Abstract
Research suggests that the use and abuse of marijuana can be especially harmful if it occurs during adolescence, a period of vast developmental changes throughout the brain. Due to the localization of cannabinoid receptors within the limbic system and the established effects of cannabinoids on emotional states and anxiety levels of rats and humans, we studied the sex- and dose-related effects of Δ9-tetrahydrocannabinol (THC, the main psychoactive component in marijuana) on behavior and anxiety during spontaneous withdrawal. Male and female Sprague Dawley rats were administered 2, 7.5 or 15 mg/kg THC or vehicle from postnatal day 35–41 (approximating mid-adolescence in humans). Locomotor activity and anxiety-related behaviors were measured during drug administration and abstinence. THC caused significant dose-dependent locomotor depression during drug administration. Locomotor depression initially abated upon drug cessation, but re-emerged by the end of the abstinence period and was greater in female than male rats. We found sensitization to the locomotor-depressing effects of THC in middle- and high-dose rats and the subsequent development of tolerance in high-dose rats. The high dose of THC increased anxiety-like behaviors while the low dose decreased anxiety-like behaviors during drug administration, with females more sensitive to the anxiogenic effects of THC than males. During abstinence, females were again especially sensitive to the anxiogenic effects of THC. This study demonstrates sexually-dimorphic effects of THC on anxiety-related behaviors and locomotor activity during and after THC administration during adolescence. This information may be useful in the development of therapeutic approaches for the treatment of marijuana withdrawal in adolescents.
Keywords: spontaneous withdrawal, tetrahydrocannabinol, sex differences, adolescence, puberty
1. Introduction
Marijuana (cannabis sativa) use and abuse remains a serious problem worldwide [1]. Research concerning the adverse effects of marijuana is especially salient today with ongoing debates over its legalization [2] as well as increasing interest in its therapeutic potential. Δ9-tetrahydrocannabinol (THC), the main psychoactive component in marijuana, is a ligand for pre-synaptically located, G-protein coupled CB1 receptors found primarily in the lungs, liver, kidneys and brain, and CB2 receptors found primarily in T-cells and macrophages [3]. CB1 receptor activation inhibits synaptic vesicle release and the adenylyl cyclase/PKA pathway [4;5;6;7]. By altering synaptic transmission at CB1 receptors located throughout the corticolimbic circuit [8;9;10;11] cannabinoids modulate emotion and anxiety. Exogenous cannabinoids (such as THC) lack the spatial and temporal acuity of endocannabinoids, which are released locally following excitation and then quickly degraded (see [12]). Therefore, acute cannabis use can produce altered emotional states and chronic use may disrupt the normal endocannabinoid regulation of emotion [13;14;15;16;17].
Chronic use can lead to tolerance to many of the behavioral effects of cannabinoids and this has been demonstrated in mice, rats and humans [18;19;20;21]. Following chronic use (or during drug deprivation or abstinence) withdrawal symptoms often develop [22]. However, a cannabis withdrawal syndrome has only recently been recognized [23;24;25] and there is still the common perception that cannabis withdrawal does not occur. Withdrawal in humans can occur within 24–48 hours of drug abstinence, peak within 2–6 days, and continue for 1–2 weeks depending upon the dose and length of exposure [22]. Symptoms include marijuana craving, anger and anxiety, depressed mood, loss of appetite and difficulty sleeping [22;26].
Although numerous studies have examined precipitated cannabis withdrawal by administering a CB1 receptor antagonist [27;28;29;30;31], few cannabis studies have been conducted using spontaneous withdrawal from THC. This may be due to difficulties in measuring robust withdrawal symptoms in a spontaneous withdrawal model [32;33;34], in part because of the long half-life of cannabinoids [35;36;37]. While hyperlocomotion and paw tremors have been identified as two somatic signs of precipitated withdrawal from THC [27; 30], humans do not typically undergo precipitated withdrawal. It may be difficult to extrapolate precipitated withdrawal results to the human population because the actions, pharmacokinetics and pharmacodynamics of the cannabinoid antagonist must be taken into account along with the effects of chronic exposure to the agonist.
Furthermore, the majority of pre-clinical research is conducted in adult male subjects despite research suggesting that there is increased vulnerability in the adolescent and/or female population. Past studies have demonstrated that an earlier age of onset of cannabis use, during mid-adolescence and especially during puberty, is associated with increased impairment including greater psychosis and cognitive and attentional deficits later in life [38;39;40;41]. Increased marijuana use during puberty may be especially damaging because the endogenous cannabinoid system is undergoing an array of developmental changes during adolescence that might make the brain vulnerable to developmental disruption by THC [42;43;44]. Additionally, there have been sex differences reported in cannabis research that warrant further investigation (see [45]). For example, sex differences have been reported in the pharmacokinetics and pharmacodynamics of the drug and in its cognitive and behavioral effects; yet data on the sexually dimorphic effects of cannabinoids on anxiety and emotions during withdrawal is generally lacking (except see Rubino and Parolaro [46] for review).
In the present study, we address these issues by examining the sexually dimorphic effects of a low, middle or high dose of THC, the primary psychoactive component of marijuana as consumed by adolescents, on behavior and anxiety during drug administration and during spontaneous withdrawal during the periadolescent period.
2. Materials and Methods
2.1.1 Subjects
Experiment 1 and 2 subjects were male and female Sprague Dawley rats (Charles River, Wilmington, MA) housed in a 12-hour reverse light/dark cycle (lights out at 11am) with ad libitum access to food and water. Pups arrived in natural litters on postnatal day (PND) 14–15, were weaned on PND 21 and then housed in same-sex pairs. For each litter, THC was randomly assigned such that a portion of the rats received one of three doses of THC, or vehicle. Approximately 1/3-1/2 of the males from each litter and 1/3-1/2 of the females from each litter were assigned to the control group; the remaining rats were split between two of the three doses of THC. Behavioral testing occurred during the drug administration period and within 2 weeks after the last administration of THC, referred to as the drug abstinence period. Body weights were measured daily during drug administration before IP injection, and before all behavioral testing.
2.1.2 Treatment
Rats were dosed once daily via IP injection with either a low (2 mg/kg), middle (7.5 mg/kg), or a high (15 mg/kg) dose of Δ9-THC (Research Triangle Park, North Carolina) in pluronic acid (Sigma-Aldrich, Inc., St. Louis, MO) /saline, or vehicle from PND 35–41, a period approximating mid-adolescence in humans. The low dose of THC (2 mg/kg) was selected for use because this dose had previously been shown to produce effects on locomotor activity [47], but does not produce catalepsy, as is demonstrated by Wiley et al, [48]. Additionally, previous research conducted in our laboratory (see supplemental material) indicated peak plasma levels after a chronic dose of 2 mg/kg THC during adolescence to be 50–100 ng/mL in females, and 40–60 ng/mL in males. Alternatively, human THC levels peak at about 140 ng/mL after inhalation of what is a relatively low dose of 34 mg marijuana; heavy chronic users may use several hundred mg per day (see [36]). Therefore, we can conclude that our dose of 2 mg/kg is indeed a low dose. The high dose of THC (15 mg/kg) was selected for use based upon the work of Wiley et al. [48] providing indication that this dose would produce effects different from those seen at 2 and 7.5 mg/kg THC, allowing us to successfully characterize a doseresponse curve. Additionally, spontaneous withdrawal from 15 mg/kg THC (administered twice daily for 6.5 days) was sufficient to cause alterations in neuronal firing rate similar to those found during precipitated withdrawal [27]. For the EPM study (Exp 2), the middle dose was excluded because we saw the greatest effects at the low and high doses and wanted to minimize animal usage.
2.1.3 Experiment 1: Locomotor Activity and Time in the Center of the Accuscan Box
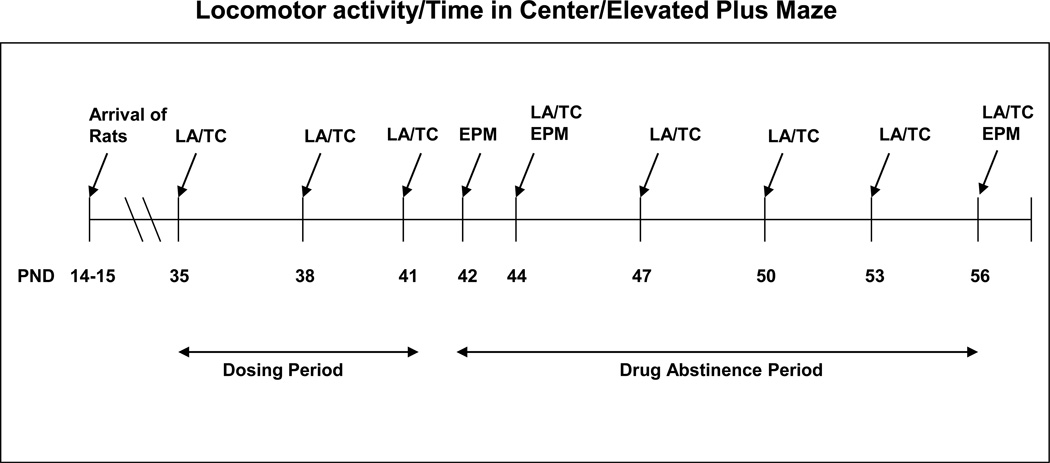
Locomotor activity was assessed using Accuscan equipment consisting of clear Plexiglas boxes (42 × 42 × 30 cm with no bedding) placed inside Versamax activity monitors (VMRXYZ16; Accuscan Instruments, Columbus, OH) that recorded the interruption of photobeams. Rats were allowed to acclimate to the testing room for at least 1 hour before the start of the trial. Both the testing room and Accuscan chambers were kept in near-darkness, with only red light illuminating the room. Animals were placed into the chambers immediately after injection with 2, 7.5 or 15 mg/kg THC or vehicle and movement was recorded for a total of one hour. Chambers were cleaned with a 30% ethanol solution after each trial. Total distance traveled in centimeters was calculated as a measure of locomotor activity, and time in the center of the box (greater than 2.5 cm from the wall) in seconds was calculated as a correlate for anxiety level. Percentage of total time spent in the center of the box was calculated with the following formula: (total time spent in the center of the box/total time spent in the box) × 100. In rats, it has been shown that spending more time in the center of an open field is a behavior indicative of less anxiety (see [49]); therefore we used time spent in the center of the Accuscan box as an indicator of level of anxiety. Behaviors were assessed on days 35, 38 and 41 during the drug administration period and on days 42, 44, 47, 50, 53 and 56 during drug abstinence (Figure 1). Rats originated from 9 litters. Approximately 10 rats per litter were used, for a total of 88 rats (two rats were omitted from the study). 10 male and 10 female rats were given 2 mg/kg THC, 10 male and 10 female rats were given 7.5 mg/kg THC, 14 male and 14 female rats were given 15 mg/kg THC and 10 male and 10 female rats were given the vehicle. Rats were re-tested on each testing day of the drug administration/drug abstinence period in the same Accuscan recording chamber.
