Abstract
BACKGROUND
No studies have comprehensively examined the prevalence of dyslipidemia, a major risk factor for cardiovascular disease, among diverse racial/ethnic minority groups. The primary aim of this study was to identify racial/ethnic differences in dyslipidemia among minorities including Asian Americans (Asian Indian, Chinese, Filipino, Japanese, Korean or Vietnamese), Mexican Americans, and African Americans compared to Non-Hispanic Whites (NHWs).
METHODS AND RESULTS
Using a three-year cross-section (2008–2011), we identified 169,430 active primary care patients (35 years or older) from an outpatient health care organization in Northern California. Age-standardized prevalence rates were calculated for three dyslipidemia subtypes: high TG (fasting lab ≥150 mg/dL), low HDL-C (fasting lab <40 [men] and <50 [women] mg/dL), and high LDL-C (fasting lab ≥130 mg/dL or taking LDL-lowering agents). Odds ratios were calculated using multivariable logistic regression, adjusting for patient characteristics (age, measured BMI, smoking). Compared to NHWs, every minority subgroup had increased prevalence of high TGs, except African Americans. Most minority groups had increased prevalence of low HDL-C, except for Japanese and African Americans. The prevalence of high LDL-C was increased among Asian Indians, Filipinos, Japanese, and Vietnamese, compared to NHWs.
CONCLUSIONS
Minority groups, except for African Americans, were more likely to have high TG/low HDL-C dyslipidemia. Further research is needed to determine how racial/ethnic differences in dyslipidemia affect racial/ethnic differences in cardiovascular disease rates.
Keywords: drugs, epidemiology, hyperlipoproteinemia, lipids, lipoproteins, risk factors
BACKGROUND
Racial/ethnic minority groups now make up 36% of the US population, and are expected to reach 53% by 2050.1,2 The two most rapidly growing racial/ethnic minority groups are Hispanic/Latino and Asian Americans (Asian Indian, Chinese, Filipino, Japanese, Korean, Vietnamese), which are expected to double in size by 2050 to 110 million and 30 million respectively.1–2 A growing body of evidence indicates variation in cardiovascular disease (CVD)burden among racial/ethnic subgroups, with African Americans,3,4 Asian Indians5,6 and Filipinos5,6 having higher coronary heart disease (CHD) burden compared to other subgroups and Non-Hispanic Whites (NHWs). Filipinos,6 Hispanics/Latinos,3,7,8 and African Americans3,8 also have a higher burden of stroke, compared to NHWs.
More than one out of every three adults in the U.S. has dyslipidemia,9 one of the major risk factors for CVD.10 The National Health and Examination Survey (NHANES) is the primary data source for national prevalence rates of dyslipidemia in the U.S., with accurate sampling data for African Americans and Mexican Americans.11 The NHANES data show higher prevalence rates of low high-density lipoprotein cholesterol (HDL-C) and high triglycerides (TG)for Mexican Americans.3 Although lower prevalence rates of low HDL-C and high TG are seen for Black/African Americans, this does not appear to be protective from CVD.3 NHANES does not currently include data specifically for Asian Americans12 and sample sizes are too small to examine specific Asian or Hispanic/Latino subgroups.11
Previous research examining the prevalence of dyslipidemia subtypes for racial/ethnic minorities has focused on the African and Mexican American population, with limited information on Asian subgroups.13–21 The majority of studies for Asians have been conducted in their country of origin with Asian Indians and Filipinos having a higher prevalence of low HDL-C and of high TG,13–16 which has been suggested as a partial explanation for their increased CHD risk.5,6 Chinese have lower levels of low-density lipoprotein cholesterol (LDL-C) and TG16–18 and Japanese have higher levels of HDL-C16 compared to NHWs, which may help explain the lower risk of CHD in these Asian subgroups. The American Heart Association,22 the US Department of Health and Human Services,23 and the Institute of Medicine24 have all acknowledged that national vital statistics data for racial/ethnic minority populations must be supplemented with population-based, local-level data in order to inform and guide the development, implementation, and evaluation of programs to address health disparities. Despite known heterogeneity in CVD risk among racial/ethnic subgroups, no studies have comprehensively examined the prevalence of dyslipidemia subtypes and treatment across the major racial/ethnic groups in the US. The primary aim of this study was to identify racial/ethnic differences in dyslipidemias in order to guide prevention, detection, and treatment efforts.
METHODS
Setting
This study was conducted in a mixed-payer, outpatient health care organization serving approximately 800,000 active patients in northern California, which has been using the Epic Care electronic health record (EHR) system since 2000. This health care organization is unique among large clinical data resources because more than 30% of the overall patient population self-identifies as Asian American. The demographic characteristics of the patients are generally similar to those of residents in the surrounding service area (Alameda, San Mateo, and Santa Clara counties). The patient population is insured (58% preferred provider organization (PPO), 23% health maintenance organization (HMO), 16% Medicare, 2% self-pay, and 1% Medicaid), and thus under represents the medically underserved. However, in this setting access to care is unlikely to confound subgroup comparisons.
Study Design
A three-year, cross-sectional sample of patients was identified and included patients age 35 years or older with at least one primary care visit occurring between June 1, 2008 to May 31, 2011. Patient race and ethnicity was identified, either by self-report25(67%) or name analysis26(33%), as Asian Indian, Chinese, Filipino, Japanese, Korean, Vietnamese, Black/African American, Hispanic/Latino, or Non-Hispanic White. Patients identified as Hispanic/Latino and who further self-classified as Mexican were included in the analysis while other Hispanic subgroups were not due to small sample sizes (N<1000). Using diagnosis codes from problem lists and encounters listed in the EHR, we excluded patients who were pregnant or had any previous history of cancer, end stage kidney disease or dialysis, or active liver disease (except for fatty liver). All datasets analyzed by the research team were de-identified according to the Health Insurance Portability and Accountability Act (HIPAA) standard; the organization’s Institutional Review Board approved the study.
