Abstract
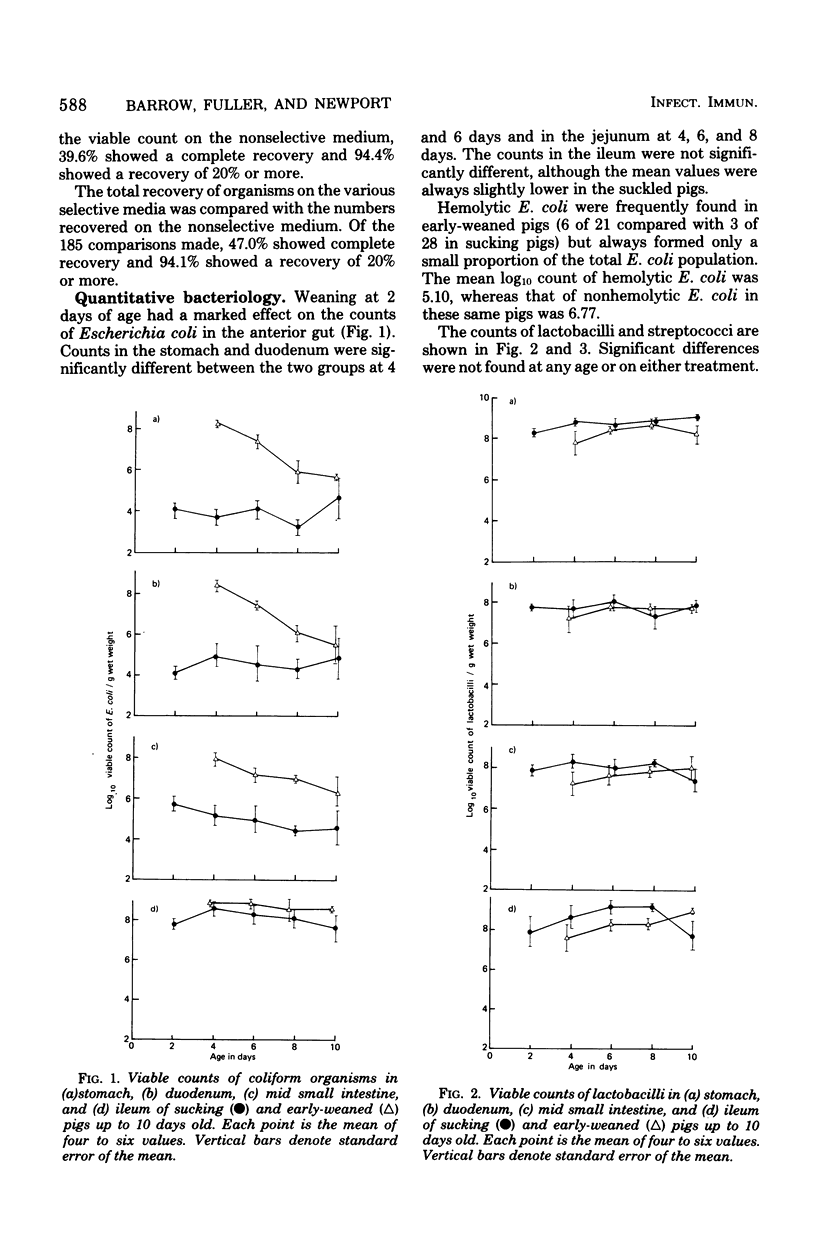
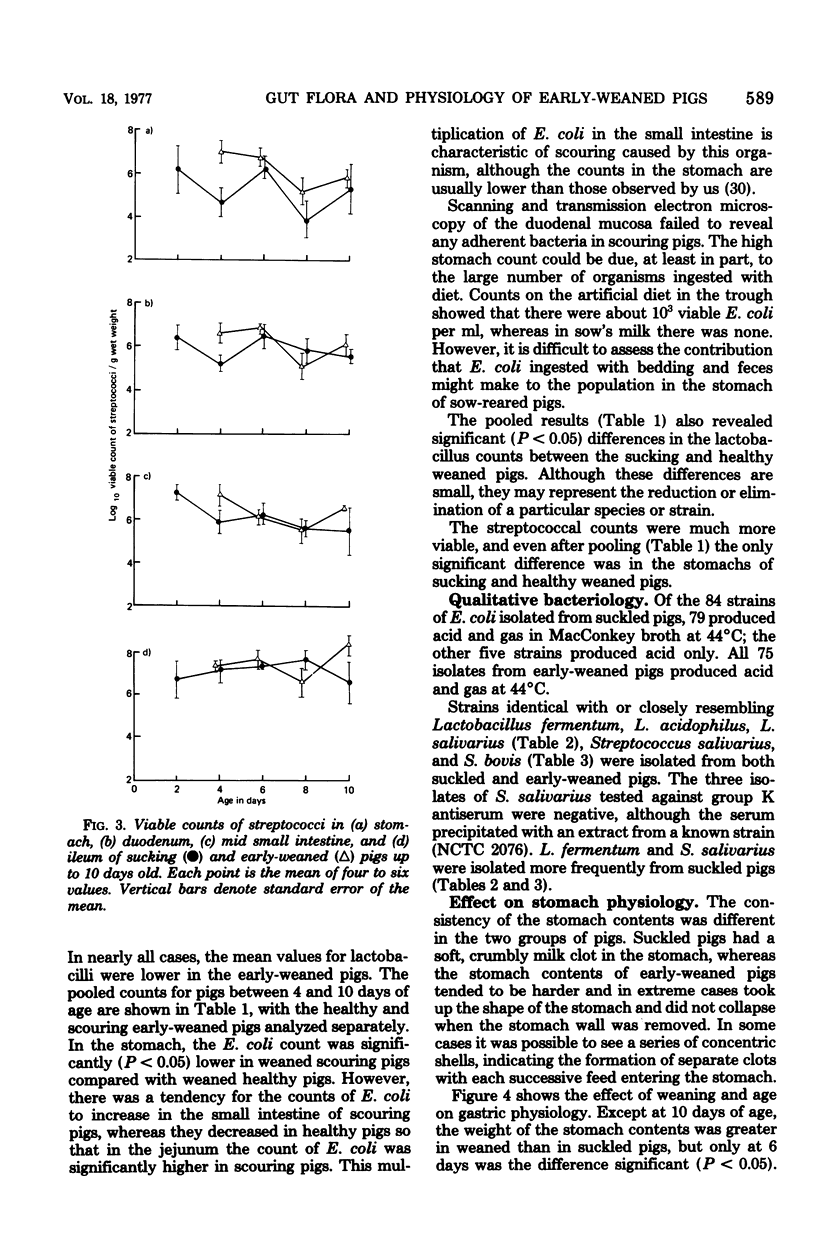
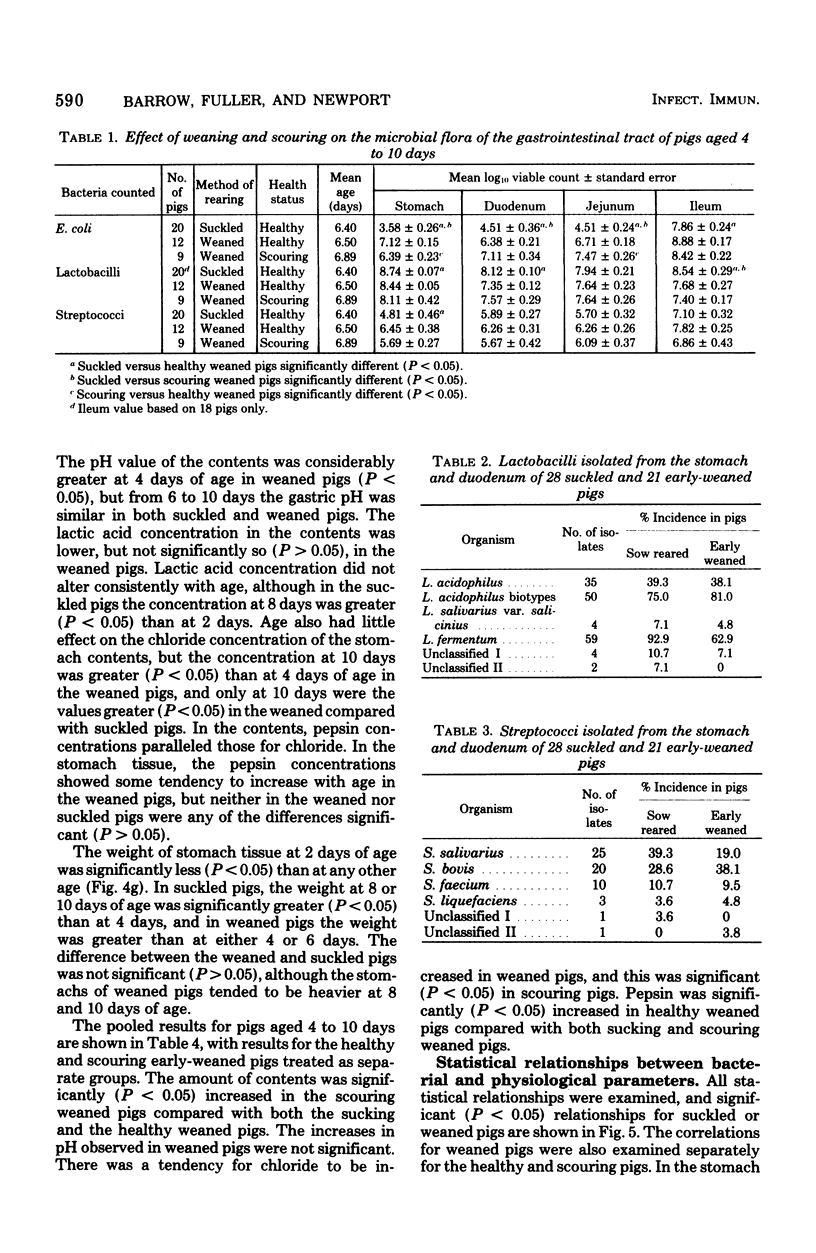
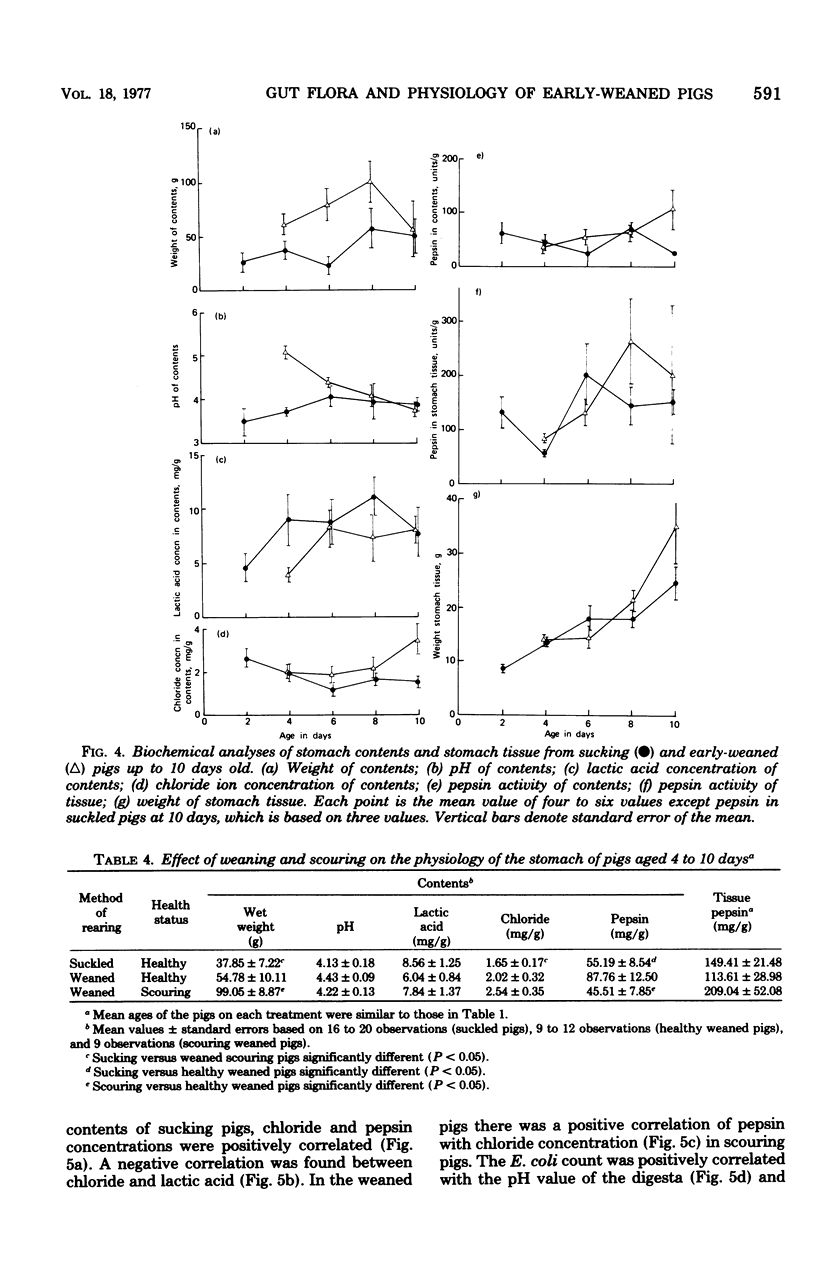
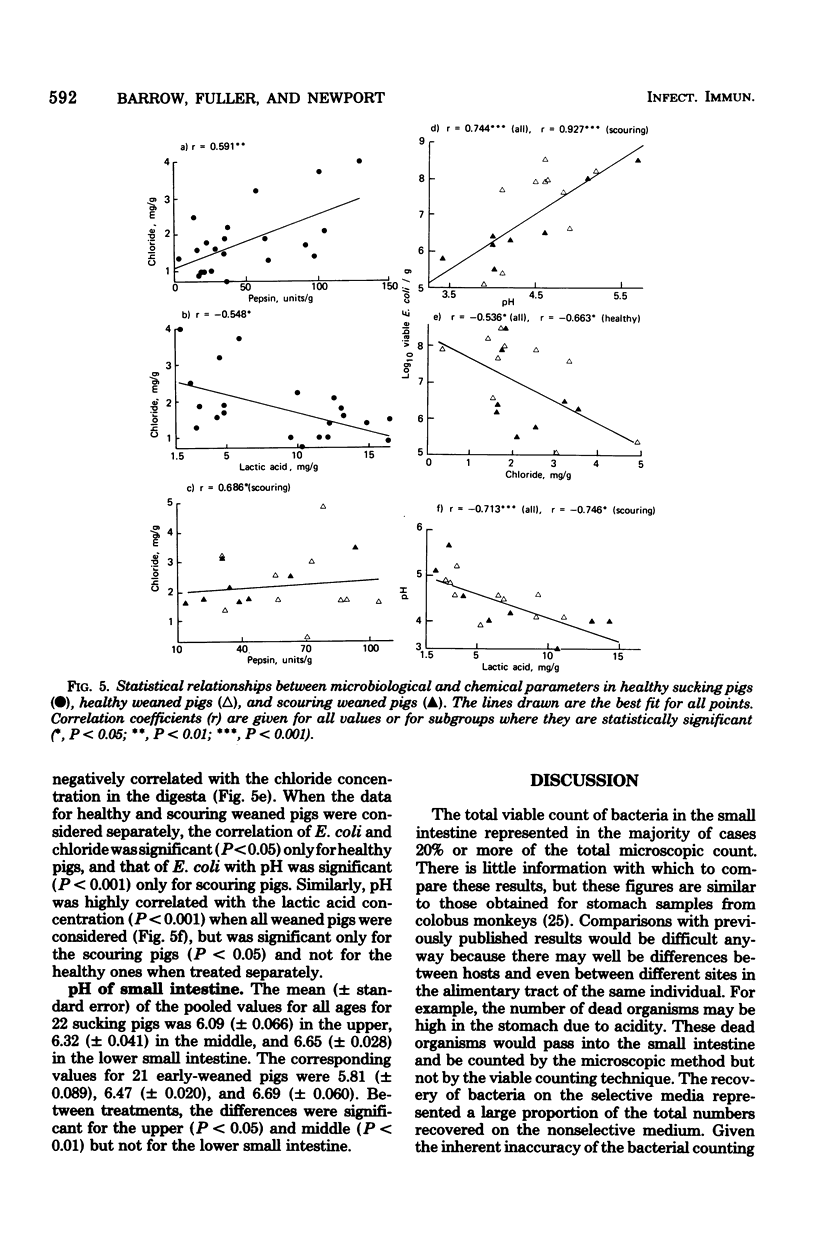
The gastrointestinal microflora and gastric physiology of piglets weaned at 2 days was compared with that of piglets allowed to continue sucking the sow. Although there was a significantly higher count of Escherichia coli in the stomach, duodenum, and jejunum of the early-weaned compared with sow-reared pigs, these differences were not detectable in samples from the ileum. There were no quantitative differences in lactobacilli and in streptococci between the two treatments. Lactobacillus fermentum, L. acidophilus, Streptococcus salivarius, S. bovis, and related biotypes were isolated from both groups of pigs. L. fermentum and S. salivarius were isolated more frequently from sow-reared piglets. The weight of digesta in the stomach was greater in weaned than in sucking pigs and was even greater in scouring weaned pigs, suggesting that in scouring pigs there may be