Figure 1.
Timeline of Experiments. LA/TC, locomotor activity/time in the center of the Accuscan box. EPM, elevated plus maze.
2.1.4 Experiment 2: Elevated Plus Maze
Elevated plus maze (EPM) equipment consisted of a black Plexiglas plus-shaped maze, elevated 55.9 cm from the floor. The maze had 2 closed arms and 2 open arms, each 50.8 cm long. The closed arms were enclosed on three sides by black Plexiglas walls 43.2 cm high, while the open arms were partially enclosed for 22.9 cm with a 6.4 cm high rail, and then continued without a rail for the remaining 27.9 cm. This design allows for analysis of time spent beyond the rail of the open arm vs. enclosed only by the short rail (combined in the present study due to lack of statistically significant difference), and has previously used by Guilinello and Smith [50] and Guilinello et al. [51]. At the intersection of the open and closed arms was the ‘center’ of the maze, a square 10.2 cm by 10.2 cm. Each arm of the maze was delineated by 3 grid lines. The test was conducted in near-darkness, with only red light illuminating the room.
Rats were allowed to acclimate to the testing room for at least 1 hour prior to the start of the test, and were kept in home cages until 10 seconds before the start of the trial session when they were placed in the center of the maze facing away from the experimenter. Each trial lasted for a total of 5 minutes. Entrance into an arm of the maze was counted if the rat crossed the grid line into the arm with all 4 legs, while entrance out of an arm was counted if the rat crossed the grid line out of the arm with 2 legs. The maze was cleaned between trials with a 30% ethanol solution. Rats were tested once on the EPM either on PND 42, 44 or on 56 (Figure 1).
Percentage of time spent in the open arm of the maze, the total number of head dips (unprotected + protected head dips) and the number of stretch-attend postures were calculated as correlates of anxiety level. An unprotected head dip occurred when the rat dipped its head towards the ground over the un-railed portion of the open arm, whereas a protected head dip occurred when the rat dipped its head over the side of the railed portion of the open arm. A stretch-attend posture occurred when the rats’ hind legs were motionless but the rat stretched its body forward. Head dipping, an exploratory open arm behavior suggests less anxiety, while a stretch-attend posture, a risk –assessment behavior, suggests the animal is more anxious [52;53]. The number of grid crossings was calculated as a measure of locomotor activity. Additionally, each session was recorded on videotape for later analysis of somatic signs of withdrawal such as wet dog shakes and forepaw tremors. Rats originated from 29 litters (each with 10 pups), with a total of 81 male and 86 female rats assessed for plus maze behaviors. 26 female and 22 male rats were given the vehicle, 27 female and 23 male rats were given 2 mg/kg THC, and 27 female and 26 male rats were given 15 mg/kg THC and included in the analysis.
All EPM behavioral testing took place between the hours of 12 and 3:30pm in a darkened room, in order to control for time of day effects reported for HPA axis responses on stress [54] and maximize time on the open arm for all groups. Estrous cycle was determined via vaginal cytology post-experiment.
3. Statistical Analysis
Data were analyzed by SAS Statistical Software, v. 9.2 (SAS Institute Inc., Cary, NC) and Systat Statistical Software, v. 13. Since this was a split litter design, litter is the statistical unit. For all tests, a significance level of p<0.05 was used; p<0.1 was considered a trend towards significance.
3.1.1 Total Distance Traveled Analysis
A mixed linear model was constructed with distance traveled as the dependent variable (data were power-transformed to stabilize variance). Two separate analyses were done: one for PND 35, 38, and 41; one for PND 42, 44, 47, 50, 53, and 56. Fixed factors were day, sex, dose and their mutual interactions. Litter was a random factor. A heterogeneous compound symmetry structure (allowing for unequal variance amongst samples) was used to model intra-subject correlation and heteroskedasticity (occurring when variables have unequal variances) across observation days. Due to unequal variances and missing data, Satterthwaite corrections to denominator degrees of freedom were applied; however, as data were essentially balanced, corrections were minimal. Model residuals were inspected for skew and for outliers.
3.1.2 Time in the Center of the Box and Elevated Plus Maze Analysis
For all other experiments missing data were minimal; therefore, an analysis of variance (ANOVA) was used followed by post-hoc Dunnett’s tests to compare the behavior of THC-treated rats to controls. Post-hoc t-tests were also conduced where appropriate, to compare male and female rats to each other and across age. Dependent variables were the individual behaviors being assessed. Fixed factors were day of testing, sex, dose and their mutual interactions. Litter was a random factor. Model residuals were inspected for skew and for outliers.
4. Results
4.1.1 Experiment 1a: Locomotor Activity
Total distance traveled in the Accuscan box differed significantly in a dose-dependent manner during the drug administration period and in a sex- and dose-dependent manner during the drug abstinence period. Locomotor activity was assessed in 12 5-minute time blocks and combined to determine the mean locomotor activity across the hour for each subject.
4.1.2 Locomotor Activity During the Drug Administration Period
Locomotor activity was depressed in a dose-dependent manner by THC, and remained depressed in both male and female THC-treated rats compared to controls across the entire hour of testing (data not shown).
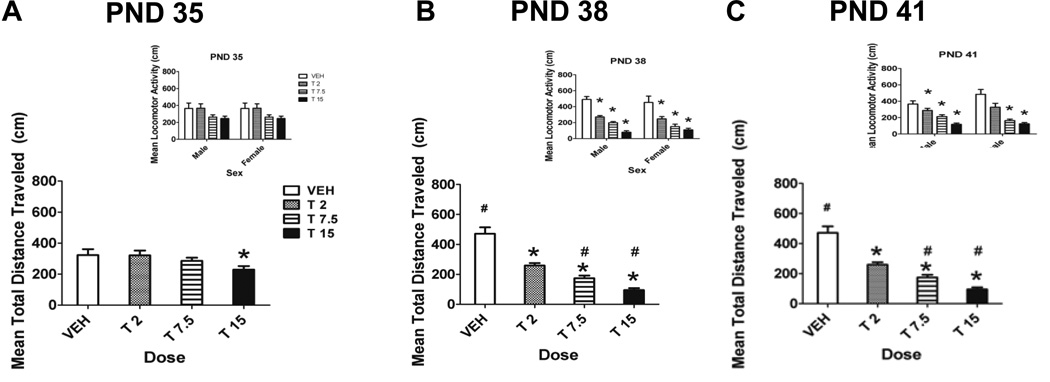
Figure 2 shows the mean total distance traveled for the testing hour collapsed across sex during drug administration. ANOVA revealed a significant main effect of dose (F[3,78]=45.96, p<0.001) and test day (F[2,120]=8.61, p<0.001). There was also a significant dose by day interaction (F[6,135]=10.32, p<0.001). There were no significant litter effects (Z=0, p=1.000). That is, litter did not significantly contribute to the variance of the dependent measure. There was no significant main effect of sex nor any significant sex by dose interactions. On the first day of drug administration, PND 35, only the high dose of 15 mg/kg caused significant locomotor depression compared to controls. However, on days 38 and 41, all three doses of THC caused significant locomotor depression compared to the control.
Figure 2.
Mean locomotor activity during drug administration. Total distance traveled (cm) for 1 hour collapsed across sex on PND 35 (A), PND 38 (B) and PND 41 (C) during the drug administration period. There were significant main effects of dose and test day (p<0.05). Insets illustrate the data by sexes.* Denotes significant difference from control and # denotes significant difference from same dose group on the previous testing day, p<0.05. n = 9–14/group
We found evidence of the development of sensitization to the locomotor-depressing effects of THC in mid- and high-dose rats. Control rat locomotor activity increased significantly between PND 35 and PND 38 (Fig. 2, #). In contrast, rats dosed with 7.5 mg/kg and 15 mg/kg THC showed a significant decrease in locomotor activity between PND 35 and 38 (Fig. 2, #). This suggests that there was sensitization to the locomotor-depressing effects of THC in mid- and high-dose rats between PND 35 and 38. There was also evidence of the subsequent development of tolerance to the locomotor-depressing effects of THC in rats dosed with 15 mg/kg. While THC initially caused a significant decrease in locomotor activity in high-dose rats from PND 35 to 38, locomotor activity of high-dose rats increased significantly from PND 38 to 41 (Fig. 2, #), while the locomotor activity of control rats did not significantly change. Therefore, although the locomotor activity levels of high-dose rats did not return to control levels, there is evidence of the development of tolerance in these rats.
4.1.3 Locomotor Activity during the Drug Abstinence Period
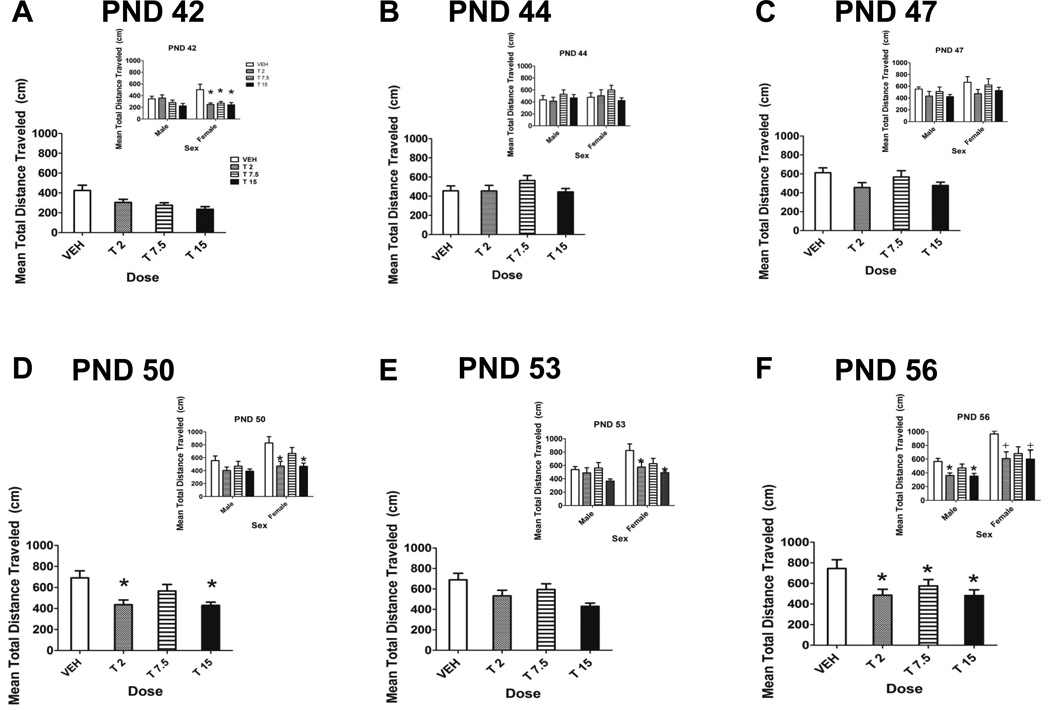
Within each testing session, the locomotor activity of all rats decreased (data not shown). However, the locomotor activity of THC-treated rats tended to stay depressed across the entire testing hour compared to controls. There was a sexually-dimorphic pattern of THC-induced locomotor depression (Fig. 3 and insets). Analysis of variance revealed a significant main effect of day (F[5,208]=31.96, p<0.001), a significant sex by day interaction (F[5,207]=4.82, p<0.001) and dose by day interaction (F[15,274]=3.39, p<0.001). There was also a significant main effect of sex (F[1,70]=8.31, p=0.005). There were no significant litter effects (Z=0.64, p=0.261).
