Abstract
High levels of reactive oxygen species (ROS) may cause a change of cellular redox state towards oxidative stress condition. This situation causes oxidation of molecules (lipid, DNA, protein) and leads to cell death. Oxidative stress also impacts the progression of several pathological conditions such as diabetes, retinopathies, neurodegeneration, and cancer. Thus, it is important to define tools to investigate oxidative stress conditions not only at the level of single cells but also in the context of whole organisms. Here, we consider the zebrafish embryo as a useful in vivo system to perform such studies and present a protocol to measure in vivo oxidative stress. Taking advantage of fluorescent ROS probes and zebrafish transgenic fluorescent lines, we develop two different methods to measure oxidative stress in vivo: i) a “whole embryo ROS-detection method” for qualitative measurement of oxidative stress and ii) a “single-cell ROS detection method” for quantitative measurements of oxidative stress. Herein, we demonstrate the efficacy of these procedures by increasing oxidative stress in tissues by oxidant agents and physiological or genetic methods. This protocol is amenable for forward genetic screens and it will help address cause-effect relationships of ROS in animal models of oxidative stress-related pathologies such as neurological disorders and cancer.
Keywords: Developmental Biology, Issue 89, Danio rerio, zebrafish embryos, endothelial cells, redox state analysis, oxidative stress detection, in vivo ROS measurements, FACS (fluorescence activated cell sorter), molecular probes
Introduction
Oxidative stress is specifically defined as a condition that results from an unbalanced cellular redox state. The complex redox reactions that routinely occur inside cells determine the cellular redox-state. Redox reactions consist of all chemical reactions that consist in the transfer of electrons between atoms of biological molecules producing reduction and oxidation of molecules (i.e. redox reactions). These reactions are catalyzed by electronically activated species (i.e. pro-oxidative species), which are characterized by an extreme structural instability and spontaneous activation of unbalanced electrons that exchange with neighboring biomolecules. These irregular reactions result into DNA damage, protein carboxylation, and lipid oxidation, and eventually lead to cell death1. Increased levels of oxidative stress have been associated with aging and the progression of different pathological states2. Oxidative stress has been reported to be responsible for vascular alterations in diabetes and cardiovascular diseases3,4. It also plays a critical role in neuronal degeneration in Alzheimer's disease and Parkinson's disease5. Moreover, oxidative stress has been demonstrated as a critical factor in governing cancer progression and metastatic events6,7. In addition, inflammation and immune responses may elicit and further support oxidative stress8.
In living cells, pro-oxidative species are derived from oxygen (ROS; reactive oxygen species) or nitrogen (RNS; reactive nitrogen species). ROS include the hydroxyl radical (.OH), the superoxide anion (O2-), and the hydrogen peroxide (H2O2). The primary RNS is nitrous oxide (NO.). A series of secondary reactive species can be generated by spontaneous interactions between ROS and RNS or free metals ions9. For example, the superoxide anion reacts with nitrous oxide to form peroxynitrate (ONOO-), while H2O2 reacting with Fe2+ generates hydroxyl radicals. ROS and RNS, due to their ability to react with several biomolecules, are considered a dangerous threat for the maintenance of the physiological redox state10. To maintain the redox state cells are equipped with a series of detoxifying anti-oxidant molecules and enzymes. The superoxide dismutase (SOD), Catalase, Glutathione peroxidase and Peroxiredoxins essentially constitute the anti-oxidant enzymatic-arsenal that provides cellular protection from pro-oxidative species including H2O2 , .OH and OONO- 11. Also anti-oxidant molecules like vitamin C and E, polyphenols and CoenzymeQ10 (CoQ10) are of critical importance to quench ROS and their dangerous derivatives12,13. However, an excessive production of ROS and RNS, or a dysfunction in the anti-oxidant system, shifts the cellular redox-state toward oxidative stress14.
Besides their negative connotation, ROS can play various physiological roles in cells of different origin. Cells normally produce ROS as signaling molecules to mediate normal biological events such as host defense and wound repair15-17. Reactive species are normally produced in cells by intracellular enzymes such as NOX (NADPH Oxidase) and XO (Xantine Oxidase) in response to signaling factors, growth factors, and intracellular fluctuations of calcium levels18,19. It has been reported that ROS may differentially modulate the activity of important nuclear factors such as p53 or cellular components such as the ATM-kinase, a master regulator of the response to DNA damage20. Analogously ROS strongly influence cellular signaling by mediating the oxidation and inactivation of protein tyrosine phosphatases (PTPs), which are established as critical regulators of signal transduction21. Moreover, proteomic based methodologies demonstrate that RNS are also responsible for specific protein modifications and alterations of molecular signaling. RNS react with the cysteine thiol groups modifying them into S-nitrothiols (SNO) and triggering molecular pathways concomitant with pathological states such as inflammatory and autoimmune diseases22,23.
Since cell culture experiments only partially reproduce the multitude of factors acting in vivo, it is of great interest to perform redox studies in animal models24,25. To achieve this, the zebrafish has been considered a suitable vertebrate animal model to study oxidative stress dynamics26. The zebrafish is a new model system that grants several advantages to study cellular and genetic events during vertebrate development and disease. Large clusters of embryos can be generated and available weekly for experimental needs. Moreover the extraordinary optical clarity of zebrafish embryos, as well their small size, enables single cell imaging and dynamic tracking in a whole organisms27. In the last decade, a considerable number of zebrafish mutants have been generated to model human pathological conditions such as cancer and genetic diseases28-31. Most importantly, a multitude of transgenic lines has been produced to allow extensive opportunities of genetic and biological manipulations32. For example, transgenic tissue-specific zebrafish lines are regularly utilized for in vivo studies. These lines express a fluorescent protein under the control of a selected promoter, offering the ability to identify single cells in vivo, as well as the anatomical structure they comprise.
Several toxicological studies have already used the zebrafish to evaluate the in vivo effect of chemicals on redox homeostasis, suggesting the suitability of this vertebrate as an animal model for the field of drug discovery and oxidative stress33-35. Even though some fluorescent probes have been tested to monitor oxidative stress in zebrafish larvae36,37, there are no established assays to detect and measure the levels of oxidative stress in zebrafish tissues and living cells. Here we describe a procedure for in vivo quantification of oxidative stress in living cells of zebrafish embryos. Imaging tools, FACS sorting, fluorescent probes and pro-oxidative conditions are all combined to generate a simple assay for the detection and quantification of oxidative species in zebrafish embryos and tissues.
Protocol
1. Preparation of Instruments and Working Solutions
Prepare the fish water solution. Make a stock solution by dissolving 2 g of sea salts 'Instant Ocean' in 50 ml of distilled water. Add 1.5 ml of stock fish water to 1 L distilled water to prepare ready to use fish water (60 µg/ml sea salts final concentration). Autoclave the ready to use fish water before usage. This solution is used as zebrafish embryo medium.
