Abstract
Background
Lung clearance index (LCI) derived from sulfur hexafluoride (SF6) multiple breath washout (MBW) is a sensitive measure of lung disease in people with cystic fibrosis (CF). However, it can be time-consuming, limiting its use clinically.
Aim
To compare the repeatability, sensitivity and test duration of LCI derived from washout to 1/30th (LCI1/30), 1/20th (LCI1/20) and 1/10th (LCI1/10) to ‘standard’ LCI derived from washout to 1/40th initial concentration (LCI1/40).
Methods
Triplicate MBW test results from 30 clinically stable people with CF and 30 healthy controls were analysed retrospectively. MBW tests were performed using 0.2% SF6 and a modified Innocor device. All LCI end points were calculated using SimpleWashout software. Repeatability was assessed using coefficient of variation (CV%). The proportion of people with CF with and without abnormal LCI and forced expiratory volume in 1 s (FEV1) % predicted was compared. Receiver operating characteristic (ROC) curve statistics were calculated. Test duration of all LCI end points was compared using paired t tests.
Results
In people with CF, LCI1/40 CV% (p=0.16), LCI1/30 CV%, (p=0.53), LCI1/20 CV% (p=0.14) and LCI1/10 CV% (p=0.25) was not significantly different to controls. The sensitivity of LCI1/40, LCI1/30 and LCI1/20 to the presence of CF was equal (67%). The sensitivity of LCI1/10 and FEV1% predicted was lower (53% and 47% respectively). Area under the ROC curve (95% CI) for LCI1/40, LCI1/30, LCI1/20, LCI1/10 and FEV1% predicted was 0.89 (0.80 to 0.97), 0.87 (0.77 to 0.96), 0.87 (0.78 to 0.96), 0.83 (0.72 to 0.94) and 0.73 (0.60 to 0.86), respectively. Test duration of LCI1/30, LCI1/20 and LCI1/10 was significantly shorter compared with the test duration of LCI1/40 in people with CF (p<0.0001) equating to a 5%, 9% and 15% time saving, respectively.
Conclusions
In this study, LCI1/20 was a repeatable and sensitive measure with equal diagnostic performance to LCI1/40. LCI1/20 was shorter, potentially offering a more feasible research and clinical measure.
Keywords: Cystic Fibrosis, Equipment Evaluations
Key messages.
Lung Clearance Index (LCI) can be time-consuming, limiting its use clinically.
Investigation of the flexibility of current multiple breath washout test end points is an important area for future research.
LCI1/20 is a repeatable and sensitive test that is shorter than LCI1/40, potentially offering a more feasible research and clinical measure.
Introduction
Lung Clearance Index (LCI) derived from multiple breath washout (MBW) is a sensitive measure of ventilation inhomogeneity1 2 and a robust surrogate outcome measure of the severity of lung disease in cystic fibrosis (CF)3 which has begun to be incorporated into clinical trials.4 5 It also shows promise as a sensitive outcome measure in idiopathic bronchiectasis6 and asthma.7 A drawback of the test is that it can be time-consuming, especially in patients with advanced disease, limiting its feasibility within the clinical environment. By convention a MBW test involves performing a minimum of three inert tracer gas washout runs, ending the washout when end-tidal tracer gas concentration falls below 1/40th of the initial concentration.8 The end point of 1/40th is based on historic studies and has not been systematically validated.8 9 The European Respiratory Society/American Thoracic Society (ERS/ATS) consensus statement highlights investigation of the flexibility of current MBW test end points as an important area for future research, which could potentially improve the utility of this test.8 Assessment of the clinimetric properties of shortened LCI in CF using nitrogen (N2) MBW testing (100% as the inert gas), have reported good diagnostic performance in children with mild disease, offering a measure of ventilation inhomogeneity which may be more practical in the clinical setting.10 However, there are no studies to assess the performance of shortened LCI using sulfur hexafluoride (SF6) MBW (another common MBW method), or studies of shortened LCI in adult patients with more moderate to advanced disease. Differences in gas diffusion and molecular mass of the inert gases used mean that results of the two types of test are not comparable.11 Study of the sensitivity of shortened MBW tests using SF6 could be useful in improving the clinical utility of these tests.