Figure 3.
Mean locomotor activity during drug abstinence. Total distance traveled (cm) for 1 hour collapsed across sex on PND 42 (A), 44 (B), 47 (C), 50 (D), 53 (E) and 56 (F). There was a main effect of dose on PND 50 and 56 (p<0.05). Insets depict locomotor activity of male and female rats on PND 42 (A), 44 (B), 47 (C), 50 (D), 53 (E) and 56 (F). * denotes significant difference from control (Dunnett’s), p<0.05. + denotes a trend towards a significant difference from control, 0.05<p<0.1. n = 9–14/group.
Post-hoc Dunnett’s tests (used to compare THC-treated rats to controls) indicated significant locomotor depression in female THC-treated rats more frequently during the drug abstinence period than in male THC-treated rats. For example, on PND 42, the first day of drug abstinence, significant locomotor depression was seen in all three THC dose groups of female rats, while there was no significant locomotor depression seen in male THC-treated rats. On PND 50 and 53, locomotor depression was seen in both the low and high dose groups of female rats; there was no significant THC-induced locomotor depression in male rats on these days. However, on PND 56 both low-and high-dose groups of male rats showed significantly reduced locomotor activity compared to controls, while female low- and high-dose rats only showed a trend towards significant locomotor depression. Collapsed across sex, there were significant differences among dose groups on PND 50 and 56 (p=0.048 and p=0.018). On PND 50, significant locomotor depression was seen in the 2 mg/kg and 15 mg/kg THC groups compared to the control, while on PND 56 all three doses of THC caused significant locomotor depression compared to the control. Activity in the control group gradually increased from PND 42 to 56.
4.2.1 Experiment 1b: Time in the Center of the Accuscan Box
Overall, for time spent in the center of the box during drug administration and drug abstinence combined, there was no significant litter effect (Z=1.26, p=0.104).
4.2.2 Time in the Center of the Box during the Drug Administration Period
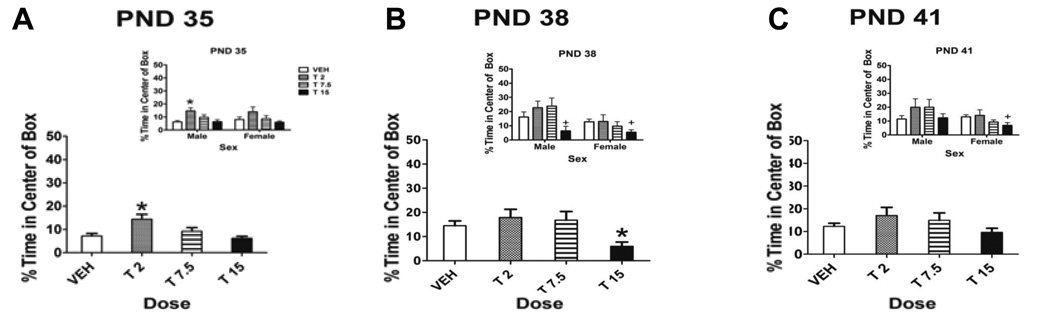
Data were analyzed as the percentage of time spent in the center of the Accuscan box (Fig. 4). A two-way ANOVA revealed a significant main effect of test day (F[2,82]=7.559, p=0.001) and a significant sex by test day interaction (F[2,82]=4.410, p=0.014). There was a significant main effect of dose on PND 35 (F[3,82]=6.570, p=0.001) and a significant main effect of dose (F[3,84]=5.019, p=0.003) and sex on PND 38 (F[1,86]=7.271, p=0.009) and 41 (F[1,85]=3.976, p=0.050).
Figure 4.
Percentage of time spent in the center of the Accuscan box. Data are for male and female rats (insets) and collapsed across sex on PND 35 (A), PND 38 (B) and PND 41 (C) during the dosing period. There was a significant main effect of dose on PND 35, a significant main effect of dose and sex on PND 38 and a significant main effect of sex on PND 41 (p<0.05). Insets depict percentage of time spent in the center of the box in male and female rats on PND 35 (A), PND 38 (B) and PND 41 (C). Note that the maximum of the y-axis is 50%. * denotes significant difference from controls, p<0.05. + indicates trend towards significant difference from control, 0.05< p<0.10. n = 9–14/group
Post-hoc Dunnett’s tests revealed that on PND 35, rats administered the low dose of 2 mg/kg THC spent significantly more time in the center of the box than control rats (p=0.002), indicating an anxiolytic-like effect of THC. Conversely, on PND 38, rats administered the high dose of 15 mg/kg spent significantly less time in the center of the box than control rats (p=0.014), indicating an anxiogenic-like effect of THC.
Post-hoc Dunnett’s tests revealed that the effects of THC during drug administration on anxiety-like behaviors were sexually dimorphic (Fig. 4 insets). On PND 35, low-dose male rats spent significantly more time in the center of the box than controls (p<0.05), with no significant differences in female rats. In contrast, although on PND 38 both male and female high dose rats showed a trend towards spending less time in the center of the Accuscan box than controls, on PND 41, only female high-dose rats showed a trend towards spending less time in the center of the box than controls (p<0.1). These results indicate that females were especially sensitive to the anxiogenic properties of THC (on PND 41) while males were more sensitive to the anxiolytic properties (on PND 35).
4.2.3 Time in the Center of the Box during the Drug Abstinence Period
There were no significant main effects of dose from PND 42 through PND 53 of the drug abstinence period (data not shown). A two-way ANOVA revealed a significant main effect of test day (F[5,58]=4.772, p<0.001), and a significant day by dose interaction (F[15,48]=1.785, p=0.036). The percentage of time spent in the center of the box by male and female control rats during drug abstinence increased overall with age, consistent with increased exploration in rats with age [50].
However, Dunnett’s tests indicated that on PND 56 (collapsed across sex), rats that had been administered the 15 mg/kg high dose spent significantly less time in the center of the box than controls (p=0.033), suggesting that this dose had long-term effects on anxiety-like behavior in males and females (data not shown).
4.3 Experiment 2A: Elevated Plus Maze Behaviors (Drug Abstinence Period)
4.3.1 Percentage of Time Spent in the Open Arm
Figure 5 depicts the time spent in the open arm of the EPM across the three testing days during drug abstinence. Results were analyzed according to percentage of time spent in the open arm of the EPM. There was no significant difference between time spent within the short rail of the open arm and time spent beyond the rail, therefore these were combined during analysis of time spent in the open arm. A two-way ANOVA revealed a significant main effect of sex (F[1,150]=11.099, p=0.001), a significant sex by dose interaction (F[2, 149]=4.505, p=0.013) and a significant sex by dose by day interaction (F[4, 148]=2.555, p=0.042).
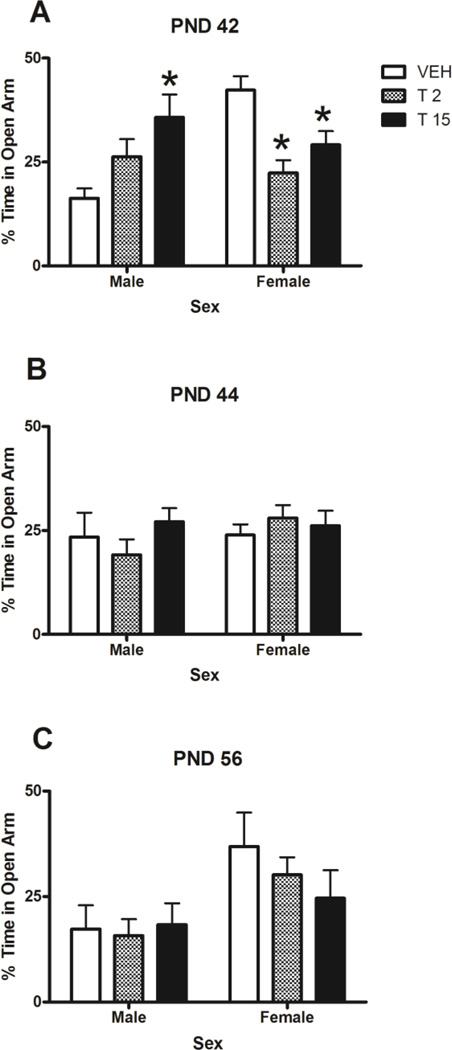
Figure 5.
Percentage of time spent in the open arm of the EPM during drug abstinence on PND 42, 44 and 56. On PND 42, there was a significant sex by dose interaction (p<0.05). Note that the scale is out of 50%. * denotes significant difference from same-sex control and # denotes significant difference from male control (p<0.05). n = 7–9/group.
Post-hoc Dunnett’s tests showed that on PND 42, both female low- and high-dose groups spent significantly less time in the open arm than female controls (p=0.001 and p=0.017, respectively), suggesting an anxiogenic effect of these doses in females. In contrast to this, male high-dose rats spent significantly more time in the open arm than male controls (p=0.007) on PND 42, suggesting an anxiolytic effect of this dose in males. On both PND 44 and 56 there were no significant differences between male controls and male THC rats, nor female controls and female THC groups. However, in general, females spent more time on the open arm than males.
In order to determine if time spent in the open arm was correlated with stage of estrous cycle, a Pearson’s correlation coefficient was calculated. Overall, no significant correlation between estrous cycle and percent time spent in the open arm was found. (Data not shown)
4.3.2 Total Number of Grid Crossings - Locomotor Activity
We found significant dose- and sex-dependent alterations in the number of grid crossings, a measure of locomotor activity on the EPM during withdrawal. On PND 42 (Fig. 6), a two-way ANOVA revealed a significant main effect of dose (F[2, 44]=5.393, p=0.008). Collapsed across sex, both the low and high dose of THC significantly decreased the number of grid crossings compared to the control. Post-hoc Dunnett’s tests indicated that this effect was driven by female treated rats making significantly fewer grid crossings than female controls on PND 42 (Fig. 6A inset). On PND 44 and PND 56 there were no significant differences found in the number of grid crossings between male and female THC-treated rats and controls.