Clinical Definitions
Data on patient demographics, anthropometric measures, physician diagnoses, laboratory results and prescription medications were extracted from the EHR. Three dyslipidemia subtypes were identified based on fasting laboratory results for each individual found during the study period: high TG (≥150 mg/dL), low HDL-C (<40 [men] and <50 [women] mg/dL), and high LDL-C (≥130 mg/dL or ever taking LDL-lowering agents). LDL-lowering agents(LLAs) including statins, bile acid resins, cholesterol absorption inhibitors and combinations) accounted for 78% of all lipid-modifying agents (LMAs) prescribed. LLA use was included in the definition of high LDL-C to avoid missing treated patients with controlled LDL-C after initial analyses indicated that using only laboratory data produced prevalence rates below those found in national data27, and that treatment rates differed by subgroup. Treatment with other LMAs (fibric acid derivatives, niacin, and omega 3 fish oil)was not included in the definition of high LDL-C, low HDL-C, or high TG, as they are not specific to one dyslipidemia subtype,10 which could lead to misclassification. The following patient characteristics were identified: self-reported race/ethnicity, age in 2008, primary insurance (PPO, HMO, and other), and self-reported smoking status (ever or never). Weight and height data recorded in the EHR were used to calculate body mass index (BMI) in kg/m2 and categorized as follows: underweight: <18.5, normal weight: ≥18.5 and <25, overweight: ≥25 and <30, and obese: ≥30). The prevalence of CVD was identified using physician recorded ICD-9 diagnosis codes (CHD 410–414, stroke 430–434, and peripheral vascular disease (PVD) 415, 440.2, 440.3, 443.9, 451, 453) during the study period. Type 2 diabetes was identified using ICD-9 codes (250.X0, 250.X2) or two abnormal glucose measurements according to American Diabetes Association guidelines (hemoglobin A1c results>6.5%, fasting blood glucose results >126 mg/dL, random blood glucose >200 mg/dL, or oral glucose tolerance test >200 mg/dL)28 or use of any anti-diabetic medications.
Statistical Methods
For our primary race/ethnic subgroup comparisons we calculated age-adjusted prevalence rates of dyslipidemia subtypes (high LDL-C, low HDL-C, and high TG) among men and women using direct standardization to the NHW population. Broad age categories (35–44, 45–54, 55–64, 65–74, 75+ (years) were used to achieve stratum-specific rates for direct standardization. Categorical variables (Table 1) were compared using pairwise chi-square tests, and non-parametric Wilcoxon tests were used to compare ages and lipid results between racial/ethnic groups that did not follow a normal distribution. Odds ratios were calculated using multivariable logistic regression adjusting for patient age, BMI, and smoking status. In secondary analyses we examined prevalence rates stratifying patients with and without a history of vascular disease. We also conducted additional analyses adjusting the model for prevalent type 2 diabetes or treatment with any LMAs. Results are presented by sex across all racial/ethnic minority groups, and all comparisons are to NHWs. Using logistic regression models with the product terms, we tested whether race/ethnicity is a significant effect modifier of the association between dyslipidemia subtypes and CVD after adjusting for common CVD risk factors. Because of the multiple comparisons included in this descriptive study, 99.9% confidence intervals are reported when appropriate. Statistical analyses were performed using SAS 9.3 (Cary, NC).
Table 1.
Patient Characteristics (N=169,430)
| Non-Hispanic Whites | Asian Indians | Chinese | Filipinos | Japanese | Koreans | Vietnamese | Mexicans | Black/ Africans | |
|---|---|---|---|---|---|---|---|---|---|
| Women (N=90,285) | |||||||||
| N | 58,117 | 6,808 | 12,895 | 3,300 | 2,284 | 1,114 | 1,401 | 2,795 | 1,571 |
| Age, y, mean (SD) | 52.9(12.9) | 44.5(10.2)* | 49.1(12.0)* | 50.5(12.2)* | 53.0(14.0) | 45.4(10.6)* | 46.7(10.3)* | 49.6(12.0)* | 51.8(12.0) |
| Measured BMI (mean, SD) | 26.7(6.1) | 25.7(4.4)* | 22.8(3.4)* | 26.0(4.6) | 23.8(4.6)* | 23.0(3.4)* | 22.8(3.5)* | 29.6(6.6)* | 31.0(7.3)* |
| Underweight (%) | 2 | 2 | 4* | 1 | 6* | 3* | 5* | 1* | 0* |
| Normal weight (%) | 41 | 45* | 69* | 45* | 56* | 67* | 68* | 24* | 20* |
| Overweight (%) | 26 | 32* | 16* | 32* | 19* | 16* | 16* | 30* | 28 |
| Obese (%) | 21 | 14* | 3* | 16* | 8* | 3* | 3* | 39* | 45* |
| Missing BMI (%) | 11 | 8* | 8* | 7* | 11 | 11 | 8* | 7* | 7* |
| Last available measure (median, IQR) | |||||||||
| LDL-C | 111(40) | 108(37)* | 105(38)* | 110(43) | 110(43) | 106(40.0)* | 109(39) | 111(39) | 112(42) |
| HDL-C | 61(22) | 51(16)* | 60(19)* | 58(18)* | 65(21)* | 59(18) | 59(18)* | 54(18)* | 58(21)* |
| TG | 89(64) | 97(69)* | 84(61)* | 100(73)* | 97(71)* | 87(56) | 93(60) | 109(78)* | 78(51)* |
| Total cholesterol | 195(48) | 182.0(44)* | 187(45)* | 193(48) | 198(48)* | 186(45)* | 192(45) | 191(49)* | 189(50)* |
| Type 2 diabetes (%) | 6 | 10* | 6 | 18* | 9* | 5 | 6 | 15* | 16* |
| Vascular Disease (%) (CHD, stroke, PVD) | 5 | 2* | 2* | 3 | 4 | 1* | 1* | 4 | 8* |
| Last available smoking status (%) | |||||||||
| Ever smoked | 23 | 2* | 3.0* | 13* | 14* | 11* | 2* | 18* | 26 |
| Never smoked | 64 | 89* | 87* | 80* | 73* | 76* | 87* | 74* | 67 |
| Missing | 14 | 10* | 10* | 7* | 13 | 13 | 11 | 8* | 7* |
| Primary Insurance (%) | |||||||||
| PPO | 71 | 73 | 69* | 61* | 70 | 75 | 68 | 56* | 60* |
| HMO | 27 | 23* | 29* | 37* | 30 | 23 | 31* | 41* | 36* |
| Other | 2 | 5* | 2 | 2 | 1* | 1 | 1 | 3 | 4* |
| Men (N=79,145) | |||||||||
| N | 50,571 | 9,728 | 10,310 | 2,088 | 1,264 | 835 | 1,098 | 2,055 | 1,196 |
| Age, y, mean (SD) | 50.5(11.4) | 43.3(9.0)* | 48.8(11.8)* | 49.3(11.2)* | 52.6(12.8)* | 43.9(10.1)* | 46.9(9.8)* | 48.1(11.1)* | 49.7(10.4) |
| Measured BMI (mean, SD) | 28.1(4.8) | 25.9(3.5)* | 25.2(3.3)* | 27.5(4.0)* | 26.6(4.1)* | 25.9(3.5)* | 25.0(3.1)* | 30.4(5.4)* | 30.1(5.5)* |
| Underweight (%) | 0 | 0* | 1* | 0 | 0 | 0 | 1* | 0 | 1 |
| Normal weight (%) | 22 | 39* | 47* | 23 | 33* | 37* | 48* | 10* | 12* |
| Overweight (%) | 41 | 42 | 37* | 49* | 41 | 44 | 37 | 40.0 | 39 |
| Obese (%) | 24 | 10* | 6* | 19* | 15* | 9* | 5* | 41* | 41* |
| Missing BMI (%) | 12 | 8* | 9* | 9* | 11 | 11 | 9* | 9* | 7* |
| Last available measure (median, IQR) | |||||||||
| LDL-C | 113(42) | 114(39) | 111(39)* | 112(45) | 112(44) | 113(36) | 119(42)* | 115(45) | 114(42) |
| HDL-C | 47(16) | 41(12)* | 47(14) | 46(14) | 50(17)* | 47(13) | 48(14) | 43(14)* | 47(16) |
| TG | 107(79) | 131(85)* | 114(80)* | 125(89)* | 118(83)* | 130(105)* | 125(90)* | 127(92)* | 88(63)* |
| Total cholesterol | 186(47) | 186(46) | 185(43) | 188(50) | 190(48)* | 189(40) | 196(46)* | 189(54) | 186(47) |
| Type 2 diabetes (%) | 8 | 13* | 8 | 23* | 16* | 9 | 9 | 18* | 16* |
| Vascular Disease (%)(CHD, stroke, PVD) | 6 | 3* | 4* | 7 | 7 | 3* | 4 | 6 | 7 |
| Last available smoking status (%) | |||||||||
| Ever smoked | 25 | 18* | 18* | 38* | 24 | 35* | 31* | 29* | 32* |
| Never smoked | 59 | 72* | 72* | 53* | 64 | 54* | 59 | 61 | 59 |
| Missing | 16 | 10* | 11* | 9* | 12* | 11* | 11* | 11* | 9* |
| Primary Insurance (%) | |||||||||
| PPO | 71 | 75* | 68* | 55* | 67 | 72 | 64* | 55* | 59* |
| HMO | 28 | 23* | 30* | 43* | 33* | 27 | 35* | 42* | 38* |
| Other | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 3* | 3 |
statistically significant (p<0.001) compared with Non-Hispanic Whites, by pairwise Chi-square test for categorical variables and pairwise Wilcoxon test for continuous variables. Percentages may not total 100% due to rounding.