Prepare methylcellulose for embryo mounting. Dissolve 1.5 g of methylcellulose in 50 ml of sterile fish water. Facilitate the dissolution by using a magnet on a stir plate. The complete dissolution of the powder may require several hours. Check the solution for clarity and aliquot into small tubes. Store at -20 °C for months and thaw out aliquots at use. Centrifuge the methylcellulose at 950 x g for 5 min before using. Avoid freeze-thaw cycles of aliquots.
Prepare 50 ml of tricaine/ethyl 3-aminobenzoate methanesulphonate salt (stock solution) by dissolving 200 mg of tricaine in 100 ml water and adjust pH to 7.0 using Tris-HCl 1 M (pH 9). Store this stock at 4 °C for no more than 30 days. CAUTION: tricaine is toxic. Use in accordance with appropriate handling guidelines.
- Inducing oxidative stress in zebrafish embryos
- Prepare an oxidant solution for generic oxidative stress induction: Make 50 ml of oxidant solution by adding H2O2 stock solution (hydrogen peroxide; 100 mM) to fish water. Use H2O2 of a final concentration between 2 mM and 100 μM. Prepare this solution shortly before usage. The oxidant solution can be applied to both whole mount ROS-detection and single-cell ROS-detection methods. Do not store this solution. CAUTION: H2O2 is dangerous, and harmful by inhalation and if swallowed. Contact with combustible material may cause fire. Handle under a fume hood and wear appropriate personal protective equipment.
- Prepare an oxidant solution for mitochondria-derived oxidative stress induction: Make a oxidant stock solution (5 mM) by dissolving 3.9 mg of rotenone in 2 ml of dimethyl sulfoxide (DMSO). Keep this solution at room temperature in the dark.
- Dissolve rotenone stock solution to 10 ml of fish water to make a ready to use solution. Use rotenone at a concentration between 5-50 μM. Do not use rotenone at concentrations higher than 100 μM. At appropriate concentrations, this oxidant solution can be applied to both whole mount ROS-detection and single-cell ROS-detection methods. CAUTION: Rotenone is toxic and hazardous. Handle according appropriate precautionary statements.
- Induce oxidative stress by gene knock-down: Knock-down nrf2a gene expression in zebrafish embryos by morpholino microinjection as previously reported by Timme-Laragy A. et al., 201238.
- Induce oxidative stress after tissue damage: generate a wound margin at the tail fin of zebrafish embryos at 72 hpf as previously described by Niethammer et al., 200939.
- Prepare 5 ml of ROS-detection solution for single cell ROS-detection method:
- Solution for general ROS detection: Dissolve the generic ROS-sensitive molecular probe in HBSS (Hanks’s Balanced Salt Solution) in order to prepare a working solution (concentration range: 2.5-10 μM). This solution must be prepared shortly before usage.
- Solution for specific detection ROS induced by mitochondria: Shortly before use, dissolve a mitochondrial ROS-sensitive probe with DMSO making a 5 mM stock solution. Dissolve stock solution in HBSS in order to prepare a working solution (concentration range: 2.5-10 μM). NOTE: Avoid light and oxygen exposure of ROS-detection working solutions and their respective stock solutions. Protect the tube from light by using an aluminum foil. Do not store or re-use probes dissolved in HBSS. Store the stock solutions at -20 °C for a month. CAUTION: Handle molecular probes and DMSO under a fume hood in accordance with appropriate guidelines.
Prepare 5 ml of ROS-detection solution for the whole mount ROS-detection method: Dissolve the generic ROS-sensitive probe stock solution (stabilized in DMSO) in HBSS in order to prepare a working solution (concentration range: 2.5-5 μM). Avoid light and oxygen exposure. Protect the tube from light. Prepare this solution before use. Do not store or re-use this solution. CAUTION: Handle molecular probes under a fume hood in accordance with appropriate guidelines.
Make 25 ml of Stop Solution by adding 10 ml of fetal bovine serum to 15 ml of PBS 1x. Keep solution sterile. Store this solution at 4 °C. This solution is required only for single cell - ROS detection method.
Set the zebrafish air incubator at 28 °C.
Set the centrifuge at 4 °C for the single cell ROS-detection method.
2. Mating of Adult Fishes and Selection of Zebrafish Embryos
Set-up adult zebrafish pair crosses according to standard protocols40. Select the appropriate transgenic line in accordance with specific experimental needs.
Collect eggs and place them into a 90-mm dish with fish water/embryo medium. Keep eggs at 28 °C until embryos will develop and grow to the desired developmental stage (e.g., 48 hpf, 72 hpf; hpf: hours post fertilization).
Screen for developing embryos. Exclude all unfertilized eggs or underdeveloped embryos.
Anesthetize embryos by adding 1 ml of tricaine (stock solution) in 50 ml fish water.
Select Tg fluorescent embryos under a stereomicroscope.
3. Treatment of Embryos with Oxidant Agent
Use at least 30 embryos between 48 hpf and 72 hpf per different condition. Wash embryos twice with fresh fish water in order to remove tricaine.
Split fluorescent embryos into three dishes. Put no more than 30 embryos/dish.
Remove the washing solution and add 10 ml of the oxidant solution or 10 ml of fish water as control solution.
Incubate embryos for 10 min to 1 hr at 28 °C. The incubation period depends by the level of oxidative stress to be induced in specific tissues. Short periods are the best as cells affected by oxidative stress progressively undergo cell death.
Transfer embryos into a new dish containing HBSS and wash embryos by swirling. Pre-warm HBSS at 28 °C.
4. Whole Mount ROS Detection Method
Collect embryos after oxidant treatment and put no more than 10 embryos in a small tube. Rinse with HBSS as much as possible. Protect the tube from light using aluminum foil.
Add 1 ml of ROS-detection solution for each tube.
Incubate embryos in the dark for 15 min at 28 °C to avoid light exposure.
During the incubation time prepare a glass slide for whole embryo analysis. Put 300 μl of methylcellulose on a “depression” glass slide and spread it on the glass surface with a pipette tip. Avoid bubbles while releasing the methylcellulose.
At the end of the incubation time, immediately remove the ROS-detection solution and wash twice with 2 ml of HBSS. Wash embryos by inverting the tube several times. Do not pipette or vortex.
Aspirate embryos up into a glass Pasteur pipette. Position embryos near the opening of the pipette and gently eject them into the methylcellulose. Orientate the embryos appropriately with a fine nylon line.
Compare the fluorescence of control embryos with treated embryos. Alternatively, fix parameters of the fluorescence stereomicroscope or confocal microscope so that all embryos are imaged using the same imaging settings.
5. Single Cell ROS-detection Method
Start from step: 3.5. Make sure to have at least 35 embryos for each condition.
Transfer embryos into a new dish. Remove fish water as much as possible. Add 10 ml of ice-cold PBS 1x and 400 μl of Protease Inhibitors Cocktail. Manually dechorionate embryos with forceps and remove the yolk sack with a fine needle. Note: Removing yolk sack ensures clean samples for the following FACS analysis. This step can be avoided according to FACS instrument.
Transfer de-yolked embryos into a 24-well multiwell plate (15 embryos/well) and remove all fish water.
Add 300 μl of HBSS, 30 μl collagenase P, 50 μl Trypsin-EDTA in each well.