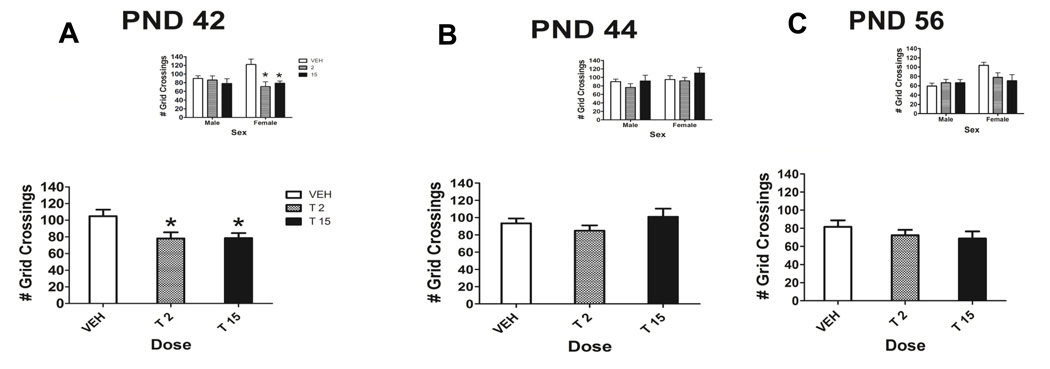
Figure 6.
Total number of grid crossings during drug abstinence collapsed across sex on PND 42 (A), PND 44 (B) and PND 56 (C), the spontaneous withdrawal period. On PND 42, there was a main effect of dose, (p<0.05). Insets depict total number of grid crossings on PND 42, 44 and 56 for male and female rats. * denotes significant difference from control, p<0.05. n = 7–9/group.
A Pearson’s correlation coefficient was computed to determine if the decrease in locomotor activity (number of grid crossings) contributed to the decrease in open arm time in female THC-treated rats on PND 42. No significant correlation was found between the number of grid crossings made by female treated rats and the time spent on the open arm by female treated rats, suggesting that the decrease in open arm time was not simply due to decreased locomotor activity.
4.3.3 Total Number of Head Dips and Stretches
The total number of head dips suggested that THC had anxiolytic-like effects in males, and anxiogenic-like effects in females (Fig. 7). ANOVA indicated that there was a significant sex by dose by day interaction (F[4,138]=7.525, p<0.001). Female control rats made more head dips than male control rats on all three testing days (significant only on PND 42, p<0.01).
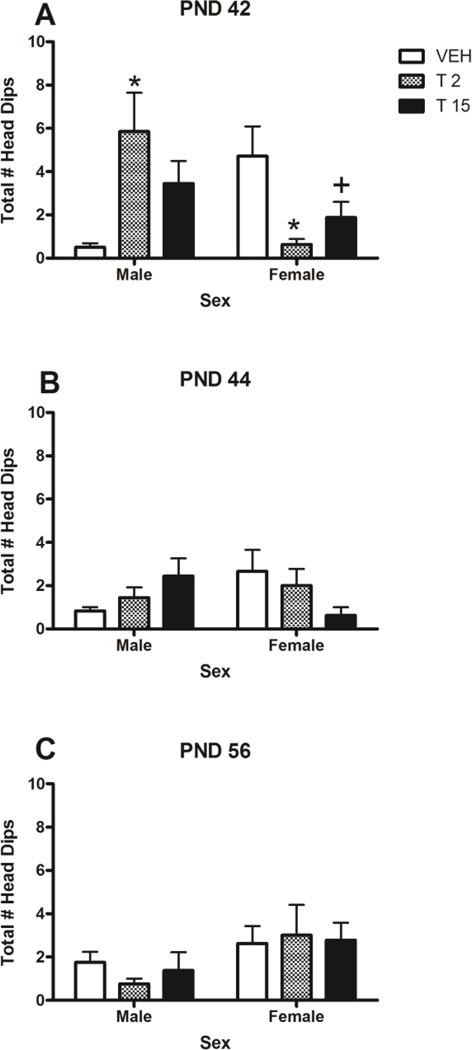
Figure 7.
Total number of head dips for male and female rats on the elevated plus maze on PND 42 (A), PND 44 (B) and PND 56 (C). There was a significant sex by dose by day interaction on PND 42 (p<0.05). * denotes significant difference from controls, p<0.05. + denotes trend towards a significant difference from controls, p<0.10. n = 7–9/group.
The only significant THC-induced alteration in the number of head dips was found on PND 42. Post-hoc Dunnett’s tests revealed that female low-dose rats made significantly fewer head dips than female controls (p=0.006), and that female high-dose rats showed a trend towards making significantly fewer head dips than female controls (p=0.056), an indication of anxiogenic effects of THC in the females. Conversely, male low-dose rats made significantly more head dips than male controls (p=0.008), an anxiolytic-like effect. No dose-related effects were found on PND 44 or 56.
The only significant dose-related difference in the number of stretch-attend postures was found on PND 44, where a two-way ANOVA revealed a significant main effect of dose (F[2,51]=3.577, p=0.036) as well as a significant dose by sex interaction (F[2,51]=4.119, p=0.022). Post-hoc Dunnett’s tests revealed that female low-dose rats made a greater number of stretch-attend postures than female controls (Data Not Shown).
5. Discussion
In the present study, we looked at the effects of low, middle and high doses of THC on locomotor activity and anxiety-related behaviors during drug administration and during a subsequent drug abstinence period. Our main goal was to determine the time-course of spontaneous withdrawal and to examine sex- and dose-related differences within the drug abstinence period. Results indicate that spontaneous withdrawal is substantially different from precipitated withdrawal and that measures of anxiety-related behavior support a physiological withdrawal that is not manifested in hyperlocomotion. Withdrawal is not the same in males and females since it is associated with sexually dimorphic changes in anxiety-related behaviors.
5.1.1 Locomotor Activity during Drug Administration and Withdrawal
The Accuscan recording chamber is an open field environment enabling free exploration. In general, the locomotor activity of control rats increased with age across the drug administration period, a reflection of increased exposure to the testing environment as well as increased exploration that occurs in rats during adolescence [55]. Based upon previous results examining the effects of chronic 2 mg/kg THC on locomotor activity in early and late adolescence (PND 21–40 and 41–60; [44]), we hypothesized that THC administration would significantly reduce locomotor activity in both male and female rats. All three doses of THC caused significant dose-dependent locomotor depression during drug administration. Acute THC-induced locomotor depression has also been widely reported elsewhere using a variety of different doses of THC [56;57]. However, while the vast majority of studies report locomotor depression after cannabinoid administration, increased locomotor activity after cannabinoid administration has also been previously reported, often at low doses of THC [58]. Such hyperlocomotion may be caused by differences in experimental procedure such as species of animal used, prior handling and type of locomotor activity box in addition to differences in age of animal at testing.
The largest alterations in locomotor activity during drug abstinence were seen in female THC-treated rats. In both experiment 1 and experiment 2, female rats that had been treated with THC showed greater locomotor depression than male THC-treated rats. Few studies have examined the sex differences of chronic THC exposure on locomotor activity during a withdrawal period, especially during adolescence. However, while intracerebroventricular THC decreased locomotion in both male and female rats, only male rats showed hyperlocomotion at 4 hours following the injection [59], providing evidence that THC may affect locomotor activity differently in male and female rats. Wiley et al. [48] also found that both male and female rats showed significant locomotor depression 24 hours following 10 days of chronic exposure to THC. Differences in age at drug administration/testing may account for these differential effects. The averseness of the testing paradigm may also account for differences in locomotor activity between testing paradigms, as bright light (highly aversive) has been demonstrated to impact locomotor activity in CB1 receptor knockout mice [60;61;62]. In the present study, nonaversive low lighting conditions were used; locomotor activity may have been different with greater test-paradigm averseness. Importantly, we found neither signs of hyperlocomotion nor any significant head shaking, paw tremors or wet-dog shakes as is commonly seen in studies of precipitated withdrawal [27;63]. Possibly, the use of a greater dose, a more potent CB1 receptor agonist, a longer dosing period or a different level of test averseness may have produced these somatic signs of withdrawal.
The endocannabinoid system plays a crucial role in regulating motor control due to the large number of cannabinoid receptors located throughout the basal ganglia. The basal ganglia integrate sensory and decision-making information, and are involved in selecting, filtering, and initiating motor programs such as those involving locomotion [64]. Information from the cortex is first relayed to the dorsal striatum, which either projects directly to the substantia nigra or internal globus pallidus (the direct pathway), or projects to the external globus pallidus, through the subthalamic nucleus, and then to the substantia nigra (the indirect pathway). The substantia nigra then sends inhibitory projections to the thalamus. CB1 receptors are located on glutamatergic cortical projection neurons synapsing on striatal medium spiny neurons [65;66] and on the presynaptic terminals of GABAergic striatal medium spiny neurons synapsing in the internal globus pallidus and the substantia nigra [67;68;69;70]. Increased excitation of the indirect pathway is primarily responsible for decreasing movement, while increased excitation of the direct pathway increases movement. However, CB1 receptor activation in these pathways has the opposite effect; in the indirect pathway, CB1 receptor activation can increase locomotor activity, while in the direct pathway CB1 receptor activation can decrease locomotor activity [71].
Endocannabinoids are synthesized in the basal ganglia upon depolarization of post-synaptic neurons, and act through retrograde signaling to inhibit both excitatory and inhibitory neurotransmission [72;73;74]. It is the balance of cannabinoid signaling at excitatory and inhibitory synapses in both the direct and indirect pathways that determines whether or not locomotor activity will be increased or decreased; activation of CB1 receptors in the indirect pathway can cause increased locomotor activity, while activation of CB1 receptors in the direct pathway can inhibit locomotor activity [71]. As a result, the administration of exogenous cannabinoids such as THC can heavily impact motor functioning and locomotion, as seen in the present study. Interestingly, the greatest effects on locomotor activity were seen with the low and high dose of THC, but not the middle dose. It is possible that the low dose of THC may have been sufficient to activate CB1 receptors in the indirect pathway, while the high dose interacted with CB1 receptors in the direct pathway; the middle dose may have struck a balance between the two, resulting in no significant change in locomotor activity.
No significant sex differences were found in the effects of THC on locomotor activity during the dosing period. As demonstrated by previous results [47], the locomotor system may be equally sensitive during THC administration in males and females at this age. In contrast, the sexually differential effects of THC during drug abstinence may be caused by greater CB1 receptor desensitization and down-regulation in the striatum and PFC of adolescent female rats compared to male rats after chronic THC administration [75;76], causing a potential decrease in CB1 receptor activation by endocannabinoids in female rats during drug abstinence. Further research must be conducted to determine the specific subpopulations of neurons in these areas that are potentially down-regulated.
Locomotor depression re-emerged in experiment 1 in treated rats by PND 50, and was also present on PND 56. On the other hand, there were no long term effects on grid crossings in experiment 2 in either sex. The long-term locomotor depression seen in male and female rats in Experiment 1 was an unexpected result. Pilot data from our own laboratory showed no evidence of long-term locomotor depression in rats administered 2 mg/kg THC from PND 41–60 (mid-late adolescence), and then tested in adulthood at approximately PND 75 (unpublished results). However, the difference in age of administration and length of dosing interval are most likely the cause of this discrepancy. Similarly, Amal et al. [77] and Rubino et al. [75] found no evidence of locomotor depression 3–4 weeks after the last dose of THC in adult male and female rats. Since behavioral assessment in the present study was not continued past PND 56, we do not know if the locomotor depression was an isolated finding or the beginning of a trend. It may be that treated rats habituated to the activity chamber following repeated exposure more so than did the controls. Importantly, we found no effect of litter origin on locomotor activity, a factor that has previously been reported to alter behavior [78].