BMI = body mass index; HDL-C = high density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; TG= triglycerides; CHD = coronary heart disease; PVD = peripheral vascular disease; PPO = preferred provider organization; HMO = health maintenance organization
RESULTS
169,430 patients were included in the analysis with patient characteristics reported in Table 1. Mexicans and every Asian subgroup except for Japanese were younger than NHWs. With the exception of Filipino women, all Asian subgroups had significantly lower mean BMI compared to NHW, whereas Mexicans and Black/Africans had higher mean BMI. Filipino, Korean, Vietnamese, and Black/African men were more likely to have ever smoked, and all Asian and Mexican women were less likely to have ever smoked, compared to NHW. Three Asian subgroups (Asian Indians, Filipinos, and Japanese) as well as Mexicans and Black/Africans had higher rates of type 2 diabetes compared to NHWs.
Age-adjusted prevalence rates of dyslipidemia subtypes and LDL-lowering treatment across racial/ethnic subgroups are described in Table 2. Filipino and Mexican women had the highest prevalence rates of both high LDL-C and high TG. With regards to low HDL-C, Asian Indian (54.9%)and Mexican (50.9%) women in particular stood out, having over twice the prevalence of Japanese women(23.7%). While Mexican women (45.4%) and nearly every Asian subgroup (except Korean women) had increased prevalence of high TG compared to NHW women (27.6%), Black/African women (18.2%) had the lowest prevalence. Treatment rates with LLAs were highest among Filipino, Mexican, and Black/African women, and lowest in Chinese women.
Table 2.
Age-Standardized Prevalence Rates(Percent) of Dyslipidemia and Treatment across Racial/Ethnic groups
| Dyslipidemia Subtypes | Non-Hispanic Whites | Asian Indians | Chinese | Filipinos | Japanese | Koreans | Vietnamese | Mexicans | Black/ Africans |
|---|---|---|---|---|---|---|---|---|---|
| Women (N=90,285) | |||||||||
| High LDL-C Ever | 52.6 (51.9, 53.3) | 54.8 (52.0, 57.6) | 45.8 (44.3, 47.4) | 63.0 (60.3, 65.8) | 54.2 (50.5, 57.9) | 51.7 (45.4, 58.0) | 56.1 (50.9, 61.4) | 56.8 (53.5, 60.1) | 57.2 (52.9, 61.5) |
| Low HDL-C ever | 30.7 (30.0, 31.5) | 54.9 (51.7, 58.0) | 31.8 (30.2, 33.4) | 37.3 (34.2, 40.3) | 23.7 (20.2, 27.1) | 34.6 (27.5, 41.6) | 37.2 (31.7, 42.8) | 50.9 (47.4, 54.5) | 39.8 (35.3, 44.3) |
| High TG Ever | 27.6 (26.9, 28.3) | 37.4 (34.3, 40.5) | 29.9 (28.4, 31.5) | 41.5 (38.5, 44.6) | 36.0 (32.2, 39.8) | 29.8 (23.1, 36.4) | 39.0 (33.5, 44.5) | 45.4 (42.0, 48.9) | 18.2 (14.7, 21.7) |
| Ever Treated by LLAs | 18.7 (18.2, 19.2) | 20.9 (18.6, 23.1) | 15.3 (14.2, 16.4) | 30.3 (27.8, 32.8) | 19.5 (16.9, 22.0) | 17.9 (12.9, 22.9) | 20.7 (16.4, 25.1) | 24.7 (22.0, 27.4) | 25.2 (21.9, 28.6) |
| Men (N=79,145) | |||||||||
| High LDL-C Ever | 62.2 (61.4, 62.9) | 65.9 (63.7, 68.0) | 55.3 (53.6, 57.0) | 73.1 (69.8, 76.4) | 65.4 (60.7, 70.2) | 55.4 (47.4, 63.3) | 71.3 (66.3, 76.3) | 66.0 (62.2, 69.7) | 63.1 (58.2, 68.0) |
| Low HDL-C ever | 35.7 (34.9, 36.5) | 52.7 (50.3, 55.2) | 34.0 (32.3, 35.7) | 37.1 (33.3, 40.8) | 26.3 (21.8, 30.7) | 28.2 (21.2, 35.3) | 33.6 (28.2, 39.0) | 47.8 (43.7, 51.9) | 34.3 (29.4, 39.2) |
| High TG Ever | 42.5 (41.7, 43.3) | 55.3 (52.9, 57.7) | 48.7 (46.9, 50.5) | 60.3 (56.6, 64.1) | 52.5 (47.5, 57.6) | 52.8 (44.7, 60.9) | 55.6 (50.0, 61.3) | 55.9 (51.9, 60.0) | 29.5 (24.8, 34.2) |
| Ever Treated by LLAs | 26.2 (25.6, 26.8) | 32.9 (30.9, 35.0) | 21.5 (20.1, 22.8) | 39.0 (35.6, 42.3) | 28.4 (24.5, 32.3) | 23.2 (16.7, 29.7) | 30.3 (25.6, 35.1) | 30.5 (27.2, 33.8) | 31.0 (26.9, 35.2) |
Data are percent with 99.9% CI
HDL-C = high density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; TG= triglycerides; LLAs=LDL-lowering agents.