Homogenize embryos by gently pipetting up and down with a tight pipette tip (1,000 μl).
Incubate at 28 °C for 20 min and homogenize samples by pipetting every 5 min.
Check tissue disruption by observing an aliquot of homogenate at the compound microscope. Facilitate single cell suspension by pipetting. Do not incubate embryos for more than 30 min.
Stop the reaction by adding 200 μl of Stop Solution. Mix by pipetting gently.
Transfer cell suspension into a pre-chilled tube with tight bottom. Keep tube on ice and avoid light exposure.
Centrifuge for 5 min at 250 x g at 4 °C.
Remove the upper phase and re-suspend cells with ice cold HBSS by gently pipetting.
Count cells and make sure you have the same number of cells in all of your samples. Do not use less than 2 x 106 cells.
Centrifuge cells for 5 min at 250 x g at 4 °C.
Remove supernatant and suspend the pellet with 1 ml of ice chilled HBSS containing the ROS-sensitive probe.
Incubate the tube at room temperature for 3 min in the dark. Vary incubation time according to the level of oxidative stress and to the sensitivity of the probe.
Transfer cells to a FACS tube. Keep tubes on ice and avoid light exposure before FACS analysis.
Sort cells with a fluorescence-activated cell sorter (FACS). Set wavelengths in accordance with Tg tissue fluorescence (e.g., GFP) and the excitation/emission spectra of the molecular probe used for ROS detection (e.g., specific mitochondrial ROS-sensitive probes, generic ROS-sensitive probes).
For each condition, measure all samples within the same experimental session by applying the same FACS settings. Calculate the percentage of fluorescent cells as the mean of different biological replicates. Analyze at least two replicates for each condition.
Representative Results
By applying the method here described, we can easily measure and detect oxidative stress (and ROS levels) in zebrafish embryonic tissues. After crossing adult zebrafish, eggs are collected and allowed to develop at 28 °C to 72 hr post fertilization (hpf). In order to induce oxidative stress, we propose two different approaches: 1) the treatment of embryos with strong pro-oxidant reagents or 2) promoting ROS formation after tissue injury.
In the first approach, we employed two different reagents according to the specific needs: hydrogen peroxide (H2O2), as the generic cellular ROS forming agent, and rotenone, as the specific mitochondrial ROS driver. Rotenone is an inhibitor of complex I of the ETC, which deregulations are causative of oxidative stress41.
In the second approach, we induced accumulation of ROS by creating a wound at the tail fin of a zebrafish embryo39. Alternatively, oxidative stress conditions can be promoted in zebrafish tissues by knocking down with morpholino injection the Nrf2 antioxidant response pathway that is the primary cellular defense against the cytotoxic effects of oxidative stress38.
Once the oxidative stress is induced in zebrafish embryos, the accumulation of reactive oxygen species (ROS) can be measured by using generic or mitochondrial specific ROS-sensitive probes, which become fluorescent upon activation (i.e. oxidation).
Treated embryos can be analyzed by using the whole mount ROS-detection method or by applying the single cell-ROS detection method as summarized in Figure 1. The selection between methods relies on the need to perform qualitative or quantitative measurement of oxidative stress. Representative results of both methods are represented in Figure 2 and Figure 3.
Whole Mount ROS-detection Method
Figure 2 shows the application of the whole mount ROS-detection method for in vivo imaging of the oxidative stress. In particular, this method has been applied to follow both “strong” oxidative stress generated by exogenous pro-oxidant treatments as well as “low” oxidative stress generated by more physiological conditions such as wound injuries or gene deletion.
Physiological levels of oxidative species are induced by generating a micro injury or a wide wound at the tail fin of a live zebrafish embryo at 72 hpf. It has been demonstrated that after injury, H2O2 accumulates at the wound margin 20 min after the wound39 . In order to visualize the accumulation of oxidative stress at the wound margin, embryos were incubated with a generic ROS-sensitive probe and imaged at 20 min after wounding39. By comparing intact tail fins with injured tails, it is possible to distinguish an accumulation of the fluorescent probe at the wound margin (Figure 2A). Non-specific weak fluorescence signal is detected all around the tail fin tissue in both conditions.
In order to validate the specific accumulation of the ROS-sensitive probe at the wound margin, the H2O2 levels at the wound have been lowered by a pharmacological approach. Since it has been demonstrated that the wound margin H2O2 accumulation is extremely sensitive to the DUOX-inhibitor VAS287039, embryos were pre-treated with this inhibitor before wounding. Comparison of the fluorescence of the ROS-sensitive probe, from VAS pre-treated embryos and respective controls, indicates that the signal is dependent on ROS accumulation (i.e. H2O2 ) (Figure 2B).
In addition, we generated high levels of oxidative species in zebrafish tissues by treating zebrafish embryos with rotenone. Rotenone-treated embryos and controls were incubated with a generic ROS-sensitive probe to specifically detect ROS species. Afterwards, the probe was washed out and the embryos were imaged under a fluorescence-stereo microscope. The oxidative stress was detected in the whole body of the embryos (Figure 2C). High magnification images show anatomical regions where the probe is successfully metabolized (Figure 2D). Bright field images (upper panels) distinguish anatomical structures, while fluorescence images (lower panels) indicate ROS-positive cells. As shown by the fluorescent images the results are mainly a qualitative report of oxidative stress detection, which is achieved by comparing control with treated embryos.
Single Cell-ROS Detection Method
Figure 3 reports representative FACS plots and quantification of oxidative stress by applying the single cell ROS detection method. This method has been adapted to measure oxidative stress levels in zebrafish cells that were subjected to pro-oxidant conditions as reported in the protocol and detailed in figure legends. As described, the oxidative stress has been measured by incubating zebrafish tissues dissociated into single cells with a ROS-sensitive molecular probe. Since the dissociation procedure and the FACS itself may cause cell damage, the analysis of samples requires that only “live cells” are considered for oxidative stress quantitation. Accordingly, dissociated cells are analyzed by selecting the fraction of cells showing “live cell” physical parameters (Figure 3A) and by excluding dead cells (Figure 3B). Thus, the samples are quantitated for oxidative stress levels according to the fluorescence of the ROS-sensitive probe and for showing an endogenous fluorescence such as the positivity for the GFP (Figure 3C). A negative control sample for the ROS-sensitive probe should be included in order to evaluate the oxidative stress induced by handling and technical procedures (Figure 3C; panel: Control -). Relative FACS plot quantification demonstrated in histograms showing measurements of different biological replicates (Figure 3D-E).
Besides oxidative stress level quantitation upon hydrogen peroxide treatments, this method has been applied to quantitate oxidative stress levels within physiological conditions such us in nrf2a morphants and respective controls (Figure 3F).
Moreover, by combining the single-cell ROS detection method with specific ROS-sensitive probes such us mitochondrial ROS-sensitive probes, it is also possible to measure oxidative stress in the context of specific mitochondria-targeted pro-oxidant treatments (Figure 3G).