5.1.2 Tolerance and Sensitization during Drug Administration
We found the development of tolerance to the locomotor-depressing effects of THC in Experiment 1 rats administered the high dose of 15 mg/kg THC (males and females combined) during the latter part of the dosing period. No tolerance was seen with the low and middle doses of THC. Tolerance has previously been demonstrated in both pre-clinical and clinical settings to many of the behavioral effects of cannabinoids [19;79;80], and may be produced through receptor desensitization, internalization and down-regulation [81;82;83;84]. The low and middle doses of 2 and 7.5 mg/kg THC may not have been adequate to produce maximum CB1 receptor downregulation or internalization.
In contrast to the tolerance at high doses, we found sensitization to the locomotor-depressing effects of THC in rats administered the 7.5 mg/kg middle dose and, initially, in high-dose rats. Sensitization describes an increase in drug effect with repeated administration, and may be an important initial step in addiction [85]. Sensitization to THC has been previously demonstrated in rodents [86;87] and may be attributed in part to increased dopamine levels and dendritic arborization in the nucleus accumbens shell and core [87;88]. Sensitization may also be the result of the accumulation of THC in the brain. Due to the long half-life of THC [35], increased levels of THC or its metabolites may be present by the third day of dosing in this study (PND 38), leading to increased effects on locomotor activity.
5.1.3 Anxiety-Related Measures during Drug Administration and Withdrawal
Results from experiment 1 indicate that the low dose of 2 mg/kg THC decreased anxiety-like behaviors while the high dose of 15 mg/kg THC increased anxiety-like behaviors as assessed by center time in the Accuscan box during drug administration. These results are consistent with previous studies reporting that lower doses of THC are anxiolytic while higher doses are anxiogenic [89;90]. In humans, low doses of THC also decrease anxiety and increase ratings of ‘high’ and ‘euphoria’, while high doses produce greater anxiety, irritability and paranoia [13;14;91]. Additionally, during withdrawal, these effects were sexually differential; THC exposure increased anxiety-like behavior in females and decreased anxiety-like behavior in males on the EPM.
Interestingly, female control rats spent significantly more time in the open arm than male controls on PND 42. Female control rats also made significantly more head dips than male control rats on all three testing days. Increased time spent in the open arm and the greater number of head dips in control females most likely reflects both the increase in exploration and risk-taking with age and the earlier onset of puberty in females [55].
We examined the possibility that the changes in time spent on the open arm of the EPM were simply due to decreases in locomotor activity (the number of grid crossings). We found no significant correlation between open arm time and the number of grid crossings for female low- and high-dose rats on PND 42. Therefore, the decrease in open arm time in female THC-treated rats on PND 42 was not likely due to decreased locomotor activity.
The endocannabinoid system is essential in the regulation of stress [92;93;94] and the localization of CB1 receptors throughout the corticolimbic circuit can account for the paradoxical effects of THC administration on anxiety and emotion seen in the present study. CB1 receptors are located on both glutamatergic and GABAergic neurons within the amygdala, and activation of CB1 receptors has been shown to decrease both excitatory and inhibitory neurotransmission [95]. CB1 receptors are also located in the PFC on both GABAergic and glutamatergic cells (see [96]) and are found throughout the hippocampus [97], primarily on GABAergic synapses [98]. The PFC is important in executive control of emotional behavior, while the hippocampus is important in the consolidation and retrieval of memories with an emotional weight [3]. The amygdala makes reciprocal connections with both the PFC and the hippocampus, and it is the balance of CB1 receptor-mediated excitatory and inhibitory transmission that maintains normal levels of emotion/anxiety [91;94]. The increase and decrease in anxiety-like behaviors seen in this study during drug administration might be produced by differential corticolimbic CB1 receptor activation by THC. Furthermore, alterations in endocannabinoid levels after chronic cannabinoid administration [75;99] may account for changes in anxiety-related behaviors seen during drug abstinence. Cannabinoid withdrawal has also been associated with increased levels of corticotropin-releasing hormone in the amygdala [100;101], which is likely associated with changes in anxiety.
A key finding of the present study is that THC exposure increased anxiety-like behavior in females and decreased anxiety-like behavior in males early in withdrawal (on the EPM). CB1 receptor levels in the limbic system are modulated by estrogen in a sexually-dimorphic manner. Riebe et al. [102] found that CB1 receptor binding site density was reduced in the hypothalamus and increased in the amygdala of normal adult female rats and ovariectomized females receiving estradiol compared to ovariectomized female rats and male rats. Estrogens in female rats in the present study may have altered the density and distribution of CB1 receptors in the amygdala and hypothalamus, thereby altering CB1 receptor binding by THC during drug administration and by endocannabinoids during withdrawal. Further research concerning the exact population of neurons in which CB1 receptors are affected by estrogen must be conducted, for example by use of mice that are CB1 receptor-deficient in certain neuronal populations (for example, principal cells, GABAergic cells or dopamine D1-expressing neurons; as has been investigated by Monory et al. [103]). It has also been shown that the stage of estrous cycle can impact the endocannabinoid system [104]; however, we found no correlation between stage of the estrous cycle and behavior.
Sex differences in the pharmacokinetic and pharmacodynamic processing of THC may contribute to the increased anxiety-like behaviors in females. Previous data suggest that peak plasma levels of both Δ9-THC and 9-Carboxy-THC, an inactive metabolite, are greater in adolescent females than males (see supplemental material). Therefore, there may be greater residual THC in female rats compared to male rats during the drug administration and drug abstinence period, increasing anxiety-like behaviors in females. Additionally, adult female rats metabolize Δ9-THC preferentially to 11-hydroxy-Δ9-THC (a form equivalent or greater than Δ9-THC in its potency), while adult male rats metabolize Δ9-THC to a number of different compounds [105], a factor that may also contribute to the greater behavioral effects seen in females rats in the present study.
A further possibility is that increased levels of the neurosteroid, allopregnanolone, contributed to increased female sensitivity to the anxiogenic effects THC. There is evidence that THC administration can increase cortical levels of allopregnanolone in rats [106]. Shen et al. [107] demonstrated that increased levels of allopregnanolone in pubertal females leads to increased anxiety-like behaviors. In our own lab, we found elevated levels of allopregnanolone in female rats, control and treated rats combined, compared to male rats, control and treated rats combined (see supplemental material). While there were no treatment differences, this may have contributed to a vulnerability to greater anxiety-like behaviors in female rats compared to males in general.
It is important to note that THC was administered during puberty (typically occurring about PND 35 in female rats and PND 39 in male rats [55; 108;109;110]. Puberty is a period that has been shown to be especially sensitive to the effects of THC [40;111;112]. During the pubertal period, the endogenous cannabinoid system is undergoing an array of developmental changes; for example, the density of cannabinoid receptors in the limbic forebrain, striatum and ventral mesencephalon increases until around PND 40, and then subsequently decreases until reaching adult receptor levels by PND 60 [42]. In the present study, the THC administration period from PND 35–41 coincided with puberty in females but only partially overlapped with the start of puberty in males. This discrepancy may contribute to the sex differences seen locomotor activity and anxiety-like behavior. Importantly, puberty often occurs earlier in human female adolescents than male adolescents (see [55]). Additionally, similar developmental changes in the endocannabinoid system occur in the human brain from the prenatal period to adulthood [43].
There was some indication of long-term effects of THC on anxiety-like behavior. In experiment 1, rats administered the high dose of THC spent significantly less time in the center of the Accuscan box on PND 56, suggesting a long-term anxiogenic effect of this dose. In contrast, there were no long-term effects seen in EPM behavior on PND 56. There was no correlation between locomotor activity and time in the center of the Accuscan box on PND 56, suggesting that this anxiogenic effect was not likely due to reduced locomotion. Differences in the anxiolytic/anxiogenic properties of the two paradigms (Accuscan box and EPM) might explain these results. The EPM may have been more sensitive to changes in anxiety-related behaviors as it was a novel environment and may have produced more fear and anxiety compared to the Accuscan box since rats were repeatedly placed inside the Accuscan box over the course of the 21 day experiment.
6. Implications
The current study has several important implications for both pre-clinical and clinical research. This study has further demonstrated that the behavioral measures of precipitated withdrawal and spontaneous withdrawal are not equivalent. While precipitated withdrawal produces robust symptoms, the more subtle symptoms of spontaneous withdrawal can be measured and may be more human-relevant.
Furthermore, the present study highlights the importance of examining sex differences in THC effects. We found opposite effects in male and female rats in anxiety-related behaviors. Currently, few pre-clinical and clinical studies examine cannabinoid effects in female animals, especially in adolescence and concurrently with male animals. Future pre-clinical research is needed to further examine sex differences in chronic cannabinoid administration and subsequent withdrawal.
7. Limitations
It is important to note that while we are administering THC in our research, street marijuana consists of numerous cannabinoid compounds in addition to THC including cannabidiol and cannabigerol. These compounds have been shown to interact with each other, and with tobacco and nicotine found in blunts, to produce effects that will not occur with THC alone. For example, cannabidiol has been shown to potentiate catalepsy, hypothermia, hypoactivity and impairment in spatial memory after THC exposure [113,114]. Alternatively, cannabidiol attenuates THC-induced increases in anxiety [115,116]. Therefore, developmental exposure to cannabidiol could certainly act in conjunction with the THC found in marijuana to produce effects other than those seen in the present study. Furthermore, the pharmacokinetics of different cannabinoid compounds are not equivalent (see [36]). However, it is nearly impossible to study the interactions of all of the compounds in marijuana at one time. Additionally, in clinical studies, it is impossible to know the duration and level of prior cannabis exposure, as individuals typically over- or under-report their cannabis use. Thus, in the present study, THC is used alone for simplicity of experimental design and because it is important to examine its effects before studying marijuana containing various other cannabinoids. In addition, THC administration is a preferred model for human marijuana use compared to the administration of synthetic agonists which possess unique pharmacologic characteristics.
Anther potential limitation of this study is the shipping of the pups with dams at PND 14–15. Several rodent studies have shown that shipping is a stressor [117;118;119] and this may have impacted the rats in our study. However, as all rats were subject to the same shipping practices and individual litters were divided into vehicle and THC-injected cohorts, all rats would have experienced the same level of stress. Additionally, pups were allowed to acclimate to our animal facility for 20–21 days prior to any experimental procedures.
8. Conclusion
This study adds to the growing body of evidence demonstrating that the long-term effects of THC exposure are sexually dimorphic in adolescent rats. Furthermore, the results of the present study indicate that behavioral measures of a spontaneous withdrawal paradigm are manifested more in changes in anxiety-like behaviors rather than in hyperlocomotion and somatic signs of withdrawal, as seen in precipitated withdrawal. Additionally, females appear to be more sensitive to increased anxiety-like behavior during the drug administration and abstinence period than males. In conclusion, the results of the present study draw attention to the importance of studying cannabinoid withdrawal in both the adolescent and female population, and may help in the development of sex-specific therapeutics for cannabinoid withdrawal.