The prevalence rates for all three dyslipidemia subtypes were generally higher for men compared to women. Filipino (73.1%), and Vietnamese (71.3%) men had the highest rates of high LDL-C, whereas Chinese men (55.3%) had the lowest rates. Asian Indian (52.7%) and Mexican (47.8%) men had the highest prevalence rates of low HDL-C whereas Japanese men (26.3%) had lowest. Similar to women, every Asian subgroup and Mexican men had increased prevalence rates of hypertriglyceridemia compared to NHW men, while Black/African men (29.5%) had the lowest. Also similar to women, Chinese men had the lowest treatment rates with LDL-lowering medication.
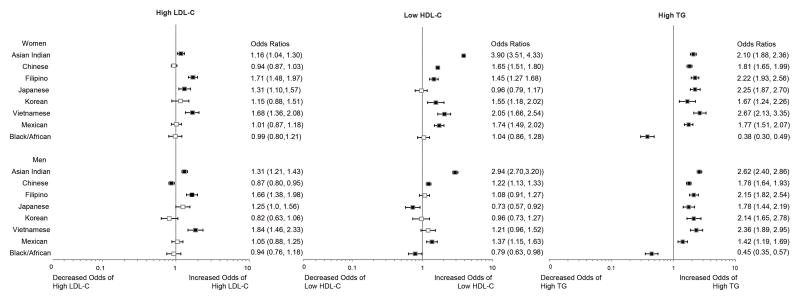
Adjusted odds ratios by race/ethnicity and sex were estimated from the multivariable logistic regression models as described previously and reported for each dyslipidemia subtype (Figure 1). Older (age >55 years) and overweight women were more likely to have combined dyslipidemias. Having ever smoked was associated with increased odds of having high LDL-C and high TG. After adjustment for patient characteristics, racial/ethnic differences among women were more pronounced for high LDL-C and low HDL-C, primarily because Asian women were younger and had lower rates of smoking and obesity. Asian Indian, Filipino and Vietnamese women had higher risk of possessing all three dyslipidemia subtypes. Mexican women and every female Asian subgroup (except Japanese women) had increased risk of having a combined dyslipidemia characterized by high TG and low HDL-C.
Figure 1.
Prevalence Odds Ratios for Racial/Ethnic Differences in Dyslipidemia Subtypes (N=169,430)
LDL-C=low-density lipoprotein cholesterol; HDL-C=high-density lipoprotein cholesterol; TG=triglycerides; Multivariable-adjusted model included age, body-mass index (BMI), primary insurance, and smoking status. The square indicates the point estimate and the bar represents the 99.9% CI. Filled squares indicate statistical significance compared with Non-Hispanic Whites at p<0.001.
Similar to women, overweight men or men who had ever smoked had higher odds of having multiple dyslipidemia subtypes. For men, older age (>55 years) and HMO insurance (versus PPO insurance) were significantly associated with greater odds of high LDL-C. Multivariable adjustment did not attenuate racial/ethnic differences in dyslipidemia for men. Odds ratios for each dyslipidemia subtype were generally similar for men compared to women; however, racial/ethnic differences for low HDL-C were smaller. Asian Indian men had higher odds of possessing all three dyslipidemia subtypes, compared to NHWs. Every Asian subgroup and Mexican men had increased risk for high TG while Black/African men had lower risk compared to NHWs.
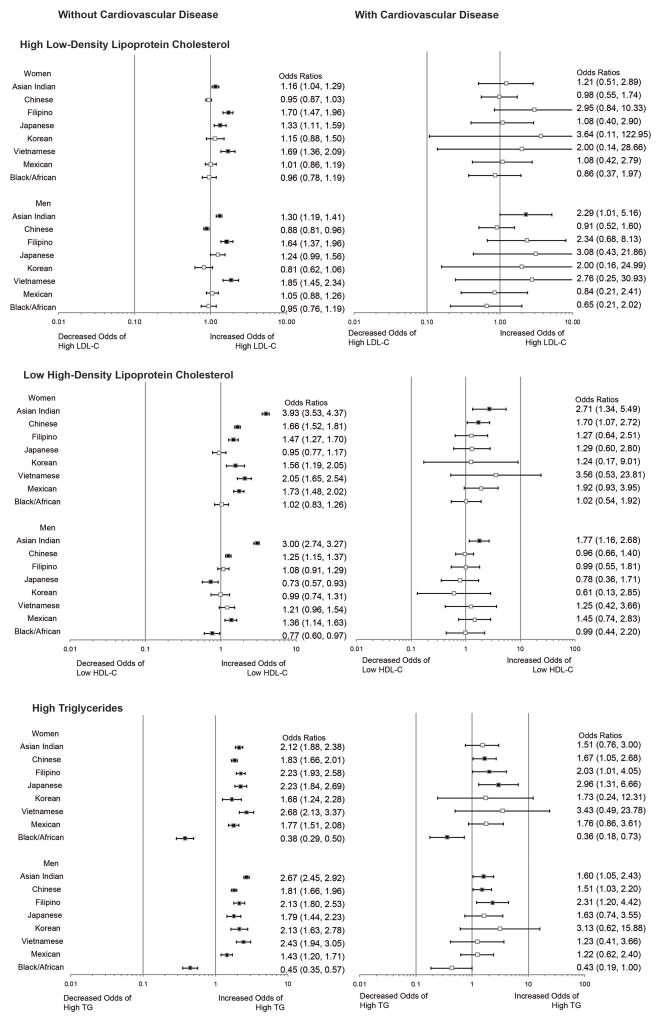
Table 3 and Figure 2 summarize racial/ethnic differences in dyslipidemia prevalence in patients with and without a history of CVD. The overall prevalence of CVD within the cohort was approximately 5%. The low number of patients with CVD made it difficult to compare dyslipidemia profiles among race/ethnic groups in those with CVD due to wide confidence intervals; only slight attenuation in prevalence odds ratios was seen in those without a history of CVD compared to the original cohort. Despite the low overall prevalence of CVD, some significant racial/ethnic differences in dyslipidemia profiles were still seen among these patients. Asian Indian men continued to have higher odds of having all three dyslipidemia subtypes, and were the only group male or female to have increased odds of high LDL-C compared to NHW men. Asian Indian women and men as well as Chinese women had greater odds of having low HDL-C compared to NHW women and men. Finally, Chinese and Filipino men and women, Japanese women, and Asian Indian men had greater odds of having high TG compared to NHWs, while African American women were the only group to have significantly lower odds of high TG. We tested for formal interaction between each dyslipidemia subtype and race/ethnicity on prevalent CVD and found no significant interaction (p values of the product terms in the model are 0.11, 0.18 and 0.36 for high LDL-C, low HDL-C and high TG, respectively).
