols as inhibitors of the arachidonic acid biosynthetic pathway. In one study, ginger rhizome extract (Zingiber officinale Roscoe, Zingiberaceae) was investigated for inhibition of COX-1 enzyme and antiplatelet aggregation activity. [8]-Paradol, a natural constituent of ginger, was the most active inhibitor and stronger than aspirin.24 Similar studies on COX-2 enzyme inhibitory activity revealed that ginger constituents [8]-paradol and [8]-shogaol had strong inhibitory activity.25 Additionally, structure–activity study on gingerols and COX-2 inhibition has shown that the paradols showed the highest affinity for COX-2 followed by the shogaols and then the gingerols.26 Our data, together with the above-mentioned studies on compounds from ginger, suggest that compounds from the gingerol family are cyclooxygenase inhibitors. When the gingerols from the extract were tested for the inhibition of expression of pro-inflammatory genes (COX2, iNOS, TNFα, IL6, and IL1β), the whole extract and [6]-shogaol exhibited inhibitory activity on IL1β gene expression, whereas other genes have not been affected.
To evaluate how these in vitro activities at enzyme and gene level transpond into in vivo activity, we used the cyclooxygenase-dependent carrageenan-induced paw edema assay,27 a standard model to reproduce acute inflammation in vivo. The ethanolic extract of grains of paradise exhibited anti-inflammatory activity in this assay. This also confirmed the anti-inflammatory activity reported earlier for the aqueous extract of grains of paradise.5,12 Additionally, the three major gingerols present in the extract were also effective in reducing the inflammation with [6]-shogaol at a level comparable to that of aspirin.
Because grains of paradise ethanolic extract and major gingerols present in it showed in vitro inhibition of COX-2 enzyme activity and ethanolic extract and [6]-shogaol reduction in expression of pro-inflammatory IL1β gene, we could hypothesize that the extract may have a dual mode of action, at the gene and enzyme levels. Inhibition by [6]-paradol and other gingerols present in the extract of COX-2 enzyme activity and inhibition by [6]-shogaol of pro-inflammatory gene expression could impair prostaglandin (the end product of the cyclooxygenase pathway) production, thereby reducing the inflammatory process in vivo. Gingerols from ginger (Z. officinale) have been previously reported to inhibit prostaglandin biosynthesis.28 However, to put more light on this, our extract and its compounds require further studies, especially on how the whole ethanolic extract and its three major isolated gingerols affect prostaglandin levels. In conclusion, this study with in vitro and in vivo assays has demonstrated that the ethanolic extract of grains of paradise and its major compounds have anti-inflammatory potential at the enzyme and in vivo levels, but they need to be further evaluated.
Acknowledgments
Excellent technical assistance was provided by Reneta Pouleva and Ruth Dorn. We thank Cheryl Lyn Dybas for a critical reading of the manuscript.
Research supported by Phytomedics Inc. (Jamesburg NJ, USA); NIH Center for Dietary Supplements Research on Botanicals and Metabolic Syndrome, Grant 1-P50 AT002776-01; Fogarty International Center of the NIH under U01 TW006674 for the International Cooperative Biodiversity Groups; and Rutgers University. Additional support came from the National Center for Complementary and Alternative Medicine of NIH under 1-K99 AT004245-01.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Gallin J. I.; Snyderman R.. Inflammation: Basic Principles and Clinical Correlates, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1999; pp 1–3. [Google Scholar]
- Dannenberg A. J.; Altorki N. K.; Boyle J. O.; Dang C.; Howe L. R.; Weksler B. B.; Subbaramaiah K. Cyclo-oxygenase 2: a pharmacological target for the prevention of cancer. Lancet Oncol. 2001, 2, 544–551. [DOI] [PubMed] [Google Scholar]
- Huss U.; Ringbom T.; Perera P.; Bohlin L.; Vasaenge M. Screening of ubiquitous plant constituents for COX-2 inhibition with a scintillation proximity based assay. J. Natl. Prod. 2002, 65, 1517–1521. [DOI] [PubMed] [Google Scholar]
- Surh Y. J. Anti-tumor promoting potential of selected spice ingredients with antioxidative and anti-inflammatory activities: a short review. Food Chem. Toxicol. 2002, 40, 1091–1097. [DOI] [PubMed] [Google Scholar]
- Umukoro S.; Ashorobi R. B. Further studies on the antinociceptive action of aqueous seed extract of Aframomum melegueta. J. Ethnopharmacol. 2007, 109, 501–504. [DOI] [PubMed] [Google Scholar]
- Akendengue B.; Louis A. M. Medicinal plants used by the Masango people in Gabon. J. Ethnopharmacol. 1994, 41, 193–200. [DOI] [PubMed] [Google Scholar]