gastric stasis. The gastric pH was higher in the weaned pigs at 4 days of age, but gradually decreased up to 10 days, during which time the lactic acid concentration rose. In weaned pigs there was a highly significant negative correlation between pH and lactic acid concentration in the stomach digesta, and also a positive correlation between pH and number of E. coli. These correlations suggest that lactic acid, from bacterial fermentation, is the major component in the regulation of gastric pH in weaned pigs. Three of twenty sucking pigs, but none of the weaned pigs, were secreting HCl (chloride concentration > 3 mg/g, pH < 3.5). In sucking pigs there was an inverse relationship between the chloride and lactic acid concentrations in the digesta. In weaned scouring pigs there was a nonsignificant increase in pepsin concentration in the stomach tissue. There was a threefold increase in the total proteolytic activity of the stomach tissue.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop R. F., Davidson G. P., Holmes I. H., Ruck B. J. Virus particles in epithelial cells of duodenal mucosa from children with acute non-bacterial gastroenteritis. Lancet. 1973 Dec 8;2(7841):1281–1283. doi: 10.1016/s0140-6736(73)92867-5. [DOI] [PubMed] [Google Scholar]
- Braude R., Mitchell K. G., Newport M. J., Porter J. W. Artificial rearing of pigs. 1. Effect of frequency and level of feeding on performance and digestion of mk proteins. Br J Nutr. 1970 Jun;24(2):501–516. doi: 10.1079/bjn19700049. [DOI] [PubMed] [Google Scholar]
- Braude R., Newport M. J. Artificial rearing of pigs. 4. The replacement of butterfat in a whole-milk diet by either beef tallow, coconut oil or soya-bean oil. Br J Nutr. 1973 May;29(3):447–455. doi: 10.1079/bjn19730120. [DOI] [PubMed] [Google Scholar]
- Braude R., Newport M. J., Porter J. W. Artificial rearing of pigs3. The effect of heat tratment on the nutritive value of spray-dried whole-milk powder for the baby pig. Br J Nutr. 1971 Jan;25(1):113–125. doi: 10.1079/bjn19710069. [DOI] [PubMed] [Google Scholar]
- Collee J. G., Watt B., Fowler E. B., Brown R. An evaluation of the Gaspak system in the culture of anaerobic bacteria. J Appl Bacteriol. 1972 Mar;35(1):71–82. doi: 10.1111/j.1365-2672.1972.tb03675.x. [DOI] [PubMed] [Google Scholar]