Table 3.
Age-Standardized Prevalence Rates (Percent) of Dyslipidemia and Treatment stratified by Cardiovascular Disease (CHD, stroke, PVD)
| Outcomes | Non-Hispanic Whites | Asian Indians | Chinese | Filipinos | Japanese | Koreans | Vietnamese | Mexicans | Black/ Africans |
|---|---|---|---|---|---|---|---|---|---|
| History of Cardiovascular Disease (N=8,016) | |||||||||
| Women | |||||||||
| High LDL-C Ever | 83.6 (81.1, 86.0) | 85.0 (73.3, 96.8) | 79.6 (71.7, 87.4) | 92.7 (84.2, 100.0) | 81.0 (68.0, 94.0) | 98.2 (93.0, 100.0) | 88.0 (--, --) | 86.3 (76.1, 96.6) | 84.2 (73.6, 94.8) |
| Low HDL-C ever | 44.2 (40.6, 47.7) | 60.8 (43.4, 78.3) | 47.9 (37.6, 58.2) | 44.6 (28.4, 60.8) | 49.0 (31.2, 66.8) | 44.6 (0.0, 92.4) | 70.2 (34.1, 100.0) | 64.6 (48.7, 80.5) | 47.3 (31.7, 62.9) |
| High TG Ever | 46.5 (42.9, 50.0) | 51.9 (34.0, 69.9) | 48.8 (38.4, 59.2) | 55.2 (38.8, 71.6) | 68.3 (51.6, 84.9) | 62.4 (14.6, 100.0) | 58.6 (10.7, 100.0) | 63.8 (47.8, 79.9) | 28.9 (14.5, 43.3) |
| Ever Treated by LLAs | 64.9 (61.9, 67.8) | 73.2 (59.1, 87.4) | 62.8 (53.7, 71.9) | 83.8 (71.4, 96.2) | 70.6 (55.6, 85.7) | 87.4 (68.2, 100.0) | 76.4 (50.1,100.0) | 72.8 (59.4, 86.2) | 71.5 (58.0, 84.9) |
| Men | |||||||||
| High LDL-C Ever | 90.3 (88.6, 92.0) | 94.8 (90.4, 99.1) | 87.9 (82.6, 93.3) | 96.8 (92.5, 100.0) | 96.1 (89.0, 100.0) | 98.6 (95.8, 100.0) | 95.5 (85.2, 100.0) | 89.4 (79.6,99.2) | 89.7 (80.6,98.8) |
| Low HDL-C ever | 48.4 (45.3, 51.4) | 56.7 (46.6, 66.7) | 42.5 (34.0, 51.1) | 46.8 (30.8, 62.9) | 40.0 (21.4, 58.5) | 41.1 (16.7, 65.5) | 46.5 (20.1,72.9) | 61.1 (45.3, 76.9) | 49.0 (28.1, 69.9) |
| High TG Ever | 49.6 (46.6, 52.6) | 53.3 (43.3, 63.4) | 52.9 (44.4, 61.4) | 65.9 (50.5, 81.3) | 58.7 (40.8,76.7) | 76.9 (57.0, 96.7) | 45.0 (19.5,70.5) | 58.6 (42.8, 74.5) | 33.0 (12.7, 53.3) |
| Ever Treated by LLAs | 79.4 (77.1, 81.7) | 89.3 (83.3, 95.2) | 80.7 (74.3, 87.1) | 93.8 (87.8, 99.8) | 89.2 (77.5,100.0) | 97.6 (94.2, 100.0) | 79.8 (60.6, 99.0) | 78.3 (65.9, 90.8) | 82.6 (71.7, 93.6) |
|
| |||||||||
| No History of Cardiovascular Disease (N=161,414) | |||||||||
| Women | |||||||||
| High LDL-C Ever | 50.9 (50.1,51.6) | 53.1 (50.2, 56.0) | 44.2 (42.6, 45.8) | 61.4 (58.5, 64.2) | 52.7 (48.9, 56.5) | 49.5 (43.0,55.9) | 54.7 (49.5,60.0) | 54.8 (51.3, 58.3) | 54.9 (50.3, 59.5) |
| Low HDL-C ever | 30.0 (29.2,30.8) | 54.5 (51.3, 57.7) | 31.1 (29.5, 32.7) | 36.8 (33.7, 40.0) | 22.7 (19.2, 26.1) | 33.8 (26.9,40.7) | 36.3 (30.9,41.7) | 49.8 (46.1,53.6) | 38.6 (33.9,43.3) |
| High TG Ever | 26.6 (25.9,27.3) | 36.6 (33.5, 39.7) | 29.0 (27.5, 30.6) | 40.7 (37.6, 43.9) | 34.6 (30.7, 38.4) | 28.3 (21.8,34.8) | 37.9 (32.5,43.2) | 44.3 (40.7, 48.0) | 17.5 (13.8, 21.2) |
| Ever Treated by LLAs | 16.5 (16.0, 17.0) | 18.4 (16.2, 20.6) | 13.4 (12.4, 14.5) | 27.9 (25.4, 30.3) | 17.4 (14.9, 19.9) | 15.4 (10.8, 20.1) | 18.7 (14.5, 22.9) | 22.0 (19.2, 24.8) | 21.6 (18.1, 25.0) |
| Men | |||||||||
| High LDL-C Ever | 59.9 (59.1, 60.7) | 63.0 (60.7, 65.3) | 53.4 (51.6, 55.2) | 71.0 (67.5, 74.6) | 63.4 (58.4, 68.4) | 52.8 (44.6, 61.0) | 69.4 (64.2, 74.7) | 63.8 (59.7, 67.8) | 61.0 (55.8, 66.2) |
| Low HDL-C ever | 34.7 (33.9, 35.5) | 52.1 (49.6, 54.6) | 33.7 (32.0, 35.4) | 36.2 (32.3, 40.1) | 25.6 (21.0, 30.2) | 28.1 (20.9, 35.2) | 32.7 (27.3, 38.2) | 46.9 (42.6, 51.3) | 33.0 (28.0, 38.1) |
| High TG Ever | 42.0 (41.1, 42.8) | 55.3 (52.8, 57.8) | 48.6 (46.8, 50.4) | 59.8 (55.9, 63.7) | 52.3 (47.0, 57.5) | 52.3 (44.1,60.6) | 56.7 (51.0, 62.4) | 56.0 (51.7, 60.3) | 29.2 (24.3, 34.1) |
| Ever Treated by LLAs | 22.6 (22.0, 23.2) | 28.4 (26.3, 30.4) | 18.5 (17.2, 19.8) | 34.7 (31.3, 38.1) | 24.8 (20.9, 28.7) | 19.6 (13.3, 25.9) | 27.0 (22.2,31.7) | 27.0 (23.5, 30.4) | 27.3 (23.0, 31.5) |
Data are percent with 99% CI; --small sample size
CHD = Coronary Heart Disease, PVD = Peripheral Vascular Disease, HDL-C = high density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; TG= triglycerides; LLAs=LDL-lowering agents.