In this study we aimed to assess and compare the repeatability, sensitivity, specificity and test duration of LCI derived from washout to 1/30th (LCI1/30), 1/20th (LCI1/20) and 1/10th of the initial concentration (LCI1/10) to ‘standard’ LCI derived from washout to 1/40th initial concentration (LCI1/40), using 0.2% SF6 as the tracer gas, in school age—adolescent children and adults with CF and healthy controls.
Methods
Subject recruitment
Cross-sectional data from 30 people with CF (n=15 aged 6–17 years old; n=15 aged ≥18 years old) and 30 healthy control participants (n=15 aged 6–17 years old; n=15 aged ≥18 years old) with three valid and repeatable MBW tests were analysed. Thirty anonymised CF and 30 healthy control data sets, as consecutively listed in a database of results collected in a large prospective project investigating the clinimetric and clinical relevance of LCI in CF were used. People with CF were recruited at a routine outpatient visit to the Northern Ireland paediatric and adult CF centres at Belfast Health and Social Care Trust (BHSCT), when clinically stable (no pulmonary exacerbation requiring intravenous antibiotics in the previous 4 weeks), between October 2010 and June 2013. Control participants were recruited by means of email circulation among people employed in Queen's University Belfast (QUB) and BHSCT between September 2011 and August 2012. All adult participants provided written informed consent. All child participants provided child or young person assent and parental consent.
MBW testing
The MBW test to measure LCI was carried out using a modified Innocor device and 0.2% SF6 using the open-circuit technique in accordance with the standard operating procedure developed by the UK CF Gene Therapy Consortium (UKCFGTC; see online supplementary file 1) as described and validated by Horsley et al2 and used in a recent CF clinical trial and observational study.4 12 Participants breathed through a mouthpiece at tidal volumes, while in a seated position and wearing a nose clip. Participants breathed 0.2% SF6 in air via a flowpast circuit until washin was complete, at which point the flowpast was disconnected and the participant breathed room air until the end tidal expired SF6 concentration fell below 1/40th of the initial concentration before disconnection. Three washouts were performed for each participant. Analysis of MBW data was performed using the SimpleWashout programme developed by Dr Nicholas Bell (UKCFGTC) and used with his permission (see online supplementary file 1). For each washout, four values for functional residual capacity (FRC) and LCI were calculated:
FRC1/40 and LCI1/40 were derived from washout data from flowpast disconnection until the first breath with end tidal SF6 concentration below 1/40th (≤0.005%) of the starting SF6 concentration (0.2%).
FRC1/30 and LCI1/30 were derived from washout data from flowpast disconnection until the first breath with end tidal SF6 concentration below 1/30th (≤0.007%) of the starting SF6 concentration (0.2%).
FRC1/20 and LCI1/20 were derived from washout data from flowpast disconnection until the first breath with end tidal SF6 concentration below 1/20th (≤0.01%) of the starting SF6 concentration (0.2%).
FRC1/10 and LCI1/10 were derived from washout data from flowpast disconnection until the first breath with end tidal SF6 concentration below 1/10th (≤0.02%) of the starting SF6 concentration (0.2%).
Mean LCI and FRC values and test duration (minutes) for each end point were calculated from each of the three washouts in each testing session.
Spirometry
Spirometry was measured according to American Thoracic Society/European Respiratory Society ATS/ERS guidelines13 using a Microlab (ML3500 MK8) spirometer (CareFusion, Kent, UK). Predicted values were calculated from reference ranges for all ages.14
Statistical analysis
Data were analysed using PASW Statistics (V.18, IBM software, USA) and Prism (V.5.01 GraphPad Software Inc.) packages. CF and control participant characteristics were summarised using descriptive statistics.