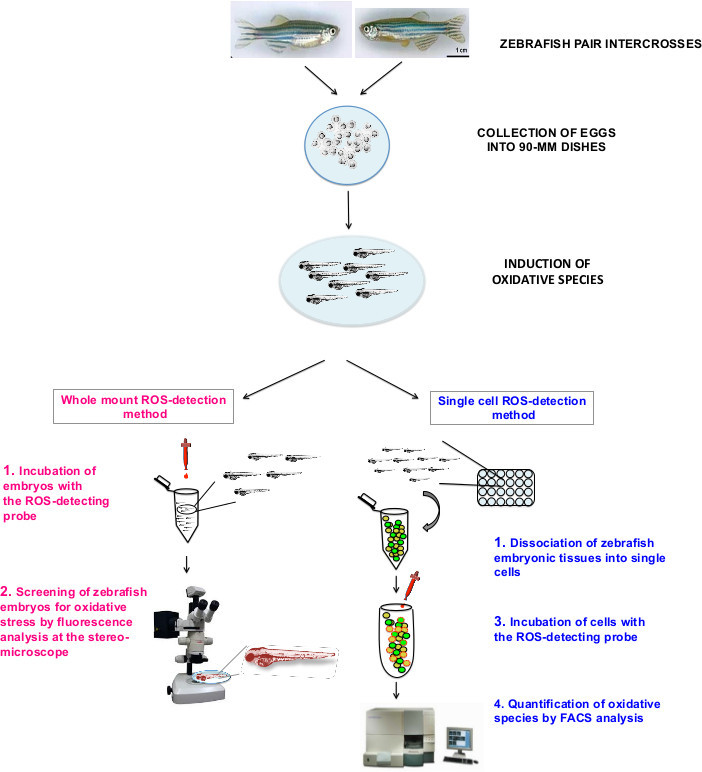
Figure 1. Schematic figure of the method for measuring ROS levels in zebrafish embryos. Zebrafish adults are crossed in the appropriate breeding tanks. Fertilized eggs are then collected into dishes and stored at 28 °C to allow embryo to fully develop. Induction of oxidative stress can be achieved in different ways, either by pharmaceutical treatments or by genetic or physical insults. Alternatively, in case a genetic mutant is analyzed, it is possible to skip this incubation and move forward. At this point, it is possible to proceed following two different methods. According to the “whole mount ROS-detection” method (left), zebrafish embryos are immediately incubated with a fluorescent ROS-sensitive probe (1) and then analyzed with fluorescence or confocal microscope (2). In the “single cell ROS-detection” method (right), zebrafish embryos are dissociated into single cells (1), incubated with a fluorescent ROS-sensitive probe (2) and analyzed by FACS for fluorescence detection and quantitation (3). While the “whole mount-ROS detection” method allows oxidative stress detection in living embryos primarily as a qualitative assay, “single-cell based” method grants both qualitative and quantitative measurements of oxidative stress levels.
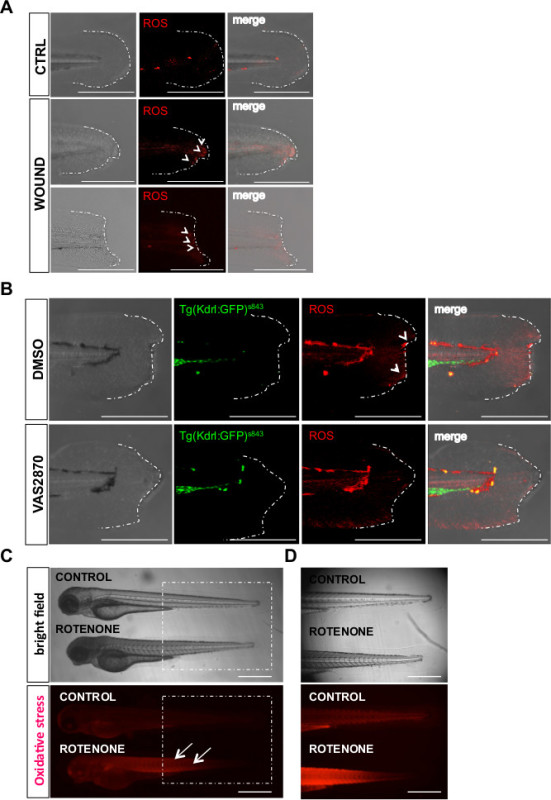 Figure 2. Representative results of the whole mount ROS-detection method. A) Zebrafish embryos at 72 hpf were subjected to wounding as previously described by Niethammer et al., 200939. Representative confocal images show pro-oxidative species (ROS) accumulation (arrowhead) at the wound margin of the wounded caudal fin. Oxidative species have been detected with the generic ROS probe (CellROX; 2.5 μM) 20 min after the wound has been made. Scale bar 20 μm. B) Representative confocal images showing wound margin of zebrafish embryos at 72 hpf. Embryos were pretreated with VAS2870 (20 μM) or DMSO for 90 min before the wound has been made. ROS have been detected with a generic ROS-sensitive probe (CellROX; 2.5 μM) 20 min after wound. Scale bar 20 μm. C) Whole body image showing rotenone-induced oxidative stress in zebrafish embryos. Oxidative stress is strongly detected in the caudal region of the embryos as shown in panel D). Rotenone is a potent inhibitor of mitochondrial ETC affecting mainly skeletal muscle cells in zebrafish. Scale bar, 180 μm.
Figure 2. Representative results of the whole mount ROS-detection method. A) Zebrafish embryos at 72 hpf were subjected to wounding as previously described by Niethammer et al., 200939. Representative confocal images show pro-oxidative species (ROS) accumulation (arrowhead) at the wound margin of the wounded caudal fin. Oxidative species have been detected with the generic ROS probe (CellROX; 2.5 μM) 20 min after the wound has been made. Scale bar 20 μm. B) Representative confocal images showing wound margin of zebrafish embryos at 72 hpf. Embryos were pretreated with VAS2870 (20 μM) or DMSO for 90 min before the wound has been made. ROS have been detected with a generic ROS-sensitive probe (CellROX; 2.5 μM) 20 min after wound. Scale bar 20 μm. C) Whole body image showing rotenone-induced oxidative stress in zebrafish embryos. Oxidative stress is strongly detected in the caudal region of the embryos as shown in panel D). Rotenone is a potent inhibitor of mitochondrial ETC affecting mainly skeletal muscle cells in zebrafish. Scale bar, 180 μm.