Figure 2.
Prevalence Odds Ratios for Racial/Ethnic Differences in Dyslipidemia Subtypes Stratified by Cardiovascular Disease
We conducted additional analyses (see supplemental appendix) to examine prevalence odds ratios for dyslipidemia subtypes after adjusting the multivariable model for: 1)prevalent type 2 diabetes and 2) treatment with lipid modifying (LMAs) and LDL-lowering (LLAs) agents (supplemental figure 1 and 2). Type 2 diabetes was positively associated with all three dyslipidemia subtypes with only slight attenuation of the observed racial/ethnic differences. Similarly, adjusting for treatment status with any lipid lowering medication did not significantly change observed racial/ethnic differences in either low HDL-C or high TG. However, there were notable differences in the prevalence odd ratios for high LDL-C by race/ethnicity. Finally, we also examined the prevalence of isolated HDL-C, and in men only found significantly higher prevalence odds ratios for Asian Indians (OR 1.72, CI 1.50–1.98) compared with NHWs (Figure 3 of supplemental appendix). For women, higher prevalence odds ratios for isolated HDL-C were found for Asian Indians, Chinese, and Vietnamese as well as Mexicans and Black/African-American women.
DISCUSSION
To our knowledge, this is the first study to examine dyslipidemia prevalence across all major racial/ethnic subgroups in the United States. Our findings indicate that there are marked differences in the prevalence of dyslipidemia subtypes among racial/ethnic subgroups. Most of the minority racial/ethnic subgroups studied, particularly women, had increased prevalence of low HDL-C and elevated TG. Chinese men had lower odds of having high LDL-C, Japanese men had lower odds of having low HDL-C, and Black/African Americans had lower odds of having either low HDL-C or high TG. Asian Indian, Filipino and Vietnamese women and Asian Indian men stood out as the groups with the highest risk for possessing all three dyslipidemia subtypes as compared to NHWs.
Our results are similar to previous studies of dyslipidemia among minority groups, which have shown lower prevalence rates for any dyslipidemia subtype in Black/African Americans3,29 and higher rates of low HDL-C and high TG in Mexican Americans.3,21 Our analysis showed increased prevalence of dyslipidemia subtypes among Mexican Americans compared to national data, likely due to the inclusion of LDL-lowering medications in our definition of high LDL-C as well as the older age of our patient cohort (inclusion criteria was 35 years and older compared to 20 years in NHANES).
Few studies have examined the prevalence of dyslipidemia subtypes among Asian American subgroups, with most of those focusing on only one subgroup. Previous studies have found dyslipidemia patterns similar to ours: lower levels of LDL-C for Chinese,16,18 low HDL-C for Asian Indians,16,19 and higher levels of HDL-C for Japanese.16 A large international meta-analysis conducted by Huxley et al. demonstrated a higher prevalence of isolated low HDL-C in Asians, which was associated with increased risk of coronary heart disease when compared to non-Asians.13 The global case-control INTERHEART study found that there was a higher proportion of Asian cases and controls with LDL-C levels <100 mg/dl compared to non-Asians.16
Our cross-sectional analysis found an increased prevalence of high LDL-C for Asian Indians, Filipinos and Vietnamese, and an increased prevalence of high TG for all Asian American subgroups. These findings reflect important and clinically relevant differences in diet and physical activity for Asians living in the U.S. as a result of immigration and acculturation.30 The Ni-Hon-San study in Japanese demonstrated the effects of immigration and acculturation, finding that CHD and stroke mortality rates in Hawaii were intermediate between higher rates of stroke in Japan and higher rates of CHD in California.31 The Ni-Hon-San study highlights the importance of studying Asian Americans specifically, since risk factor and disease prevalence may differ from those in the native Asian populations. When compared with international data these findings demonstrate that triglyceride levels are more sensitive and susceptible to environmental influences associated with immigration and acculturation (i.e. changes in diet, decreased physical activity, weight gain) than HDL-C. Chandalia et al. found that HDL-C levels were uniformly lower in Asian Indian women living in both the U.S. and India, but TG levels were higher only for those living in the U.S.19 Our research is congruent with previous evidence32 on the relative genetic and environmental contributions to triglyceride and HDL-C levels, and extends this observation to immigrant Asian populations in the U.S. Better understanding of these contributions may provide better insight in how to direct and improve prevention efforts in minority groups.
The variation in dyslipidemia prevalence among minority groups appears to correlate with the rates of CHD. Among Asian Americans, Asian Indians and Filipinos have consistently been shown to have increased risk for CHD5,6 with higher mortality rates than other racial/ethnic groups in the U.S.33,34 In contrast, CHD incidence and mortality rates are lower for Chinese and Japanese,35,36 which may be explained in part by the protective effects of having low LDL-C and high HDL-C in these subgroups.16 Similar to Asian Americans, Mexican Americans who exhibit combined dyslipidemia, particularly the atherogenic high TG/low HDL profile, appear to have increased CHD risk. The Hispanic Community Health Study/Study of Latinos found increased CHD risk (self-reported) among Hispanic/Latino participants with hypercholesterolemia.21 The INTERHEART study also found abnormal lipids to be strongly associated with increased risk of acute myocardial infarction in subjects from Latin America.37 However, for African Americans who have relatively benign dyslipidemia patterns (low LDL-C, high HDL-C, low TGs), higher prevalence rates of hypertension, diabetes, and obesity may play a greater role in explaining CHD risk.3,21 Rates of hypertension for African Americans are almost twice as high, compared to NHWs,38 and blood pressure was shown to be more predictive of CHD in African American women than NHW women.39
LDL-C has been the primary target of CVD prevention and treatment with statins being the most widely prescribed cholesterol-lowering medications in the U.S.10,40 The national prevalence of lipid-lowering medication for all adults is 15.5% (2007–2010 NHANES),27 which is slightly lower than we found, most likely due to the older age of our cohort. Contrary to national findings, however, treatment rates were higher for the majority of minority groups, which may be a reflection of increased access to care for this insured population. The higher prevalence of elevated LDL-C seen among Asian Indians, Filipinos and Vietnamese may be an especially important target for CVD prevention in Asian Americans.
Asian and Mexican Americans, but not African Americans appear to possess the high TG/low HDL-C dyslipidemia pattern, which has been characterized as atherogenic and associated with insulin resistance/metabolic syndrome.41,42 There is currently a lack of specific treatment options for low HDL-C and elevated TG due to a lack of efficacy data. Lipid medications that specifically target TGs and HDL-C, such as fibrates and niacin, have demonstrated a reduction of CVD events in some trials but not in many others.43–47 Guidelines for dyslipidemia management and treatment have largely been derived from clinical trials comprised of mostly NHW populations, which have often under-represented minority groups. Future clinical trials should include minority subgroups to further examine the potential benefits of HDL-C and TG treatment for these groups specifically.