Intravisit repeatability of LCI1/40, LCI1/30 LCI1/20 and LCI1/10 was assessed using the coefficient of variation (CV%) of all three tests and Bland-Altman plots15 comparing tests one and three, for people with CF and healthy controls. Results between people with CF and healthy controls were compared using an independent samples t test. Mean LCI and FRC values for each end point from people with CF or healthy controls were compared using paired samples t tests. The relationship between mean LCI1/40 and mean LCI1/30, LCI1/20 and LCI1/10 was assessed using scatter plots and the Spearman's rank correlation coefficient. This analysis was also used to assess the relationship between FEV1% predicted and all LCI end points. Sensitivity of all LCI end points compared with FEV1% predicted was assessed using scatter plots and limits of normal of respective tests calculated from healthy controls (mean +1.96 SD). Sensitivity and specificity were further analysed using receiver operating characteristic (ROC) curves, and by comparing area under the ROC curve (AUCROC) and 95% CI for LCI1/40, LCI1/30, LCI1/20, LCI1/10 and FEV1% predicted. Mean test duration (minutes) of LCI1/40, LCI1/30, LCI1/20 and LCI1/10 was compared using a paired samples t test. As multiple comparisons were being made, a Bonferroni adjustment was incorporated. A p value of <0.01 was considered statistically significant.
Results
LCI1/40, LCI1/30, LCI1/20, LCI1/10 and FEV1% predicted were significantly different between the CF and control group. However, there was no difference between the CF and control group in age, sex, LCI1/40 CV%, LCI1/30 CV%, LCI1/20 CV%, LCI1/10 CV% or test duration of any LCI end point (table 1).
Table 1.
CF and healthy control participant characteristics
| People with CF | Healthy controls | p Value (CF vs controls) | |
|---|---|---|---|
| N | 30 | 30 | – |
| M/F | 14/16 | 16/14 | 0.61 |
| Age (years) | 20.7 (11.1) (6–51) | 20.8 (10.7) (7–44) | 0.93 |
| FEV1 (% predicted) | 79.3 (17.9) (46.0–116.0) | 92.9 (11.3) (68.0–116.0) | 0.009 |
| FRC1/40 (L) | 1.95 (0.76) | 2.23 (1.0) | 0.22 |
| LCI1/40 (number of turnovers) | 9.0 (2.4) | 6.4 (0.5) | <0.0001 |
| LCI1/40 CV% | 5.2 (2.8) | 4.3 (2.0) | 0.16 |
| LCI1/40 triplicate test duration (min) | 21.8 (9.7) (8.0–57.1) | 19.4 (6.5) (10.1–31.1) | 0.26 |
| FRC1/30 (L) | 1.93 (0.76) | 2.24 (1.02) | 0.18 |
| LCI1/30 (number of turnovers) | 7.7 (1.6) | 5.9 (0.4) | <0.0001 |
| LCI1/30 CV% | 4.6 (3.2) | 4.1 (2.8) | 0.53 |
| LCI1/30 triplicate test duration (min) | 20.9 (9.1) (7.3–53.2) | 18.9 (6.2) (9.9–29.9) | 0.34 |
| FRC1/20 (L) | 1.90 (0.75) | 2.24 (1.0) | 0.15 |
| LCI1/20 (number of turnovers) | 6.6 (1.2) | 5.2 (0.4) | <0.0001 |
| LCI1/20 CV% | 5.7 (3.4) | 4.6 (2.3) | 0.14 |
| LCI1/20 triplicate test duration (min) | 20.0 (8.9) (6.7–51.7) | 18.3 5.9 (9.6–28.3) | 0.38 |
| FRC1/10 (L) | 1.84 (0.73) | 2.22 (1.00) | 0.09 |
| LCI1/10 (number of turnovers) | 4.7 (0.6) | 4.0 (0.3) | <0.0001 |
| LCI1/10 CV% | 5.4 (3.8) | 4.3 (2.8) | 0.25 |
| LCI1/10 triplicate test duration (min) | 18.6 (8.1) (5.7–46.9) | 17.1 (5.5) (8.8–26.6) | 0.41 |
All values summarised as mean (SD)±(range).
CF, cystic fibrosis; CV, coefficient of variation, F, female; FEV1, forced expiratory volume in 1 s; FRC, functional residual capacity; LCI, Lung Clearance Index; M, male.