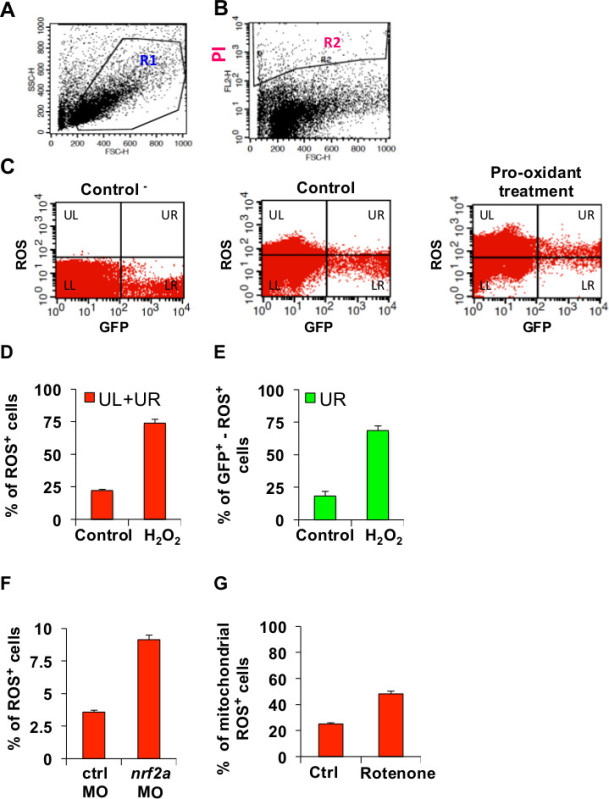 Figure 3. Representative results of the single cells ROS-detection method. A) Representative FACS plot showing a typical sample of zebrafish embryos dissociated to single cells. Live cells are gated in R1 region according with FSC-H and SSC-H parameters. SSC-H: 539 (Voltage), 1.0 (AmpGain), Mode: linear; FSC-H: E00 (Voltage), 2.1 (AmpGain). B) Representative FACS plot showing a typical sample of zebrafish embryos dissociated to single cells. Before FACS analysis, the sample has been incubated with Propidium Iodide (PI; 1 μg/ml) for 5 min. Dead cells are gated on R2 region according with PI fluorescence (FL2-H channel). FSC-H: E00 (Voltage), 2.1 (AmpGain), Mode: linear, SSC-H: 539 (Voltage), 1.0 (AmpGain), Mode: linear. Voltage Channels: FL-2: 613 Log. C) Representative FACS plots showing dissociated zebrafish embryos subjected to pro-oxidant treatment (H2O2) and respective controls. Endothelial cells are visualized by GFP channel. FACS plots represent all cells affected by oxidative stress (ROS) on UL and UR. Negative cells are plotted on lower quadrants (LL and LR). Tg(Kdrl:GFP)s843 zebrafish embryos at 48hpf were incubated with H2O2 (2 mM) or H2O as control for 10 min. Before FACS analysis, embryos are processed as described in the protocol. Cells affected by oxidative stress are detected by using a generic ROS-sensitive fluorescent probe (CellROX; 2.5 μM). A sample not incubated with the ROS-sensitive probe has been included as negative control. Comparing the sample subjected to pro-oxidant treatment with the respective control, the number of cells plotted on upper quadrants (UL+UR) is higher. FACS acquisition settings were as follows: Voltage channels: FL-1: 582 Log; FL-4: 410 Log. D) Histogram showing the percentage of cells (UL+UR) affected by oxidative stress in H2O2-treated embryos and respective control. Measurements are related to samples shown in C. Cells affected by oxidative stress are detected by using a generic ROS-sensitive fluorescent probe (CellROX; 2.5 μM). Results are the mean of n=2 different biological replicates ± SD. E) Histogram showing the percentage of endothelial cells (GFP+) affected by oxidative stress (ROS+) in H2O2-treated embryos and respective control. Measurements are related to samples shown in C. Results are the mean of n=2 different biological replicates ± SD. F) Histogram showing the percentage of cells affected by oxidative stress (ROS+) in nrf2a morphants (nrf2a MO) and respective controls (ctrl MO) at 24 hpf. Results are the mean of n=2 different biological replicates ± SD. G) Histogram showing the percentage of cells affected by mitochondrial oxidative stress in treated embryos (Rotenone; 10 μM) and respective controls (Ctrl; DMSO) at 72 hpf. After dissociation of embryos into single cells, mitochondrial oxidative stress (mitochondrial ROS+) was measured with a mitochondrial specific probe (MitoSOX; 5 μM). Results are the mean of n=3 different biological replicates ± SD.
Figure 3. Representative results of the single cells ROS-detection method. A) Representative FACS plot showing a typical sample of zebrafish embryos dissociated to single cells. Live cells are gated in R1 region according with FSC-H and SSC-H parameters. SSC-H: 539 (Voltage), 1.0 (AmpGain), Mode: linear; FSC-H: E00 (Voltage), 2.1 (AmpGain). B) Representative FACS plot showing a typical sample of zebrafish embryos dissociated to single cells. Before FACS analysis, the sample has been incubated with Propidium Iodide (PI; 1 μg/ml) for 5 min. Dead cells are gated on R2 region according with PI fluorescence (FL2-H channel). FSC-H: E00 (Voltage), 2.1 (AmpGain), Mode: linear, SSC-H: 539 (Voltage), 1.0 (AmpGain), Mode: linear. Voltage Channels: FL-2: 613 Log. C) Representative FACS plots showing dissociated zebrafish embryos subjected to pro-oxidant treatment (H2O2) and respective controls. Endothelial cells are visualized by GFP channel. FACS plots represent all cells affected by oxidative stress (ROS) on UL and UR. Negative cells are plotted on lower quadrants (LL and LR). Tg(Kdrl:GFP)s843 zebrafish embryos at 48hpf were incubated with H2O2 (2 mM) or H2O as control for 10 min. Before FACS analysis, embryos are processed as described in the protocol. Cells affected by oxidative stress are detected by using a generic ROS-sensitive fluorescent probe (CellROX; 2.5 μM). A sample not incubated with the ROS-sensitive probe has been included as negative control. Comparing the sample subjected to pro-oxidant treatment with the respective control, the number of cells plotted on upper quadrants (UL+UR) is higher. FACS acquisition settings were as follows: Voltage channels: FL-1: 582 Log; FL-4: 410 Log. D) Histogram showing the percentage of cells (UL+UR) affected by oxidative stress in H2O2-treated embryos and respective control. Measurements are related to samples shown in C. Cells affected by oxidative stress are detected by using a generic ROS-sensitive fluorescent probe (CellROX; 2.5 μM). Results are the mean of n=2 different biological replicates ± SD. E) Histogram showing the percentage of endothelial cells (GFP+) affected by oxidative stress (ROS+) in H2O2-treated embryos and respective control. Measurements are related to samples shown in C. Results are the mean of n=2 different biological replicates ± SD. F) Histogram showing the percentage of cells affected by oxidative stress (ROS+) in nrf2a morphants (nrf2a MO) and respective controls (ctrl MO) at 24 hpf. Results are the mean of n=2 different biological replicates ± SD. G) Histogram showing the percentage of cells affected by mitochondrial oxidative stress in treated embryos (Rotenone; 10 μM) and respective controls (Ctrl; DMSO) at 72 hpf. After dissociation of embryos into single cells, mitochondrial oxidative stress (mitochondrial ROS+) was measured with a mitochondrial specific probe (MitoSOX; 5 μM). Results are the mean of n=3 different biological replicates ± SD.
Discussion
Critical Steps
The procedure for oxidative stress detection in zebrafish embryos herein described comprises two different methods. The whole mount ROS-detection method is mainly a qualitative assay for ROS-detection, while the single cell ROS-detection method allows more specific quantitative measurements (Figure 1). Both methods offer a quick and easy way to assess in vivo ROS-detection on zebrafish embryos. However, they both present some critical steps.