Study limitations include using data from a single geographic area (Northern California) with smaller sample sizes in the African American, Mexican, Korean and Vietnamese populations. However, this region has the most diversity in minority subgroups in the U.S. The study population is also insured and under-representative of the medically underserved, but these geographic and socioeconomic limitations also minimize unmeasured confounding between subgroups. Because clinical and administrative records rarely include any socioeconomic data on the individual level, this relative homogeneity in economic status improves the internal validity of our comparisons. We adjusted the significance level to p<0.001 to account for multiple comparisons. We were not able to include lifestyle information, such as physical activity or dietary habits for comparison between minority groups. As a cross-sectional analysis, we were unable to examine the temporal relationships between dyslipidemias and development of comorbidities such as cardiovascular disease. Finally, we could not account for patients who during the study period may have been started on LLAs for reasons other than high LDL (CVD, stroke, peripheral vascular disease, etc.), which may have overestimated prevalence rates. Our findings regarding high LDL-C must be interpreted with caution due to the inclusion of LLAs in our definition of the high LDL-C subtype. When stratified by treatment (supplemental figure 2), there are differences in the prevalence odds ratios by race/ethnicity for having high LDL-C lab test that are difficult to explain given our cross-sectional design. Unlike LDL-C, the racial/ethnic differences in prevalence odds ratios for having low HDL-C or high TG are consistent with and without medication adjustment, and therefore may offer unique opportunities for improving prevention efforts and further research in minority populations.
CONCLUSION
Most minority subgroups have higher prevalence rates of dyslipidemia than do NHWs. Asian Indian, Filipino, Vietnamese women, and Asian Indian men stood out as Asian subgroups with increased risk of having combined dyslipidemias (high LDL-C, low HDL-C, and high TG). While variation was seen among minority groups for the high LDL-C and low HDL-C dyslipidemia subtypes, every minority group except African Americans had high triglycerides when compared to NHWs. Further research is needed to determine the role of dyslipidemia subtypes and other risk factors in explaining the higher risk of CVD for Asian Indians, Filipinos, Hispanics, and Black/African Americans. Additionally, clinical trials should recruit and include participants belonging to minority subgroups in order to increase our understanding of dyslipidemia in CVD risk, as well as treatment goals and medication efficacy for these groups.
Supplementary Material
Acknowledgments
This study was supported by grant from the National Institutes of Diabetes, Digestive and Kidney Diseases (1 R01 DK081371–01A1 Identifying Disparities in Type 2 Diabetes Among Asian Americans: The Pan Asian Cohort Study).
Footnotes
CONFLICTS OF INTEREST
All authors declare they have no conflicts of interest.
BIBLIOGRAPHY AND REFERENCES CITED
- 1.Humes KR, Jones NA, Ramirez RR. Overview of Race and Hispanic Origin: 2010. Washington, D.C: U.S. Census Bureau; 2011. [Google Scholar]
- 2.U.S. Census Bureau. Table 4. Projections of the Population by Sex, Race, and Hispanic Origin for the United States: 2015 to 2060. Washington, D.C: U.S. Census Bureau; 2012. [Google Scholar]
- 3.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillum RF, Mehari A, Curry B, Obisesan TO. Racial and geographic variation in coronary heart disease mortality trends. BMC Public Health. 2012;12:410. doi: 10.1186/1471-2458-12-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klatsky AL, Tekawa I. Health problems and hospitalizations among Asian-American ethnic groups. Ethn Dis. 2005;15:753–760. [PubMed] [Google Scholar]
- 6.Holland AT, Wong EC, Lauderdale DS, Palaniappan LP. Spectrum of cardiovascular diseases in Asian-American racial/ethnic subgroups. Ann Epidemiol. 2011;21:608–614. doi: 10.1016/j.annepidem.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgenstern LB, Smith MA, Lisabeth LD, Risser JM, Uchino K, Garcia N, Longwell PJ, McFarling DA, Akuwumi O, Al-Wabil A, Al-Senani F, Brown DL, Moye LA. Excess stroke in Mexican Americans compared with non-Hispanic Whites: the Brain Attack Surveillance in Corpus Christi Project. Am J Epidemiol. 2004;160:376–383. doi: 10.1093/aje/kwh225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White H, Boden-Albala B, Wang C, Elkind MS, Rundek T, Wright CB, Sacco RL. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2005;111:1327–1331. doi: 10.1161/01.CIR.0000157736.19739.D0. [DOI] [PubMed] [Google Scholar]
- 9.Cohen JD, Cziraky MJ, Cai Q, Wallace A, Wasser T, Crouse JR, Jacobson TA. 30-year trends in serum lipids among United States adults: results from the National Health and Nutrition Examination Surveys II, III, and 1999–2006. Am J Cardiol. 2010;106:969–975. doi: 10.1016/j.amjcard.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 10.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention NCfHS. [Accessed April 11, 2013];National Health and Examination Survey: Note on 2007–2010 Sampling Methodology. 2011 http://www.cdc.gov/nchs/nhanes/nhanes2007-2008/sampling_0708.htm.