There was no difference between the CF and control group in any of the FRC values (FRC1/40, FRC1/30, FRC1/20 or FRC1/10). Within the CF group, as expected, FRC was incrementally lower with each earlier end point. Although FRC1/40, and FRC1/30 were not significantly different (1.95 vs 1.93, p=0.07) FRC1/40 and FRC1/20 (1.95 vs 1.90, p=0.002) and FRC1/40 and FRC1/10 (1.95 vs 1.84) were significantly different.
Intravisit repeatability
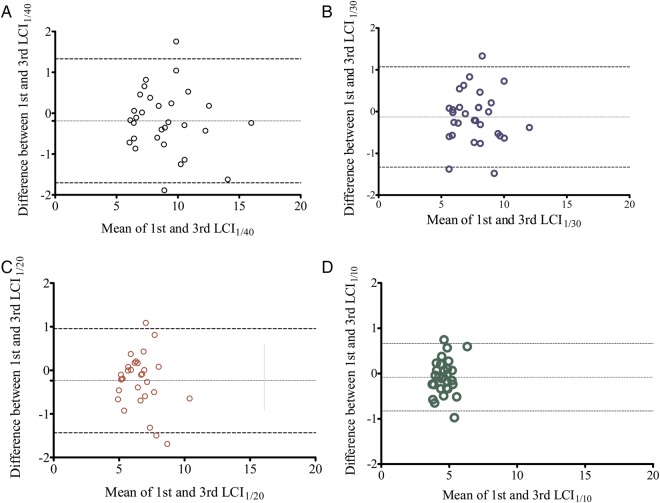
LCI1/40 CV%, LCI1/30 CV%, LCI1/20 CV% and LCI1/10 CV% in people with CF were not significantly different to values in healthy controls (table 1). There was also no significant difference between LCI1/40 CV% and the CV% of any other LCI end point in people with CF. A Bland-Altman plot15 of the mean versus the difference between tests one and three of LCI1/40, LCI1/30, LCI1/20 and LCI1/10 for people with CF showed no evidence of greater variability in participants with more advanced disease (ie, a higher LCI reading; figure 1A–D).
Figure 1.
(A) Lung Clearance Index (LCI)1/40; (B) LCI1/30; (C) LCI1/20; (D) LCI1/10 first and third test in people with cystic fibrosis (dotted horizontal lines represent the bias and 95% limits of agreement).
For LCI1/40, the 95% limits of agreement between the two measurements were −1.70 to 1.33 lung turnovers, compared with −1.33 to 1.07 (LCI1/30), −1.43 to 0.96 (LCI1/20) and −0.83 to 0.66 (LCI1/10) lung turnovers. Therefore the intravisit repeatability of the LCI1/40, LCI1/30, LCI1/20 and LCI1/10 measurements was 1.5, 1.2, 1.2 and 0.7 lung turnovers respectively.
Relationship between shortened LCI and ‘standard’ LCI1/40
In people with CF, LCI1/30 (r=0.98, p<0.0001), LCI1/20 (r=0.95, p<0.0001) and LCI1/10 (r=0.88, p<0.0001) correlated significantly with LCI1/40 (figure 2).
Figure 2.
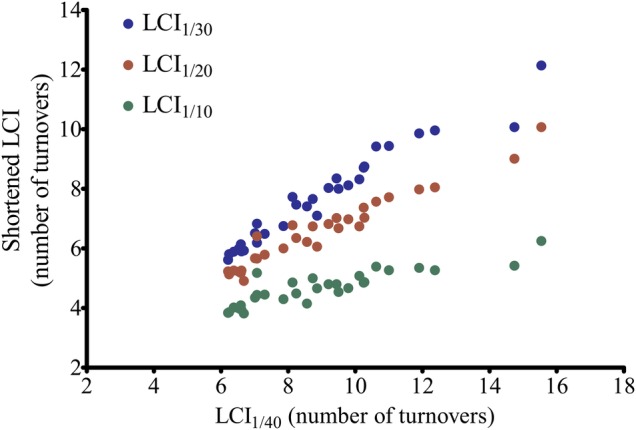
Shortened Lung Clearance Index (LCI) versus standard LCI1/40 in people with cystic fibrosis.