Critical Steps Related to Method I
The first method for whole mount ROS-detection has the great advantage to easily detect the oxidative stress in all tissues of living embryos (Figure 2). However there are three important critical aspects that must be considered and might influence the outcome of the assay: 1) the permeability of the probe to embryo tissues, 2) the probe toxicity and 3) the probe sensitivity to low ROS concentration.
The first aspect is specifically related to the in vivo application of the method42. The “cell-permeability” property of the ROS-sensitive probe is an essential feature for the achievement of this assay. Ideally, all water-soluble chemicals are optimal for delivery in live zebrafish embryos; however most of the reagents for oxidative stress detection are insoluble in water. Thus it is usually recommended to use probes that are soluble in DMSO (dimethyl sulfoxide).
There are possible toxic effects on embryos linked to the concentration of the ROS probe. Side effects are normally represented by tissue damage (i.e. necrosis) or accelerated metabolism of the probe. Long incubations (days) of zebrafish embryos with the hydroethidine probe cause embryo death while high doses of 5-(and-6)-Carboxy-2',7'-Dichlorofluorescein diacetate results in non-specific accumulation of the fluorescence in the gut (unpublished data). To overcome this issue it is important to monitor embryos during the incubation with the probe. Tests of several concentrations and times of incubation with the same probe would be beneficial as well. The optimal incubation time allows delivery and oxidation of the molecular probe without superficial tissues damage. Incubation times using chemical probes (0.5-10 μM) can be generally fixed in the range of 10-30 min and should not exceed 1 hr. This condition can be quite tricky to set up especially when the amount of oxidative species is close to physiological levels or when it is restricted to internal embryonic tissues. Indeed, when a whole organism is incubated with a ROS-detection chemical probe, the most superficial tissues (i.e. skin, eyes, and heart) are where the probe is mainly oxidized and becomes fluorescent. Later, the probe is progressively delivered to internal organs, liver or vessels. As a consequence, an appropriate time for the incubation of zebrafish embryos with the ROS-detecting probe is critical to detect oxidative stress in all tissues.
Finally, when using this method it is important to consider the sensitivity of the probe to the level of oxidative stress expressed by tissues. Most of the commercially available probes are not finely characterized for their sensitivity and this means that the optimal probe for specific applications must be empirically determined. All ROS-sensitive probes generally detect high levels of oxidative stress, but most of them are not sensitive to minimal shifts of oxidative stress levels43.
In order to limit this disadvantage and allow a more detailed and precise analysis it is suggested to apply the second method based on single cells detection of oxidative stress.
Critical Steps Related to Method II
The single-cell ROS-detection method presents some critical steps. They are mainly determined by 1) the efficiency of embryonic cell dissociation and by 2) the type of ROS-sensitive probe associated to the assay.
The dissociation of embryos into single cells has been adapted from previous protocols for zebrafish cell analysis via flow cytometry44 and presents two critical aspects that strongly influence the outcome of the whole procedure. The first aspect regards the method used to dissociate embryos, while the second one is related to the recovery of dissociated cells.
The dissociation of embryos into single cells is mainly controlled by the concentration of dissociation reagents (i.e. collagenase P and trypsin). However, it must be considered that the efficiency of tissue dissociation is dependent on the number of embryos incubated and their developmental stage. Zebrafish embryos at earlier developmental stages (24 hpf-48 hpf) are more sensible to dissociation reagents than larvae (96 hpf-120 hpf) because of progressive tissues growth45. Thus, adjustments in the dissociation procedure are required when working with zebrafish embryos at different developmental stages than 72 hpf. Excessive incubation with reagents causes cell death, while reduced concentration of reagents only partially dissociates the embryos. As it is important to correctly disaggregate tissues, it is equally important to collect and preserve single cells. An important consideration is the temperature at which cells are recovered after tissue division. It is important to maintain cells on ice or at 4 °C during centrifugation in order to limit the oxidative stress secondary to mechanical disruption of tissues or handling procedures.
The second critical step regarding the associated probe is linked to the analysis on a specific tissue of interest. The easiest way to detect a cell population in zebrafish is offered by the wide array of transgenic lines that have been established in recent decades46,47. However the fluorescence of the selected transgenic line must be compatible with the ROS-detecting probe. It is crucial to avoid cross-talk between background tissue fluorescence and the low, but specific, fluorescence signal emitted by the probe.
Applications of this Protocol
Considering both methods offer an easy and cost effective assay to measure oxidative stress in vivo, it is possible to apply this protocol to several conditions:
Measurements of Oxidative Stress in Different Genetic (e.g., pathological) Conditions
Oxidant and antioxidant genes can be easily modulated in zebrafish embryos by the injection of morpholinos (loss-of-function) or mRNA (gain-of-function). Recently reported experiments of gene knock-down and microinjection of capped mRNA in zebrafish embryos established different roles for the antioxidant Nrf2a and Nrf2b genes in protecting cells from oxidative stress during development. Approaches of gene expression modulation assessed an evolutionary conserved axis between mammalian and zebrafish to hypoxia response and oxidative stress in neuronal cells38,48. In addition, a great opportunity to study the oxidative stress is offered by several zebrafish mutant lines. For example, the zebrafish ducttrip (dtp) mutant represents an interesting example for the characterization of oxidative stress in relation to a pathological condition. The dtp mutant carries a deficiency for the S-adenosylhomocysteine hydrolase (ahcy) gene and its characterization revealed that the loss of Ahcy activity causes liver degeneration by influencing ROS levels49. Equivalently, the zebrafish mutant nrf2fh318, carrying a loss-of-function mutation of the transcriptional factor nrf2 gene, has been regarded with considerable interest to investigate the protective role of this antioxidant gene in lower vertebrates50.
Measurements of Redox State Triggered by Pharmaceutical Compounds
Treatment of zebrafish embryos with small drugs can inhibit or activate oxidative stress. We recently demonstrate that statin treatment of zebrafish embryos induced oxidative stress that can be recovered by CoQ10 treatment51. Alternatively, the analysis of oxidative stress in the zebrafish animal model for human RYR1-related myopathies highlighted the role of the antioxidant drug NAC (N-acetyl cysteine) as a potential therapeutic approach for muscle disease52.
Large Scale Screening of Small Molecules and Drugs to Characterize their Oxidative Properties
The zebrafish is a well-established animal model for large chemical screening. A recent chemical screening of small molecules performed in zebrafish identified antioxidants as modulators of dopaminergic neurons survival, which is severely affected in mammalian neurodegenerative diseases such as Parkinson’s disease53. This method can be used to screen for new pro- or anti-oxidant molecules in vivo.
Significance and Limitations
The prominence of both “whole mount” and “single-cell” ROS-detection methods relies on the ability to perform in vivo measurements of oxidative stress by using simple laboratory techniques and basic equipment. The application of the zebrafish as an animal model in combination with ROS-detecting probes allows not only the mere revelation of oxidative stress, but also its detection in the context of a living organism and quantification at the level of single tissues. Importantly, these assays can be performed with commercially available tools and do not require specific expertise to be realized. In addition, both methods are not time-consuming procedures and can be applied with appropriate time to large sets of samples. All together these practical advantages make these methods of particular significance to improve oxidative stress analysis in vivo.