- Cranwell P. D., Noakes D. E., Hill K. J. Gastric secretion and fermentation in the suckling pig. Br J Nutr. 1976 Jul;36(1):71–86. doi: 10.1079/bjn19760059. [DOI] [PubMed] [Google Scholar]
- Cranwell P. D., Titchen D. A. Gastric acid secretion in newly born piglets. Res Vet Sci. 1974 Jan;16(1):105–107. [PubMed] [Google Scholar]
- Dolby J. M., Honour P., Valman H. B. Bacteriostasis of Escherichia coli by milk. I. Colonization of breast-fed infants by milk resistant organisms. J Hyg (Lond) 1977 Feb;78(1):85–93. doi: 10.1017/s0022172400055960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt J. H., Rigby J. Effect of various milk feeds on numbers of Escherichia coli and Bidifobacterium in the stools of new-born infants. J Hyg (Lond) 1976 Aug;77(1):129–139. doi: 10.1017/s0022172400055601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayne-Williams D. J. Miniaturized methods for the characterization of bacterial isolates. J Appl Bacteriol. 1975 Jun;38(3):305–309. doi: 10.1111/j.1365-2672.1975.tb00534.x. [DOI] [PubMed] [Google Scholar]
- Jayne-Williams D. J. The application of miniaturized methods for the characterization of various organisms isolated from the animal gut. J Appl Bacteriol. 1976 Apr;40(2):189–200. doi: 10.1111/j.1365-2672.1976.tb04165.x. [DOI] [PubMed] [Google Scholar]
- Kutas F., Szabó J. A study of gastric acid production in pigs in relation to early weaning. Acta Vet Acad Sci Hung. 1974;24(1):133–138. [PubMed] [Google Scholar]
- Owaki K., Hungate R. E., Lotter L., Hofmann R. R., Maloiy G. Stomach fermentation in East African Colobus monkeys in their natural state. Appl Microbiol. 1974 Apr;27(4):713–723. doi: 10.1128/am.27.4.713-723.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGOSA M., MITCHELL J. A., WISEMAN R. F. A selective medium for the isolation and enumeration of oral and fecal lactobacilli. J Bacteriol. 1951 Jul;62(1):132–133. doi: 10.1128/jb.62.1.132-133.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger S. M., Craven J. A., Williams I. Letter: Demonstration of reovirus-like particles in intestinal contents of piglets with diarrhoea. Aust Vet J. 1975 Nov;51(11):536–536. doi: 10.1111/j.1751-0813.1975.tb06917.x. [DOI] [PubMed] [Google Scholar]
- SMITH H. W., JONES J. E. OBSERVATIONS ON THE ALIMENTARY TRACT AND ITS BACTERIAL FLORA IN HEALTHY AND DISEASED PIGS. J Pathol Bacteriol. 1963 Oct;86:387–412. [PubMed] [Google Scholar]
- Watt B., Collee J. G., Brown R. The isolation of strict anaerobes: the use of an anaerobic cabinet compared with a conventional procedure. J Med Microbiol. 1974 Aug;7(3):315–324. doi: 10.1099/00222615-7-3-315. [DOI] [PubMed] [Google Scholar]
- Willis A. T., Bullen C. L., Williams K., Fagg C. G., Bourne A., Vignon M. Breast milk substitute: a bacteriological study. Br Med J. 1973 Oct 13;4(5884):67–72. doi: 10.1136/bmj.4.5884.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woode G. N., Bridger J., Hall G. A., Jones J. M., Jackson G. The isolation of reovirus-like agents (rota-viruses) from acute gastroenteritis of piglets. J Med Microbiol. 1976 May;9(2):203–209. doi: 10.1099/00222615-9-2-203. [DOI] [PubMed] [Google Scholar]