Sensitivity and specificity
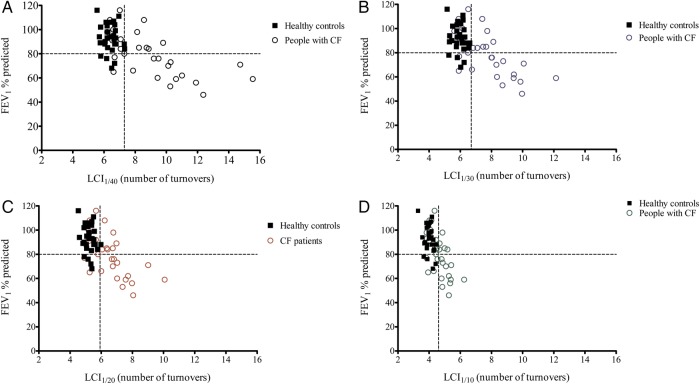
The upper limit of normal for LCI1/40, LCI1/30, LCI1/20 and LCI1/10 was 7.3, 6.7, 5.9 and 4.6 lung turnovers, respectively, (control mean +1.96 SD). The lower limit of normal of 80% for FEV1% predicted was used, as this is the level that is historically used in clinical practice.
The sensitivity of LCI1/40, LCI1/30, LCI1/20 to differentiate between people with CF and healthy controls was identical (67%). The sensitivity of LCI1/10 and FEV1% predicted was lower (53% and 47%, respectively). In people with CF, LCI1/40 (r=−0.73, p<0.0001), LCI1/30 (r=−0.70, p<0.0001), LCI1/20 (r=−0.69, p<0.0001) and LCI1/10 (r=−0.62, p=0.0003) correlated significantly with FEV1% predicted (figures 3A–D). Using LCI1/40, 6/30 (20%) people with CF had an abnormal LCI in the presence of a normal FEV1% predicted (figure 3A). Similarly, using LCI1/30 or LCI1/20, 7/30 (23%) people with CF had an abnormal LCI in the presence of a normal FEV1% predicted (figure 3B–C). LCI1/10 was less sensitive, detecting 5/30 (17%) with an abnormal LCI in the presence of a normal FEV1% predicted (figure 3D).
Figure 3.
Forced expiratory volume in 1 s (FEV1)% predicted versus (A) LCI1/40; (B) LCI1/30; (C) LCI1/20 (D) LCI1/10 (dotted horizontal lines represent the limits of normal for FEV1% predicted (80% predicted) and LCI (LCI1/40:7.3; LCI1/30:6.7; LCI1/20:5.9; LCI1/10:4.6)).
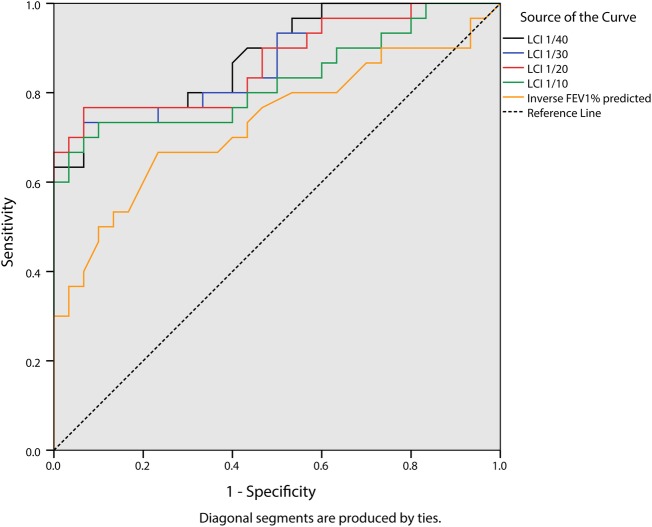
ROC curve analysis (figure 4) showed that while all LCI values and FEV1% predicted had statistically significant levels of sensitivity and specificity in determining people with CF vs control participants, LCI1/40, LCI1/30 and LCI1/20 had comparable and higher sensitivity and specificity compared with LCI1/10 and FEV1% predicted (table 2).