Supplementary Material
Research Highlights.
Withdrawal from chronic THC during adolescence in the rat
Differential effects in males and females on anxiety
Implications for treatment for marijuana withdrawal
Acknowledgements
The authors would like to thank Dr. Jeremy Weedon for his statistical analysis, and Dr. Cheryl Frye for analysis of neurosteroid levels (see supplemental material).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.SAMHSA. Results from the 2009 National survey on Drug Use and Health: Summary of National Findings. US Department of Health and Human Services 1; 2011. [Google Scholar]
- 2.Hoffmann DE, Weber E. Medical marijuana and the law. New Engl J Med. 2010;362:1453–1457. doi: 10.1056/NEJMp1000695. [DOI] [PubMed] [Google Scholar]
- 3.Iverson L. Cannabis and the brain. Brain. 2003;126:1252–1270. doi: 10.1093/brain/awg143. [DOI] [PubMed] [Google Scholar]
- 4.Misner DL, Sullivan JM. Mechanism of cannabinoid effects on long-term potentiation and depression in hippocampal CA1 neurons. Neuroscience. 1999;19:6795–6805. doi: 10.1523/JNEUROSCI.19-16-06795.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Auclair N, Otani S, Soubrie P, Crepel F. Cannabinoids Modulates Synaptic Strength and Plasticity at Glutamatergic Synapses of rat Prefrontal Cortex Pyramidal Neurons. J of Neurophysiology. 2000;83:3293. doi: 10.1152/jn.2000.83.6.3287. [DOI] [PubMed] [Google Scholar]
- 6.Robbe D, Alonso G, Manzoni OJ. Exogenous and endogenous cannabinoids control synaptic transmission in mice nucleus accumbens. Ann NY Acad Sci. 2003;1003:212–225. doi: 10.1196/annals.1300.013. [DOI] [PubMed] [Google Scholar]
- 7.Azad SC, Eder M, Marsicano G, Lutz B, Zieglgänsberger W, Rammes G. Activation of the cannabinoid receptor type 1 decreases glutamatergic and GABAergic synaptic transmission in the lateral amygdala of the mouse. Learn Memory. 2003;10:116–128. doi: 10.1101/lm.53303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, et al. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci. 2001;21:9506–9518. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan H, Lauzon NM, Bishop SF, Bechard MA, Laviolette SR. Integrated cannabinoid CB1 receptor transmission within the amygdala-prefrontal cortical pathway modulates neuronal plasticity and emotional memory encoding. Cereb Cortex. 2010;20:1486–1496. doi: 10.1093/cercor/bhp210. [DOI] [PubMed] [Google Scholar]
- 10.Haj-Dahmane S, Shen RY. Regulation of plasticity of glutamate synapses by endocannabinoids and the cyclic-AMP/protein kinase A pathway in midbrain dopamine neurons. J Physiol. 2010;588:2589–2604. doi: 10.1113/jphysiol.2010.190066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belue RC, Howlett AC, Westlake TM, Hutchings DE. The ontogeny of cannabinoid receptors in the brain of postnatal and aging rats. Neurotoxic Teratol. 1995;17:25–30. doi: 10.1016/0892-0362(94)00053-g. [DOI] [PubMed] [Google Scholar]
- 12.Lafenêtre P, Chaouloff F, Marsicano G. The endocannabinoid system in the processing of anxiety and fear and how CB1 receptors may modulate fear extinction. Pharmacol Res. 2007;56:367–381. doi: 10.1016/j.phrs.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Haney M, Comer SD, Ward AS, Foltin RW, Fischman MW. Factors influencing marijuana self-administration by humans. Behav Pharmacol. 1997;8:101–112. [PubMed] [Google Scholar]
- 14.Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following oral THC administration to humans. Psychopharmacol. 1999;141:385–394. doi: 10.1007/s002130050848. [DOI] [PubMed] [Google Scholar]
- 15.Haney M, Hart CL, Vosburg SK, Nasser J, Bennett A, Zubaran C, et al. Marijuana withdrawal in humans: effects of oral THC or divalproex. Neuropsychopharmacol. 2004;29:158–170. doi: 10.1038/sj.npp.1300310. [DOI] [PubMed] [Google Scholar]
- 16.Kelly TH, Foltin RW, Emurian CS, Fischman MW. Are choice and self-administration of marijuana related to delta 9-THC content? Experimental and Clinical Psychopharmacol. 1997;5:74–82. doi: 10.1037//1064-1297.5.1.74. [DOI] [PubMed] [Google Scholar]
- 17.Hart CL, van Gorp W, Haney M, Foltin RW, Fischman MW. Effects of acute smoked marijuana on complex cognitive performance. Neuropsychopharmacol. 2001;25:757–765. doi: 10.1016/S0893-133X(01)00273-1. [DOI] [PubMed] [Google Scholar]
- 18.Abood ME, Sauss C, Fan F, Tilton CL, Martin BR. Development of behavioral tolerance to delta 9-THC without alteration of cannabinoid receptor binding or mRNA levels in whole brain. Pharmacol Biochem Be. 1993;46:575–579. doi: 10.1016/0091-3057(93)90546-6. [DOI] [PubMed] [Google Scholar]
- 19.Bass CE, Martin BR. Time course for the induction and maintenance of tolerance to Delta(9)-tetrahydrocannabinol in mice. Drug Alcohol Depen. 2000;60:113–119. doi: 10.1016/s0376-8716(99)00150-7. [DOI] [PubMed] [Google Scholar]
- 20.Lichtman AH, Martin BR. Cannabinoid tolerance and dependence. Handb Exp Pharmacol. 2005:691–717. doi: 10.1007/3-540-26573-2_24. [DOI] [PubMed] [Google Scholar]
- 21.D'Souza DC, Ranganathan M, Braley G, Gueorguieva R, Zimolo Z, Cooper T, et al. Blunted psychotomimetic and amnestic effects of delta-9-tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacol. 2008;33:2505–2516. doi: 10.1038/sj.npp.1301643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Budney AJ, Hughes JR, Moore BA, Vandrey R. Review of the validity and significance of cannabis withdrawal syndrome. Am J Psychiat. 2004;161:1967–1977. doi: 10.1176/appi.ajp.161.11.1967. [DOI] [PubMed] [Google Scholar]
- 23.Tanda G, Goldberg SR. Cannabinoids: reward, dependence, and underlying neurochemical mechanisms--a review of recent preclinical data. Psychopharmacol (Berl) 2003;169:115–134. doi: 10.1007/s00213-003-1485-z. [DOI] [PubMed] [Google Scholar]
- 24.Budney AJ, Hughes JR. The cannabis withdrawal syndrome. Curr Opin in Psychiatr. 2006;19:233–238. doi: 10.1097/01.yco.0000218592.00689.e5. [DOI] [PubMed] [Google Scholar]
- 25.Fattore L, Altea S, Fratta W. Sex differences in drug addiction: a review of animal and human studies. Women Health (Lond Engl) 2008;4:51–65. doi: 10.2217/17455057.4.1.51. [DOI] [PubMed] [Google Scholar]
- 26.Budney AJ, Vandrey RG, Hughes JR, Moore BA, Bahrenburg B. Oral delta-9-tetrahydrocannabinol suppresses cannabis withdrawal symptoms. Drug Alcohol Depend. 2007;86:22–29. doi: 10.1016/j.drugalcdep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 27.Diana M, Melis M, Muntoni AL, Gessa GL. Mesolimbic dopaminergic decline after cannabinoid withdrawal. P Natl Acad Sci. 1998;95:10269–10273. doi: 10.1073/pnas.95.17.10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Compton DR, Aceto MD, Lowe J, Martin BR. In vivo characterization of a specific cannabinoid receptor antagonist (SR141716A): inhibition of delta 9-tetrahydrocannabinol-induced responses and apparent agonist activity. J Pharmacol Exp Ther. 1996;277:586–594. [PubMed] [Google Scholar]
- 29.Degroot A, Nomikos GG. Genetic deletion and pharmacological blockade of CB1 receptors modulates anxiety in the shock-probe burying test. European Journal of Neurosci. 2004;20:1059–1064. doi: 10.1111/j.1460-9568.2004.03556.x. [DOI] [PubMed] [Google Scholar]
- 30.Huang P, Liu-Chen LY, Kirby LG. Anxiety-like effects of SR141716-precipitated delta9-tetrahydrocannabinol withdrawal in mice in the elevated plus-maze. Neurosci Lett. 2010;475:165–168. doi: 10.1016/j.neulet.2010.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stewart JL, McMahon LR. Rimonabant-induced Delta9-tetrahydrocannabinol withdrawal in rhesus monkeys: discriminative stimulus effects and other withdrawal signs. J Pharmacol Exp Ther. 2010;334:347–356. doi: 10.1124/jpet.110.168435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leite JR, Carlini EA. Failure to obtain "cannabis-directed behavior" and abstinence syndrome in rats chronically treated with cannabis sativa extracts. Psychopharmacologia. 36:133–145. doi: 10.1007/BF00421785. 174. [DOI] [PubMed] [Google Scholar]
- 33.Lichtman AH, Poklis JL, Poklis A, Wilson DM, Martin BR. The pharmacological activity of inhalation exposure to marijuana smoke in mice. Drug Alcohol Depend. 2001;63:107–116. doi: 10.1016/s0376-8716(00)00205-2. [DOI] [PubMed] [Google Scholar]
- 34.Wilson DM, Varvel SA, Harloe JP, Martin BR, Lichtman AH. SR 141716 (Rimonabant) precipitates withdrawal in marijuana-dependent mice. Pharmacol Biochem Behav. 2006;85:105–113. doi: 10.1016/j.pbb.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 35.Klausner HA, Dingell JV. The metabolism and excretion of delta 9-tetrahydrocannabinol in the rat. Life Sci. 1971;10:49–59. doi: 10.1016/0024-3205(71)90245-1. [DOI] [PubMed] [Google Scholar]
- 36.Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. 2003;42:327–360. doi: 10.2165/00003088-200342040-00003. [DOI] [PubMed] [Google Scholar]
- 37.Toennes SW, Ramaekers JG, Theunissen EL, Moeller MR, Kauert GF. Comparison of cannabinoid pharmacokinetic properties in occasional and heavy users smoking a marijuana or placebo joint. J Anal Toxicol. 2008;32:470–477. doi: 10.1093/jat/32.7.470. [DOI] [PubMed] [Google Scholar]
- 38.Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, et al. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacol (Berl) 1999;142:295–301. doi: 10.1007/s002130050892. [DOI] [PubMed] [Google Scholar]
- 39.Pope HG, Jr., Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend. 2003;69:303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- 40.Schneider M. Puberty as a highly vulnerable developmental period for the consequences of cannabis exposure. Addict Biol. 2008;13(2):253–263. doi: 10.1111/j.1369-1600.2008.00110.x. [DOI] [PubMed] [Google Scholar]