However these assays also show some limitations. In general, most ROS-sensitive probes now commercially available are not valued for fine qualitative and quantitative analysis of oxidative stress levels in living cells. The commonly used hydroethidine and MitoSOX probes were designed for sensing of the superoxide (O2.- ), but recently concerns about their specificity have been raised, especially when using fluorescence-based assays54. Analogously some reports advanced doubts about the dichlorofluorescein diacetate (DCFH-DA) for exclusive H2O2 sensing55,56. Moreover these redox-active fluorescent probes are characterized by partial non-specific activation (i.e. tissue auto-fluorescence) and are not reversible after oxidation. Once the fluorescent probe is oxidized by ROS it cannot come back to its non-fluorescent reduced state. Consequently the detection of ROS fluctuations with temporal resolution is limited by application of such chemical probes43.
A considerable limitation of this field is represented by the lack of probes for exclusive RNS detection. Recently, proteomic based methodologies demonstrated that RNS induce protein modifications that are considered as biomarkers of nitroso-active stress and can be evaluated via immunoblotting assays by using specific antibodies to protein oxidation/nitration (e.g., anti-SNO-cysteine, anti-3-Nitrotyrosine)22,23. However, all these assays are only indirect measurements of oxidative stress triggered by RNS and in most of cases they require cellular extracts, meaning that they are not applicable to living cells or living zebrafish embryos.
A valid alternative to conventional probes is represented by genetically-encoded redox sensitive fluorescent proteins. The major constituents of this group are: roGFP, derived from green fluorescent proteins, rxYFP and cpYFP, both derived from the yellow fluorescent protein and the HyPer probe, a specific H2O2-sensitive fluorescent probe57. All of these engineered redox probes created by fluorescent proteins allow fluorescence-based ratiometric quantifications and enable higher temporal resolution (from seconds to minutes) than chemical probes58. in vivo application of these biosensors is also possible as demonstrated by the zebrafish transgenic line expressing HyPer for real-time sensing of endogenous H2O2 levels39,59. Implementing the zebrafish with genetically encoded sensors offers an optimal system to investigate oxidative stress, but requires setting up a transgenic animal line and appropriate imaging tools. The procedure for oxidative stress detection in zebrafish presented here is less accurate than genetic methods, but is quick and provides a primary assay for in vivo detection of high levels of pro-oxidative species. Accurate measurement of individual reactive oxygen and/or nitrogen species is currently a limit of this research field. In the future, forthcoming nanoscale redox-sensors could help to solve this issue. The successful application of nanoparticle and nanotube based redox-sensors has been tested in several in vitro studies, but not available as in vivo assays until now60.
Disclosures
The authors have nothing to disclose.
Acknowledgments
Support in Massimo Santoro lab come from HFSP, Marie Curie Action, Telethon and AIRC. We thank Dafne Gays and Emiliano Panieri for critical reading of the manuscript.
References
- Alfadda AA, Sallam RM. Reactive oxygen species in health and disease. J Biomed Biotechnol. 2012;2012 doi: 10.1155/2012/936486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Finkel T. Free radicals and senescence. Exp Cell Res. 2008;314:1918–1922. doi: 10.1016/j.yexcr.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AF, et al. Free radical biology of the cardiovascular system. Clin Sci (Lond. 2012;123:73–91. doi: 10.1042/CS20110562. [DOI] [PubMed] [Google Scholar]
- Selvaraju V, et al. Diabetes, oxidative stress, molecular mechanism, and cardiovascular disease--an overview. Toxicol Mech Methods. 2012;22:330–335. doi: 10.3109/15376516.2012.666648. [DOI] [PubMed] [Google Scholar]
- Gandhi S, Abramov AY. Mechanism of oxidative stress in neurodegeneration. Oxid Med Cell Longev. 2012;2012 doi: 10.1155/2012/428010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Zhang Y, Zheng J, Pan J. Reactive oxygen species in cancer stem cells. Antioxid Redox Signal. 2012;16:1215–1228. doi: 10.1089/ars.2012.4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X. Reactive oxygen species: the achilles' heel of cancer cells. Antioxid Redox Signal. 2012;16:1212–1214. doi: 10.1089/ars.2012.4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Bourla AB, Kastner DL, Colbert RA, Siegel RM. Lighting the fires within: the cell biology of autoinflammatory diseases. Nat Rev Immunol. 2012;12:570–580. doi: 10.1038/nri3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C, Ding A. SnapShot: Reactive Oxygen Intermediates (ROI) Cell. 2010;140:951–951. doi: 10.1016/j.cell.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Brown GC, Borutaite V. Interactions between nitric oxide, oxygen, reactive oxygen species and reactive nitrogen species. Biochem Soc Trans. 2006;34:953–956. doi: 10.1042/BST0340953. [DOI] [PubMed] [Google Scholar]
- Brieger K, Schiavone S, Miller FJ, Krause KH. Reactive oxygen species: from health to disease. Swiss Med Wkly. 2012;142 doi: 10.4414/smw.2012.13659. [DOI] [PubMed] [Google Scholar]
- Littarru GP, Tiano L, Belardinelli R, Watts GF. Coenzyme Q(10), endothelial function, and cardiovascular disease. Biofactors. 2011;37:366–373. doi: 10.1002/biof.154. [DOI] [PubMed] [Google Scholar]
- Landete JM. Dietary intake of natural antioxidants: vitamins and polyphenols. Crit Rev Food Sci Nutr. 2013;53:706–721. doi: 10.1080/10408398.2011.555018. [DOI] [PubMed] [Google Scholar]
- Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarsour EH, Kumar MG, Chaudhuri L, Kalen AL, Goswami PC. Redox control of the cell cycle in health and disease. Antioxid Redox Signal. 2009;11:2985–3011. doi: 10.1089/ars.2009.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C, Shiloh MU. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl Acad Sci U S A. 2000;97:8841–8848. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autreaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- Maryanovich M, Gross A. A ROS rheostat for cell fate regulation. Trends Cell Biol. 2013;23:129–134. doi: 10.1016/j.tcb.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Tonks NK. Redox redux: revisiting PTPs and the control of cell signaling. Cell. 2005;121:667–670. doi: 10.1016/j.cell.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Antelmann H, Helmann JD. Thiol-based redox switches and gene regulation. Antioxid Redox Signal. 2011;14:1049–1063. doi: 10.1089/ars.2010.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster MW, Hess DT, Stamler JS. Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med. 2009;15:391–404. doi: 10.1016/j.molmed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht SC, Barata AG, Grosshans J, Teleman AA, Dick TP. In vivo mapping of hydrogen peroxide and oxidized glutathione reveals chemical and regional specificity of redox homeostasis. Cell Metab. 2011;14:819–829. doi: 10.1016/j.cmet.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Knoefler D, et al. Quantitative in vivo redox sensors uncover oxidative stress as an early event in life. Mol Cell. 2012;47:767–776. doi: 10.1016/j.molcel.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L, Miller YI. Emerging applications for zebrafish as a model organism to study oxidative mechanisms and their roles in inflammation and vascular accumulation of oxidized lipids. Free Radic Biol Med. 2012;53:1411–1420. doi: 10.1016/j.freeradbiomed.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RM, et al. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell. 2008;2:183–189. doi: 10.1016/j.stem.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amatruda JF, Patton EE. Genetic models of cancer in zebrafish. Int Rev Cell Mol Biol. 2008;271:1–34. doi: 10.1016/S1937-6448(08)01201-X. [DOI] [PubMed] [Google Scholar]