Figure 4.
Receiver operating characteristic ROC curve of Lung Clearance Index (LCI)1/40, LCI1/30, LCI1/20, LCI1/10 and inverse forced expiratory volume in 1 s % predicted: sensitivity and specificity to the presence of cystic fibrosis CF.
Table 2.
AUCROC and 95% CI for LCI1/40, LCI1/30, LCI1/20 LCI1/10 and inverse FEV1% predicted (1.0 indicating best performance, 0.5 indicating poor performance)
| AUCROC | 95% CI | p Value | |
|---|---|---|---|
| LCI1/40 | 0.89 | 0.80 to 0.97 | <0.0001 |
| LCI1/30 | 0.87 | 0.77 to 0.96 | <0.0001 |
| LCI1/20 | 0.87 | 0.78 to 0.96 | <0.0001 |
| LCI1/10 | 0.83 | 0.72 to 0.94 | <0.0001 |
| FEV1% predicted (inverse) | 0.73 | 0.60 to 0.86 | 0.002 |
AUC, area under the curve; FEV1, forced expiratory volume in 1 s; LCI, Lung Clearance Index, ROC, receiver operating characteristic
Test duration
Test duration of LCI1/30, LCI1/20 and LCI1/10 was significantly shorter compared with washout duration of LCI1/40 in people with CF (p<0.0001) and in healthy controls (p<0.0001; table 1). In people with CF, the mean (95% CI) time saving per triplicate MBW test was 1 (0.8 to 1.3) minutes or 5% with LCI1/30, 1.9 (1.4 to 2.3) minutes or 9% with LCI1/30 and 3.3 (2.6 to 4.2) minutes or 15% with LCI1/10.
Discussion
This study is the first to show that SF6 MBW tests can be reliably shortened. Results show that in children and adults with CF, LCI shortened to 1/30th or 1/20th (LCI1/30 or LCI1/20) of the initial concentration have comparable intravisit repeatability and sensitivity to ‘standard’ LCI at 1/40th of the starting concentration (LCI1/40) providing additional information to FEV1% predicted and offering a time saving. Although repeatable, LCI shortened to 1/10th of the starting concentration (LCI1/10) was less sensitive to lung disease, compared with the other LCI end points. It was, however, still more sensitive than FEV1% predicted.
The ‘standard’ end point of 1/40th is based on historic studies using nitrogen washout (2.5%) and has not been systematically validated for MBW tests using SF6.8 9 This study aimed to assess the performance of earlier arbitrary end points compared with the ‘standard’ end point in the SF6 washout, in an attempt to improve the clinical utility of the MBW test by reducing test duration. Like LCI1/40, LCI1/30 and LCI1/20 target the flatter tail of the washout curve, making it unsurprising that similar information can be obtained (see online supplementary file 2). In contrast, when using LCI1/10, the end point occurs before the washout curve flattens. This supports the theory that most information is contained in the tail of the washout curve.16 Therefore a cut-off before this point may provide less information about lung disease severity, as highlighted by the lower sensitivity of LCI1/10 in this study. Yammine et al10 assessed the repeatability and sensitivity of shortened N2 MBW to measure LCI, at a number of earlier end points including 1/20th of the starting concentration and as early as 1/5th of the starting concentration, in 68 children with CF (n=44 with mild disease). In agreement with results from our study, they reported good performance of shortened LCI at 1/20th of the starting concentration compared with ‘standard’ LCI. Furthermore, as with the results from our study, Yammine et al10 found that while the earlier LCI end points had good intravisit repeatability, they were less sensitive and specific to the presence of CF. The authors concluded that shortened N2 MBW to 1/20th of the starting concentration could offer a more feasible measure for use in clinical practice. In this study we extended these observations to investigate and confirm the utility of shortened ventilation indices in older patients with more advanced disease. Theoretically, in cases of severe flow asynchrony between the best and the least ventilated lung units, the end-tidal concentrations in subsequent breathing cycles can enhance the contribution of the least ventilated units toward the end of the washout where LCI is measured.16 This may lead to increased variation in results taken at an earlier end point, such as 1/20th of the starting concentration. In contrast, LCI from an earlier end point may be more precise, as an end point where the washout curve slope is greater may avoid random breath-by-breath variability that could be observed at a later end point. In this study, LCI1/30 and LCI1/20 had marginally better sensitivity to the presence of CF (23% vs 20%), compared with LCI1/40, even though there was no significant difference in variability (LCI CV%) between ‘standard’ LCI (LCI1/40) and LCI at an earlier end point (LCI1/30, LCI1/20, LCI1/10). LCI1/30 and LCI1/20 also had good diagnostic performance and superior sensitivity compared with FEV1% predicted. Importantly, these findings indicate that no further additional information was obtained using LCI1/40 compared with the shortened versions, LCI1/30 and LCI1/20.