- 41.Schneider M, Schömig E, Leweke FM. Acute and chronic cannabinoid treatment differentially affects recognition memory and social behavior in pubertal and adult rats. Addict Biol. 2008;13(3–4):345–357. doi: 10.1111/j.1369-1600.2008.00117.x. [DOI] [PubMed] [Google Scholar]
- 42.Rodríguez de Fonseca F, Ramos JA, Bonnin A, Fernández-Ruiz JJ. Presence of cannabinoid binding sites in the brain from early postnatal ages. Neuroreport. 1993;4:135–138. doi: 10.1097/00001756-199302000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Mato S, Del Olmo E, Pazos A. Ontogenetic development of cannabinoid receptor expression and signal transduction functionality in the human brain. Eur J Neurosci. 2003;17:1747–1754. doi: 10.1046/j.1460-9568.2003.02599.x. [DOI] [PubMed] [Google Scholar]
- 44.Ellgren M, Artmann A, Tkalych O, Gupta A, Hansen HS, Hansen SH, et al. Dynamic changes of the endogenous cannabinoid and opioid mesocorticolimbic systems during adolescence: THC effects. Eur Neuropsychopharmacol. 2008;18:826–834. doi: 10.1016/j.euroneuro.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fattore L, Fratta W. How important are sex differences in cannabinoid action? Br J Pharmacol. 2010;160:544–548. doi: 10.1111/j.1476-5381.2010.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rubino T, Parolaro D. Sexually dimorphic effects of cannabinoid compounds on emotion and cognition. Front Behav Neurosci. 2011;5:1–5. doi: 10.3389/fnbeh.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harte LC, Dow-Edwards D. Sexually dimorphic alterations in locomotion and reversal learning after adolescent tetrahydrocannabinol exposure in the rat. Neurotoxicol Teratol. 2010;32:515–524. doi: 10.1016/j.ntt.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wiley JL, O'connell MM, Tokarz ME, Wright MJ., Jr. Pharmacological effects of acute and repeated administration of Delta(9)-tetrahydrocannabinol in adolescent and adult rats. J Pharmacol Exp Ther. 2007;320:1097–1105. doi: 10.1124/jpet.106.108126. [DOI] [PubMed] [Google Scholar]
- 49.Ramos A. Animal models of anxiety: do I need multiple tests? Trend Pharmacol Sci. 2008;29:493–498. doi: 10.1016/j.tips.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Gulinello M, Smith SS. Anxiogenic effects of neurosteroid exposure: sex differences and altered GABAA receptor pharmacology in adult rats. J Pharmacol Exp Ther. 2003;305(2):541–548. doi: 10.1124/jpet.102.045120. [DOI] [PubMed] [Google Scholar]
- 51.Gulinello M, Gong QH, Li X, Smith SS. Short-term exposure to a neuroactive steroid increases alpha4 GABA(A) receptor subunit levels in association with increased anxiety in the female rat. Brain Res. 2001;910(1–2):55–66. doi: 10.1016/s0006-8993(01)02565-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rodgers RJ, Haller J, Holmes A, Halasz J, Walton TJ, Brain PF. Corticosterone response to the plus-maze: high correlation with risk assessment in rats and mice. Physiol Behav. 1999;68:47–53. doi: 10.1016/s0031-9384(99)00140-7. [DOI] [PubMed] [Google Scholar]
- 53.Rodgers RJ, Davli A. Anxiety, defence and the elevated plus maze. Neurosci Biobehav Rev. 1997;21:801–810. doi: 10.1016/s0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- 54.Allen-Rowlands CF, Allen JP, Greer MA, Wilson M. Circadian rhythmicity of ACTH and corticosterone in the rat. J Endocrinol Invest. 1980;3:371–377. doi: 10.1007/BF03349373. [DOI] [PubMed] [Google Scholar]
- 55.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 56.Whitlow CT, Freedland CS, Porrino LJ. Metabolic mapping of the time-dependent effects of delta 9-tetrahydrocannabinol administration in the rat. Psychopharmacology (Berl) 2002;161:129–136. doi: 10.1007/s00213-002-1001-x. [DOI] [PubMed] [Google Scholar]
- 57.Long LE, Chesworth R, Huang XF, McGregor IS, Arnold JC, Karl T. A behavioural comparison of acute and chronic Delta9-tetrahydrocannabinol and cannabidiol in C57BL/6JArc mice. Int J Neuropsychopharmacol. 2010;13:861–876. doi: 10.1017/S1461145709990605. [DOI] [PubMed] [Google Scholar]
- 58.Sañudo-Peña MC, Romero J, Seale GE, Fernandez-Ruiz JJ, Walker JM. Activational role of cannabinoids on movement. Eur J Pharmacol. 2000;391:269–274. doi: 10.1016/s0014-2999(00)00044-3. [DOI] [PubMed] [Google Scholar]
- 59.Wakley AA, Craft RM. Antinociception sedation following intracerebroventricular administration of Δ9-tetrahydrocannabinol in female vs. male rats. Behav Brain Res. 2011;216:200–206. doi: 10.1016/j.bbr.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 60.Jacob W, Yassouridis A, Marsicano G, Monory K, Lutz B, Wotjak CT. Endocannabinoids render exploratory behaviour largely independent of the test aversiveness: role of glutamatergic transmission. Genes Brain Behav. 2009;8(7):685–698. doi: 10.1111/j.1601-183X.2009.00512.x. [DOI] [PubMed] [Google Scholar]
- 61.Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418(6897):530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 62.Haller J, Varga B, Ledent C, Barna I, Freund TF. Context-dependent effects of CB1 cannabinoid gene disruption on anxiety-like and social behaviour in mice. Eur J Neurosci. 2004;19(7):1906–1912. doi: 10.1111/j.1460-9568.2004.03293.x. [DOI] [PubMed] [Google Scholar]
- 63.Huang P, Liu-Chen LY, Unterwald EM, Cowan A. Hyperlocomotion and paw tremors are two highly quantifiable signs of SR141716-precipitated withdrawal from delta9-tetrahydrocannabinol in C57BL/6 mice. Neurosci Lett. 2009;465:66–70. doi: 10.1016/j.neulet.2009.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grillner S, Hellgren J, Ménard A, Saitoh K, Wikström MA. Mechanisms for selection of basic motor programs--roles for the striatum and pallidum. Trends Neurosci. 2005;28:364–370. doi: 10.1016/j.tins.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 65.Köfalvi A, Rodrigues RJ, Ledent C, Mackie K, Vizi ES, Cunha RA, et al. Involvement of cannabinoid receptors in the regulation of neurotransmitter release in the rodent striatum: a combined immunochemical and pharmacological analysis. J Neurosci. 2005;25:2874–2884. doi: 10.1523/JNEUROSCI.4232-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, Watanabe M. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J Neurosci. 2007;27:3663–3676. doi: 10.1523/JNEUROSCI.0448-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herkenham M. Cannabinoid receptor localization in brain: relationship to motor and reward systems. Ann N Y Acad Sci. 1992;654:19–32. doi: 10.1111/j.1749-6632.1992.tb25953.x. [DOI] [PubMed] [Google Scholar]
- 68.Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hohmann AG, Herkenham M. Localization of cannabinoid CB(1) receptor mRNA in neuronal subpopulations of rat striatum: a double-label in situ hybridization study. Synapse. 2000;37:71–80. doi: 10.1002/(SICI)1098-2396(200007)37:1<71::AID-SYN8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 70.Rodriguez JJ, Mackie K, Pickel VM. Ultrastructural localization of the CB1 cannabinoid receptor in mu-opioid receptor patches of the rat Caudate putamen nucleus. J Neurosci. 2001;21:823–833. doi: 10.1523/JNEUROSCI.21-03-00823.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fernández-Ruiz J. The endocannabinoid system as a target for the treatment of motor dysfunction. Br J Pharmacol. 2009;156:1029–1040. doi: 10.1111/j.1476-5381.2008.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kreitzer AC, Regehr WG. Cerebellar depolarization-induced suppression of inhibition is mediated by endogenous cannabinoids. J Neurosci. 2001;21:174. doi: 10.1523/JNEUROSCI.21-20-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- 74.Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- 75.Rubino T, Guidali C, Vigano D, Realini N, Valenti M, Massi P, et al. CB1 receptor stimulation in specific brain areas differently modulate anxiety-related behaviour. Neuropharmacology. 2008;54:151–160. doi: 10.1016/j.neuropharm.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 76.Burston JJ, Wiley JL, Craig AA, Selley DE, Sim-Selley LJ. Regional enhancement of cannabinoid CB₁ receptor desensitization in female adolescent rats following repeated Delta-tetrahydrocannabinol exposure. Br J Pharmacol. 2010;161(1):103–112. doi: 10.1111/j.1476-5381.2010.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Amal H, Fridman-Rozevich L, Senn R, Strelnikov A, Gafni M, Keren O, et al. Long-term consequences of a single treatment of mice with an ultra-low dose of Delta9-tetrahydrocannabinol (THC) Behav Brain Res. 2010;206:245–253. doi: 10.1016/j.bbr.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 78.Okva KO, Lang A, Nevalainen T, Mauranen K, Vali M, Pokk P. Effects of litter origin and weight on behaviour of outbred NIH/S mice in plus-maze and staircase tests. Scandanavian J Lab An Sci. 2008;35(13):127–134. [Google Scholar]
- 79.Kirk JM, de Wit H. Responses to oral delta9-tetrahydrocannabinol in frequent and infrequent marijuana users. Pharmacol Biochem Behav. 1999;63:137–142. doi: 10.1016/s0091-3057(98)00264-0. [DOI] [PubMed] [Google Scholar]
- 80.Whitlow CT, Freedland CS, Porrino LJ. Functional consequences of the repeated administration of Delta9-tetrahydrocannabinol in the rat. Drug Alcohol Depend. 2003;71:169–177. doi: 10.1016/s0376-8716(03)00135-2. [DOI] [PubMed] [Google Scholar]
- 81.Oviedo A, Glowa J, Herkenham M. Chronic cannabinoid administration alters cannabinoid receptor binding in rat brain: a quantitative autoradiographic study. Brain Res. 1993;616:293–302. doi: 10.1016/0006-8993(93)90220-h. [DOI] [PubMed] [Google Scholar]
- 82.Romero J, Berrendero F, Manzanares J, Pérez A, Corchero J, Fuentes JA, et al. Time-course of the cannabinoid receptor down-regulation in the adult rat brain caused by repeated exposure to delta9-tetrahydrocannabinol. Synapse. 1998;30:298–308. doi: 10.1002/(SICI)1098-2396(199811)30:3<298::AID-SYN7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 83.Zhuang S, Kittler J, Grigorenko EV, Kirby MT, Sim LJ, Hampson RE, et al. Effects of long-term exposure to delta9-THC on expression of cannabinoid receptor (CB1) mRNA in different rat brain regions. Brain Res Mol Brain Res. 1998;62:141–149. doi: 10.1016/s0169-328x(98)00232-0. [DOI] [PubMed] [Google Scholar]