- Galal A. M. Anti-microbial activity of 6-paradol and related compounds. Int. J. Pharmacog. 1996, 31, 64–69. [Google Scholar]
- Kamtchouing P.; Mbongue G. Y. F.; Dimo T.; Watcho P.; Jatsa H. B.; Sokeng S. D. Effects of Aframomum melegueta and Piper guineense on sexual behaviour of male rats. Behav. Pharmacol. 2002, 13, 243–247. [DOI] [PubMed] [Google Scholar]
- Alo M. N.; Anyim C.; Igwe J. C.; Elom M.; Uchenna D. S. Antibacterial activity of water, ethanol and methanol extracts of Ocimum gratissimum, Vernonia amygdalina and Aframomum melegueta. Adv. Appl. Sci. Res. 2012, 3, 844–848. [Google Scholar]
- Chiejina N. V.; Ukeh J. A. Antimicrobial properties and phytochemical analysis of methanolic extracts of Aframomum melegueta and Zingiber officinale on fungal diseases of tomato fruit. J. Nat. Sci. Res. 2012, 2, 10–15. [Google Scholar]
- Mbongue G. Y. F.; Kamtchouing P.; Dimo T. Effects of the aqueous extract of dry seeds of Aframomum melegueta on some parameters of the reproductive function of mature male rats. Andrologia 2012, 44, 53–58. [DOI] [PubMed] [Google Scholar]
- Umukoro S.; Ashorobi R. B. Effect of Aframomum melegueta seed extract on thermal pain and on carrageenin-induced edema. Nig. Q. J. Hosp. Med. 2001, 11, 220–225. [Google Scholar]
- Umukoro S.; Ashorobi R. B. Further pharmacological studies on aqueous seed extract of Aframomum melegueta in rats. J. Ethnopharmacol. 2008, 115, 489–493. [DOI] [PubMed] [Google Scholar]
- Lawal B. A. S.; Aderibigbe A. O.; Essiet G. A.; Essien A. D. Hypotensive and antihypertensive effects of Aframomum melegueta seeds in humans. Int. J. Pharmacol. 2007, 3, 311–318. [Google Scholar]
- Sugita J.; Yoneshiro T.; Hatano T.; Sayuri A.; Takeshi I.; Hideyo U.; Toshihiko I.; Toshimitsu K.; Yuko K.; Masayuki S. Grains of paradise (Aframomum melegueta) extract activates brown adipose tissue and increases whole-body energy expenditure in men. Br. J. Nutr. 2013, 110, 733–738. [DOI] [PubMed] [Google Scholar]
- Sugita J.; Yoneshiro T.; Sugishima Y.; Ikemoto T.; Uchiwa H.; Suzuki I.; Masayuki S. Daily ingestion of grains of paradise (Aframomum melegueta) extract increases whole-body energy expenditure and decreases visceral fat in humans. J. Nutr. Sci. Vitaminol. 2014, 60, 22–27. [DOI] [PubMed] [Google Scholar]
- Kulmacz R. J.; Lands W. E. M. Requirements for hydroperoxide by the cyclooxygenase and peroxidase activities of prostaglandin H synthase. Prostaglandins 1983, 25, 531–540. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Chakravarty S.; Dey M. Phenethylisothiocyanate alters site- and promoter-specific histone tail modifications in cancer cells. PLoS One 2013, 8, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey M.; Ribnicky D.; Kurmukov A. G.; Raskin I. Transcriptional modulation and in vivo anti-inflammatory activity of a seed preparation containing phenethylisothiocyanate. J. Pharmacol. Exp. Ther. 2006, 317, 326–333. [DOI] [PubMed] [Google Scholar]
- Gröblacher B.; Maier V.; Kunert O.; Bucar F. Putative mycobacterial efflux inhibitors from the seeds of Aframomum melegueta. J. Nat. Prod. 2012, 75, 1393–1399. [DOI] [PubMed] [Google Scholar]
- Wu Q.; Cho J.; Yoo K.; Jeong T.; Park J.; Kim S.; Kang J.; Chung I.; Choi M.; Lee K.; Chung H.; Bang M.; Baek N. A new phenanthrene derivative and two diarylheptanoids from the roots of Brassica rapa ssp. campestris inhibit the growth of cancer cell lines and LDL-oxidation. Arch. Pharm. Res. 2013, 36, 423–429. [DOI] [PubMed] [Google Scholar]
- Escoubas P.; Lajide L.; Mizutani J. Part 2. Termite antifeedant activity in Aframomum melegueta. Phytochemistry 1995, 40, 1097–1099. [Google Scholar]
- Connell D. W. Natural pungent compounds. III. Paradols and associated compounds. Aust. J. Chem. 1970, 23, 369–376. [Google Scholar]
- Nurtjahja-Tjendraputra E.; Ammit A. J.; Roufogalis B. D.; Tran V. H.; Duke C. C. Effective anti-platelet and COX-1 enzyme inhibitors from pungent constituents of ginger. Thromb. Res. 2003, 111, 259–265. [DOI] [PubMed] [Google Scholar]
- Tjendraputra E.; Tran V. H.; Liu-Brennan D.; Roufogalis B. D.; Duke C. C. Effect of ginger constituents and synthetic analogues on cyclooxygenase-2 enzyme in intact cells. Bioorg. Chem. 2001, 29, 156–163. [DOI] [PubMed] [Google Scholar]
- van Breemen R. B.; Tao Y.; Li W. Cyclooxygenase-2 inhibitors in ginger (Zingiber officinale). Fitoterapia 2011, 82, 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamache D. A.; Povlishock J. T.; Ellis E. F. Carrageenan-induced brain inflammation. Characterization of the model. J. Neurosurg. 1986, 65, 679–685. [DOI] [PubMed] [Google Scholar]
- Kiuchi F.; Iwakem S.; Shibuya M.; Hanaoka A.; Sankawa U. Inhibition of prostaglandin and leukotriene biosynthesis by gingerols and diarylheptaniods. Chem. Pharm. Bull. 1992, 40, 387–391. [DOI] [PubMed] [Google Scholar]