Test duration for LCI1/30, LCI1/20 and LCI1/10 was significantly shorter than LCI1/40 in people with CF, with an average 5%, 9% and 15% time saving, respectively. As LCI1/20 offers a greater time saving than LCI1/30, while maintaining reliability and sensitivity, it provides the most attractive measure and may enhance the feasibility of MBW in the research and clinical setting. The proportion of time saved in this study is smaller than that reported in the study by Yammine et al10 as MBW tests using an exogenous gas such as SF6 require a washin and a washout phase. However, as these results are from a retrospective analysis, the time saving measurement does not take into account the total time saving per testing session. Finishing a test earlier would allow for the second and third test of the triplicate to start sooner, resulting in a larger time saving. Although the washin time is unchanged, any shortening of LCI test duration could be especially useful in younger children where long assessment periods are not feasible, and in patients with advanced disease, where the washin and/or washout periods can be prolonged. The use of MBW equipment using SF6 has been successfully used in multicentre studies and remains popular as it has advantages in terms of tracer gas estimation (measured directly rather than by subtraction as with N2 MBW) avoiding the potential confounding effects of 100% O2 on breathing pattern. This study is the first to show that MBW using SF6 can be reliably shortened.
Considering potential limitations of shortened MBW tests, one study highlights that advanced analysis of washout curves to determine the relative contribution of convective and acinar airways to ventilation heterogeneity (phase III analysis) usually requires six lung-volume turnovers.17 However, recent work suggests that the same information may be obtainable in three lung-volume turnovers18 in which case use of LCI1/20 would still enable full phase III analysis. Regardless, phase III indices may have limited utility in CF, as demonstrated by Horsley et al.19
The retrospective nature of this study is a limitation. However, the study did endeavour to avoid selection bias by use of anonymsied patient data sets as consecutively listed in a database and represents the first exploratory study to report on the clinimetric properties of an earlier end point in SF6 MBW. More data from across the disease severity range in CF are required to define normal ranges of shortened LCI.
Conclusions
LCI1/20 is a repeatable and sensitive test with equal diagnostic performance to LCI1/40 that is shorter, potentially offering a more feasible research and clinical measure.
Supplementary Material
Acknowledgments
The authors would like to thank the patients and families who participated in this study.
Footnotes
Contributors: KO'N recruited patients, collected clinical data, conducted MBW tests and performed lung function assessment; KO'N, DH, JSE and NB analysed data; IB provided statistical analysis support; KO'N, DH, JMB, NB and JSE wrote the paper.
Funding: Work funded by HSC Research and Development, Public Health Agency, Northern Ireland and the Medical Research Council through a US-Ireland Partnership Grant.
Competing interests: None.
Patient consent: Obtained.