- 84.Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Vogt LJ, Sim-Selley LJ. Chronic delta9-tetrahydrocannabinol treatment produces a time-dependent loss of cannabinoid receptors and cannabinoid receptor-activated G proteins in rat brain. J Neurochem. 1999;73:2447–2459. doi: 10.1046/j.1471-4159.1999.0732447.x. [DOI] [PubMed] [Google Scholar]
- 85.Vanderschuren LJ, Pierce RC. Sensitization processes in drug addiction. Curr Top Behav Neurosci. 2010;3:179–195. doi: 10.1007/7854_2009_21. [DOI] [PubMed] [Google Scholar]
- 86.Rubino T, Viganò D, Massi P, Parolaro D. The psychoactive ingredient of marijuana induces behavioural sensitization. Eur J Neurosci. 2001;14:884–886. doi: 10.1046/j.0953-816x.2001.01709.x. [DOI] [PubMed] [Google Scholar]
- 87.Cadoni C, Valentini V, Di Chiara G. Behavioral sensitization to delta 9-tetrahydrocannabinol and cross-sensitization with morphine: differential changes in accumbal shell and core dopamine transmission. J Neurochem. 2008;106:1586–1593. doi: 10.1111/j.1471-4159.2008.05503.x. [DOI] [PubMed] [Google Scholar]
- 88.Kolb B, Gorny G, Limebeer CL, Parker LA. Chronic treatment with Delta-9-tetrahydrocannabinol alters the structure of neurons in the nucleus accumbens shell and medial prefrontal cortex of rats. Synapse. 2006;60:429–436. doi: 10.1002/syn.20313. [DOI] [PubMed] [Google Scholar]
- 89.Valjent E, Mitchell JM, Besson MJ, Caboche J, Maldonado R. Behavioural and biochemical evidence for interactions between Delta 9-tetrahydrocannabinol and nicotine. Br J Pharmacol. 2002;135:564–578. doi: 10.1038/sj.bjp.0704479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Berrendero F, Maldonado R. Involvement of the opoid system in the anxiolytic-like effects induced by Delta(9)-tetrahydrocannabinol. Psychopharmacology (Berl) 2002;163:111–117. doi: 10.1007/s00213-002-1144-9. [DOI] [PubMed] [Google Scholar]
- 91.Phan KL, Angstadt M, Golden J, Onyewuenyi I, Popovska A, de Wit H. Cannabinoid modulation of amygdala reactivity to social signals of threat in humans. J Neurosci. 2008;28:2313–2319. doi: 10.1523/JNEUROSCI.5603-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am Psychol. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- 93.Lee TT, Gorzalka BB. Timing is everything: evidence for a role of corticolimbic endocannabinoids in modulating hypothalamic-pituitary-adrenal axis activity across developmental periods. Neuroscience. 2011 doi: 10.1016/j.neuroscience.2011.10.006. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 94.Hill MN, McLaughlin RJ, Bingham B, Shrestha L, Lee TTY, Gray JM, et al. Endogenous cannabinoid signaling is essential for stress adaptation. PNAS. 2010;107:9406–9411. doi: 10.1073/pnas.0914661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tye KM, Prakash R, Kim SU, Fenno LE, Grosenick L, Zarabi H, et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Egerton A, Allison C, Brett RR, Pratt JA. Cannabinoids and prefrontal cortical function: Insights from preclinical studies. Neuroscience and Biobebehavioral reviews. 2006;30:680–695. doi: 10.1016/j.neubiorev.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 97.Liu BDK, Darlington CL, Smith PF. Cannabinoid receptor protein expression in the rat hippocampus and entorhinal, perirhinal, postrhinal and temporal cortices: regional variations and age-related changes. Brain Res. 2003;979:235–239. doi: 10.1016/s0006-8993(03)02872-5. [DOI] [PubMed] [Google Scholar]
- 98.Tsou K, Mackie K, Sañudo-Peña MC, Walker JM. Cannabinoid CB1 receptors are localized primarily on cholecystokinin-containing GABAergic interneurons in the rat hippocampal formation. Neuroscience. 1999;93:969–975. doi: 10.1016/s0306-4522(99)00086-x. [DOI] [PubMed] [Google Scholar]
- 99.Di Marzo V, Berrendero F, Bisogno T, Gonzalez S, Cavalier P, Romero J, et al. Enhancement of anandamide formation in the limbic forebrain and reduction of endocannabinoid contents in the striatum of delta9-tetrahydrocannabinol-tolerant rats. J Neurochem. 2000;74:1627–1635. doi: 10.1046/j.1471-4159.2000.0741627.x. [DOI] [PubMed] [Google Scholar]
- 100.Rodríguez de Fonseca F, Carrera MR, Navarro M, Koob GF, Weiss F. Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science. 1997;276:2050. doi: 10.1126/science.276.5321.2050. [DOI] [PubMed] [Google Scholar]
- 101.Caberlotto L, Rimondini R, Hansson A, Eriksson S, Heilig M. Corticotropin-releasing hormone (CRH) mRNA expression in rat central amygdala in cannabinoid tolerance and withdrawal: evidence for an allostatic shift? Neuropsychopharmacology. 2004;29:15–22. doi: 10.1038/sj.npp.1300296. [DOI] [PubMed] [Google Scholar]
- 102.Riebe CJN, Hill MN, Lee TTY, Hillard CJ, Gorzalka BB. Estrogenic regulation of limbic cannabinoid receptor binding. Psychoneuroendocrinology. 2010;35:1265–1269. doi: 10.1016/j.psyneuen.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Monory K, Blaudzun H, Massa F, Kaiser N, Lemberger T, Schütz G, et al. Genetic dissection of behavioural and autonomic effects of Delta(9)-tetrahydrocannabinol in mice. PLoS Biol. 2007;5(10):2354–2368. doi: 10.1371/journal.pbio.0050269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rodríguez de Fonseca F, Cebeira M, Ramos JA, Martín M, Fernández-Ruiz JJ. Cannabinoid receptors in rat brain areas: sexual differences, fluctuations during estrous cycle and changes after gonadectomy and sex steroid replacement. Life Sci. 1994;54:159–170. doi: 10.1016/0024-3205(94)00585-0. [DOI] [PubMed] [Google Scholar]
- 105.Narimatsu S, Watanabe K, Yamamoto I, Yoshimura H. Sex difference in the oxidative metabolism of delta 9-tetrahydrocannabinol in the rat. Biochem Pharmacol. 1991;41:1187–1194. doi: 10.1016/0006-2952(91)90657-q. [DOI] [PubMed] [Google Scholar]
- 106.Grobin AC, VanDoren MJ, Porrino LJ, Morrow AL. Cortical 3 alpha-hydroxy-5 alpha-pregnan-20-one levels after acute administration of Delta-9-tetrahydrocannabino, cocaine and morphine. Psychopharmacology (Berl) 2005;179:544–550. doi: 10.1007/s00213-004-2084-3. [DOI] [PubMed] [Google Scholar]
- 107.Shen H, Gong QH, Aoki C, Yuan M, Ruderman Y, Dattilo M, et al. Reversal of neurosteroid effects at alpha4beta2delta GABAA receptors triggers anxiety at puberty. Nat Neurosci. 2007;10:469–477. doi: 10.1038/nn1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Korenbrot CC, Huhtaniemi IT, Weiner RI. Preputial separation as an external sign of pubertal development in the male rat. Biol Reprod. 1977;17(2):298–303. doi: 10.1095/biolreprod17.2.298. [DOI] [PubMed] [Google Scholar]
- 109.Engelbregt MJ, van Weissenbruch MM, Popp-Snijders C, Lips P, Delemarre-van de Waal HA. Body mass index, body composition, and leptin at onset of puberty in male and female rats after intrauterine growth retardation and after early postnatal food restriction. Pediatr Res. 2001;50(4):474–478. doi: 10.1203/00006450-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 110.Delemarre-van de Waal HA, van Coeverden SC, Engelbregt MT. Factors affecting onset of puberty. Horm Res. 2002;57(Suppl 2):15–18. doi: 10.1159/000058095. [DOI] [PubMed] [Google Scholar]
- 111.Schneider M, Drews E, Koch M. Behavioral effects in adult rats of chronic prepubertal treatment with the cannabinoid receptor agonist WIN 55,212-2. Behav Pharmacol. 2005;16(5–6):447–454. doi: 10.1097/00008877-200509000-00018. [DOI] [PubMed] [Google Scholar]
- 112.Schneider M, Koch M. Deficient social and play behavior in juvenile and adult rats after neonatal cortical lesion: effects of chronic pubertal cannabinoid treatment. Neuropsychopharmacology. 2005;30(5):944–957. doi: 10.1038/sj.npp.1300634. [DOI] [PubMed] [Google Scholar]
- 113.Fernandes M, Schabarek A, Coper H, Hill R. Modification of delta9-THC-actions by cannabinol and cannabidiol in the rat. Psychopharmacologia. 1974;38:329–338. doi: 10.1007/BF00429130. [DOI] [PubMed] [Google Scholar]
- 114.Hayakawa K, Mishima K, Hazekawa M, Sano K, Irie K, Orito K, et al. Cannabidiol potentiates pharmacological effects of Delta(9)-tetrahydrocannabinol via CB(1) receptor-dependent mechanism. Brain Res. 2008;1188:157–164. doi: 10.1016/j.brainres.2007.09.090. [DOI] [PubMed] [Google Scholar]
- 115.Zuardi AW, Shirakawa I, Finkelfarb E, Karniol IG. Action of cannabidiol on the anxiety and other effects produced by delta 9-THC in normal subjects. Psychopharmacology (Berl) 1982;76(3):245–250. doi: 10.1007/BF00432554. [DOI] [PubMed] [Google Scholar]
- 116.Fusar-Poli P, Crippa JA, Bhattacharyya S, Borgwardt SJ, Allen P, Martin-Santos R, et al. Distinct effects of {delta}9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch Gen Psychiatry. 2009;66(1):95–105. doi: 10.1001/archgenpsychiatry.2008.519. [DOI] [PubMed] [Google Scholar]
- 117.Tuli JS, Smith JA, Morton DB. Stress measurements in mice after transportation. Lab Anim. 1995;29(2):132–138. doi: 10.1258/002367795780740249. [DOI] [PubMed] [Google Scholar]
- 118.Laroche J, Gasbarro L, Herman JP, Blaustein JD. Reduced behavioral response to gonadal hormones in mice shipped during the peripubertal/adolescent period. Endocrinol. 2009;150(5):2351–2358. doi: 10.1210/en.2008-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.van Ruiven R, Meijer GW, Wiersma A, Baumans V, van Zutphen LF, Ritskes-Hoitinga J. The influence of transportation stress on selected nutritional parameters to establish the necessary minimum period for adaptation in rat feeding studies. Lab Anim. 1998;32(4):446–456. doi: 10.1258/002367798780599893. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.