- Jing L, Zon LI. Zebrafish as a model for normal and malignant hematopoiesis. Dis Model Mech. 2011;4:433–438. doi: 10.1242/dmm.006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JT, Fishman MC. From Zebrafish to human: modular medical models. Annu Rev Genomics Hum Genet. 2002;3:311–340. doi: 10.1146/annurev.genom.3.031402.131506. [DOI] [PubMed] [Google Scholar]
- Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet. 2007;8:353–367. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Wolfe SA. Forward and reverse genetic approaches for the analysis of vertebrate development in the zebrafish. Dev Cell. 2011;21:48–64. doi: 10.1016/j.devcel.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Duan J, Yu Y, Li Y, Sun Z. Cardiovascular toxicity evaluation of silica nanoparticles in endothelial cells and zebrafish model. Biomaterials. 2013;34:5853–5862. doi: 10.1016/j.biomaterials.2013.04.032. [DOI] [PubMed] [Google Scholar]
- Sobrino-Figueroa AS. Evaluation of oxidative stress and genetic damage caused by detergents in the zebrafish Danio rerio (Cyprinidae) Comp Biochem Physiol A Mol Integr Physiol. 2013;165:528–532. doi: 10.1016/j.cbpa.2013.03.026. [DOI] [PubMed] [Google Scholar]
- Xu H, et al. Oxidative stress and immune related gene expression following exposure to di-n-butyl phthalate and diethyl phthalate in zebrafish embryos. Ecotoxicol Environ Saf. 2013;93:39–44. doi: 10.1016/j.ecoenv.2013.03.038. [DOI] [PubMed] [Google Scholar]
- Hermann AC, Millard PJ, Blake SL, Kim CH. Development of a respiratory burst assay using zebrafish kidneys and embryos. J Immunol Methods. 2004;292:119–129. doi: 10.1016/j.jim.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Rieger S, Sagasti A. Hydrogen peroxide promotes injury-induced peripheral sensory axon regeneration in the zebrafish skin. PLoS Biol. 2011;9 doi: 10.1371/journal.pbio.1000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timme-Laragy AR, et al. Nrf2b, novel zebrafish paralog of oxidant-responsive transcription factor NF-E2-related factor 2 (NRF2) J Biol Chem. 2012;287:4609–4627. doi: 10.1074/jbc.M111.260125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MP, et al. Perspective. Cell Metabolism. 2011;13(4):361–366. doi: 10.1016/j.cmet.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, et al. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem. 2003;278:8516–8525. doi: 10.1074/jbc.M210432200. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Chang TS, Jeong W, Kang D. Methods for detection and measurement of hydrogen peroxide inside and outside of cells. Mol Cells. 2010;29:539–549. doi: 10.1007/s10059-010-0082-3. [DOI] [PubMed] [Google Scholar]
- Murphy MP, et al. Unraveling the biological roles of reactive oxygen species. Cell Metab. 2011;13:361–366. doi: 10.1016/j.cmet.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covassin L, et al. Global analysis of hematopoietic and vascular endothelial gene expression by tissue specific microarray profiling in zebrafish. Dev Biol. 2006;299:551–562. doi: 10.1016/j.ydbio.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Beis D, Stainier DY. In vivo cell biology: following the zebrafish trend. Trends Cell Biol. 2006;16:105–112. doi: 10.1016/j.tcb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Pan YA, et al. Zebrabow: multispectral cell labeling for cell tracing and lineage analysis in zebrafish. Development. 2013;140:2835–2846. doi: 10.1242/dev.094631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussavi Nik SH, et al. The BACE1-PSEN-AbetaPP regulatory axis has an ancient role in response to low oxygen/oxidative stress. J Alzheimers Dis. 2012;28:515–530. doi: 10.3233/JAD-2011-110533. [DOI] [PubMed] [Google Scholar]
- Matthews RP, et al. TNFalpha-dependent hepatic steatosis and liver degeneration caused by mutation of zebrafish S-adenosylhomocysteine hydrolase. Development. 2009;136:865–875. doi: 10.1242/dev.027565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaigasa K, et al. Genetic evidence of an evolutionarily conserved role for Nrf2 in the protection against oxidative stress. Mol Cell Biol. 2012;32:4455–4461. doi: 10.1128/MCB.00481-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugoni V, et al. Ubiad1 is an antioxidant enzyme that regulates eNOS activity by CoQ10 synthesis. Cell. 2013;152:504–518. doi: 10.1016/j.cell.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling JJ, et al. Oxidative stress and successful antioxidant treatment in models of RYR1-related myopathy. Brain. 2012;135:1115–1127. doi: 10.1093/brain/aws036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Dong Z, Khodabakhsh H, Chatterjee S, Guo S. Zebrafish chemical screening reveals the impairment of dopaminergic neuronal survival by cardiac glycosides. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielonka J, Kalyanaraman B. Hydroethidine- and MitoSOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: another inconvenient truth. Free Radic Biol Med. 2010;48:983–1001. doi: 10.1016/j.freeradbiomed.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zhong Z, Xu Z, Chen L, Wang Y. 2',7'-Dichlorodihydrofluorescein as a fluorescent probe for reactive oxygen species measurement: Forty years of application and controversy. Free Radic Res. 2010;44:587–604. doi: 10.3109/10715761003709802. [DOI] [PubMed] [Google Scholar]
- Karlsson M, Kurz T, Brunk UT, Nilsson SE, Frennesson CI. What does the commonly used DCF test for oxidative stress really show. Biochem J. 2010;428:183–190. doi: 10.1042/BJ20100208. [DOI] [PubMed] [Google Scholar]
- Belousov VV, et al. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods. 2006;3:281–286. doi: 10.1038/nmeth866. [DOI] [PubMed] [Google Scholar]
- Bjornberg O, Ostergaard H, Winther JR. Measuring intracellular redox conditions using GFP-based sensors. Antioxid Redox Signal. 2006;8:354–361. doi: 10.1089/ars.2006.8.354. [DOI] [PubMed] [Google Scholar]
- Yoo SK, Starnes TW, Deng Q, Huttenlocher A. Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature. 2011;480:109–112. doi: 10.1038/nature10632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusitalo LM, Hempel N. Recent Advances in Intracellular and In Vivo ROS Sensing: Focus on Nanoparticle and Nanotube Applications. Int J Mol Sci. 2012;13:10660–10679. doi: 10.3390/ijms130910660. [DOI] [PMC free article] [PubMed] [Google Scholar]