Ethics approval: This study was approved by the Office for Research Ethics Committees Northern Ireland (ORECNI) (REC reference number: 10/NIR01/41) and co-sponsored by BHSCT and QUB (research office reference number: 10067SE-OPMS).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Gustafsson PM, Aurora P, Lindblad A. Evaluation of ventilation maldistribution as an early indicator of lung disease in children with cystic fibrosis. Eur Respir J 2003;22:972–9 [DOI] [PubMed] [Google Scholar]
- 2.Horsley AR, Gustafsson PM, Macleod KA, et al. Lung clearance index is a sensitive, repeatable and practical measure of airways disease in adults with cystic fibrosis. Thorax 2008;63:135–40 [DOI] [PubMed] [Google Scholar]
- 3.Kent L, Reix P, Innes JA, et al. Lung clearance index: evidence for use in clinical trials in cystic fibrosis. J Cyst Fibros 2014;13:123–38 [DOI] [PubMed] [Google Scholar]
- 4.Davies J, Sheridan H, Bell N, et al. Assessment of clinical response to ivacaftor with lung clearance index in cystic fibrosis patients with a G551D-CFTR mutation and preserved spirometry: a randomised controlled trial. Lancet Respir Med 2013;1:630–8 [DOI] [PubMed] [Google Scholar]
- 5.Subbarao P, Stanojevic S, Brown M, et al. Lung clearance index as an outcome measure for clinical trials in young children with cystic fibrosis. A pilot study using inhaled hypertonic saline. Am J Respir Crit Care Med 2013;188:456–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rowan SA, Bradley JM, Bradbury I, et al. Lung clearance index is a repeatable and sensitive indicator of radiological changes in bronchiectasis. Am J Respir Crit Care Med 2014;189:586–92 [DOI] [PubMed] [Google Scholar]
- 7.Macleod KA, Horsley AR, Bell NJ, et al. Ventilation heterogeneity in children with well controlled asthma with normal spirometry indicates residual airways disease. Thorax 2009;64:33–7 [DOI] [PubMed] [Google Scholar]
- 8.Robinson PD, Latzin P, Verbanck S, et al. Consensus statement for inert gas washout measurement using multiple- and single- breath tests. Eur Respir J 2013;41:507–22 [DOI] [PubMed] [Google Scholar]
- 9.Bouhuys A, van Lennep HJ. Effect of body posture on gas distribution in the lungs. J Appl Physiol 1962;17:38–42 [DOI] [PubMed] [Google Scholar]
- 10.Yammine S, Singer F, Abbas C, et al. Multiple-breath washout measurements can be significantly shortened in children. Thorax 2013;68:586–7 [DOI] [PubMed] [Google Scholar]
- 11.Nielsen N, Nielsen JG, Horsley AR. Evaluation of the impact of alveolar nitrogen excretion on indices derived from multiple breath nitrogen washout. PLoS ONE 2013;8:e73335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horsley AR, Davies JC, Gray RD, et al. Changes in physiological, functional and structural markers of cystic fibrosis lung disease with treatment of a pulmonary exacerbation. Thorax 2013;68:532–9 [DOI] [PubMed] [Google Scholar]
- 13.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319–38 [DOI] [PubMed] [Google Scholar]
- 14.Stanojevic S, Wade A, Stocks J, et al. Reference ranges for spirometry across all ages. Am J Respir Crit Care Med 2008;177:253–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–10 [PubMed] [Google Scholar]
- 16.Verbanck S, Paiva M, Paeps E, et al. Lung clearance index in adult cystic fibrosis patients: the role of convection-dependent lung units. Eur Respir J 2013;42:380–8 [DOI] [PubMed] [Google Scholar]
- 17.Verbanck S, Paiva M. Gas mixing in the airways and airspaces. Compr Physiol 2011;1:809–34 [DOI] [PubMed] [Google Scholar]
- 18.Verbanck S, Paiva M, Schuermans D, et al. Relationships between the lung clearance index and conductive and acinar ventilation heterogeneity. J Appl Physiol 2012;112:782–90 [DOI] [PubMed] [Google Scholar]
- 19.Horsley AR, Macleod KA, Robson AG, et al. Effects of cystic fibrosis lung disease on gas mixing indices derived from alveolar slope analysis. Respir Physiolo Neurobiol 2008;162:197–203 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.