- 12.Holland AT, Palaniappan LP. Problems with the collection and interpretation of Asian-American health data: omission, aggregation, and extrapolation. Ann Epidemiol. 2012;22:397–405. doi: 10.1016/j.annepidem.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huxley RR, Barzi F, Lam TH, Czernichow S, Fang X, Welborn T, Shaw J, Ueshima H, Zimmet P, Jee SH, Patel JV, Caterson I, Perkovic V, Woodward M. Isolated low levels of high-density lipoprotein cholesterol are associated with an increased risk of coronary heart disease: an individual participant data meta-analysis of 23 studies in the Asia-Pacific region. Circulation. 2011;124(19):2056–64. doi: 10.1161/CIRCULATIONAHA.111.028373. [DOI] [PubMed] [Google Scholar]
- 14.Rutherford JN, McDade TW, Feranil AB, Adair LS, Kuzawa CW. High prevalence of low HDL-c in the Philippines compared to the US: population differences in associations with diet and BMI. Asia Pac J Clin Nutr. 2010;19:57–67. [PMC free article] [PubMed] [Google Scholar]
- 15.Misra A, Khurana L. Obesity-related non-communicable diseases: South Asians vs White Caucasians. Int J Obes (Lond) 2011;35:167–187. doi: 10.1038/ijo.2010.135. [DOI] [PubMed] [Google Scholar]
- 16.Karthikeyan G, Teo KK, Islam S, McQueen MJ, Pais P, Wang X, Sato H, Lang CC, Sitthi-Amorn C, Pandey MR, Kazmi K, Sanderson JE, Yusuf S. Lipid profile, plasma apolipoproteins, and risk of a first myocardial infarction among Asians: an analysis from the INTERHEART Study. J Am Coll Cardiol. 2009;53:244–253. doi: 10.1016/j.jacc.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 17.Truesdale KP, Stevens J, Cai J. Impact of body mass index levels on lipid abnormalities in Chinese Asians, American Blacks and American Whites: the People’s Republic of China (PRC) and Atherosclerosis Risk in Communities (ARIC) Studies. Atherosclerosis. 2011;218:517–523. doi: 10.1016/j.atherosclerosis.2011.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goff DC, Jr, Bertoni AG, Kramer H, Bonds D, Blumenthal RS, Tsai MY, Psaty BM. Dyslipidemia prevalence, treatment, and control in the Multi-Ethnic Study of Atherosclerosis (MESA): gender, ethnicity, and coronary artery calcium. Circulation. 2006;113:647–656. doi: 10.1161/CIRCULATIONAHA.105.552737. [DOI] [PubMed] [Google Scholar]
- 19.Chandalia M, Mohan V, Adams-Huet B, Deepa R, Abate N. Ethnic difference in sex gap in high-density lipoprotein cholesterol between Asian Indians and Whites. J Investig Med. 2008;56:574–580. doi: 10.2310/JIM.0b013e31816716fd. [DOI] [PubMed] [Google Scholar]
- 20.Derby CA, Wildman RP, McGinn AP, Green RR, Polotsky AJ, Ram KT, Barnhart J, Weiss G, Santoro N. Cardiovascular risk factor variation within a Hispanic cohort: SWAN, the Study of Women’s Health Across the Nation. Ethn Dis. 2010;20:396–402. [PMC free article] [PubMed] [Google Scholar]
- 21.Daviglus ML, Talavera GA, Aviles-Santa ML, Allison M, Cai J, Criqui MH, Gellman M, Giachello AL, Gouskova N, Kaplan RC, LaVange L, Penedo F, Perreira K, Pirzada A, Schneiderman N, Wassertheil-Smoller S, Sorlie PD, Stamler J. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. JAMA. 2012;308:1775–1784. doi: 10.1001/jama.2012.14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mensah GA. Eliminating disparities in cardiovascular health: six strategic imperatives and a framework for action. Circulation. 2005;111:1332–1336. doi: 10.1161/01.CIR.0000158134.24860.91. [DOI] [PubMed] [Google Scholar]
- 23.U.S. Department of Health and Human Services. HHS Action Plan to Reduce Racial and Ethnic Disparities: A Nation Free of Disparities in Health and Health Care. Washington, D.C: U.S. Department of Health and Human Services; 2011. [Google Scholar]
- 24.Institute of Medicine. Unequal Treatment:Confronting Racial and Ethnic Disparities in Health Care (with CD) Washington, D.C: The National Academies Press; 2003. [PubMed] [Google Scholar]
- 25.Palaniappan LP, Wong EC, Shin JJ, Moreno MR, Otero-Sabogal R. Collecting patient race/ethnicity and primary language data in ambulatory care settings: a case study in methodology. Health Serv Res. 2009;44:1750–1761. doi: 10.1111/j.1475-6773.2009.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong EC, Palaniappan LP, Lauderdale DS. Using Name Lists to Infer Asian Racial/Ethnic Subgroups in the Healthcare Setting. Med Care. 2010;48:540–546. doi: 10.1097/MLR.0b013e3181d559e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carroll MD, Kit BK, Lacher DA, Shero ST, Mussolino ME. Trends in lipids and lipoproteins in US adults, 1988–2010. JAMA. 2012;308:1545–1554. doi: 10.1001/jama.2012.13260. [DOI] [PubMed] [Google Scholar]
- 28.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35:S64–S71. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hyre AD, Muntner P, Menke A, Raggi P, He J. Trends in ATP-III-defined high blood cholesterol prevalence, awareness, treatment and control among U.S. adults. Ann Epidemiol. 2007;17:548–555. doi: 10.1016/j.annepidem.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 30.Palaniappan LP, Araneta MR, Assimes TL, Barrett-Connor EL, Carnethon MR, Criqui MH, Fung GL, Narayan KM, Patel H, Taylor-Piliae RE, Wilson PW, Wong ND. Call to action: cardiovascular disease in Asian Americans: a science advisory from the American Heart Association. Circulation. 2010;122:1242–1252. doi: 10.1161/CIR.0b013e3181f22af4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benfante R. Studies of cardiovascular disease and cause-specific mortality trends in Japanese-American men living in Hawaii and risk factor comparisons with other Japanese populations in the Pacific region: a review. Hum Biol. 1992;64:791–805. [PubMed] [Google Scholar]
- 32.Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM, Lennie TA, Levi M, Mazzone T, Pennathur S. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123(20):2292–333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- 33.Bainey KR, Jugdutt BI. Increased burden of coronary artery disease in South-Asians living in North America. Need for an aggressive management algorithm. Atherosclerosis. 2009;204:1–10. doi: 10.1016/j.atherosclerosis.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 34.Dodani S. Excess coronary artery disease risk in South Asian immigrants: can dysfunctional high-density lipoprotein explain increased risk? Vasc Health Risk Manag. 2008;4:953–961. doi: 10.2147/vhrm.s2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palaniappan L, Wang Y, Fortmann SP. Coronary heart disease mortality for six ethnic groups in California, 1990–2000. Ann Epidemiol. 2004;14:499–506. doi: 10.1016/j.annepidem.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Wild SH, Laws A, Fortmann SP, Varady AN, Byrne CD. Mortality from coronary heart disease and stroke for six ethnic groups in California, 1985 to 1990. Ann Epidemiol. 1995;5:432–439. doi: 10.1016/1047-2797(95)00058-5. [DOI] [PubMed] [Google Scholar]
- 37.Lanas F, Avezum A, Bautista LE, Diaz R, Luna M, Islam S, Yusuf S INTERHEART Investigators in Latin America. Risk factors for acute myocardial infarction in Latin America: the INTERHEART Latin American study. Circulation. 2007;115(9):1067–74. doi: 10.1161/CIRCULATIONAHA.106.633552. [DOI] [PubMed] [Google Scholar]
- 38.Hertz RP, Unger AN, Cornell JA, Saunders E. Racial disparities in hypertension prevalence, awareness, and management. Arch Intern Med. 2005;165:2098–2104. doi: 10.1001/archinte.165.18.2098. [DOI] [PubMed] [Google Scholar]
- 39.Jones DW, Chambless LE, Folsom AR, Heiss G, Hutchinson RG, Sharrett AR, Szklo M, Taylor HA., Jr Risk factors for coronary heart disease in African Americans: the atherosclerosis risk in communities study, 1987–1997. Arch Intern Med. 2002;162:2565–2571. doi: 10.1001/archinte.162.22.2565. [DOI] [PubMed] [Google Scholar]
- 40.Ma J, Sehgal NL, Ayanian JZ, Stafford RS. National trends in statin use by coronary heart disease risk category. PLoS Med. 2005;2:e123. doi: 10.1371/journal.pmed.0020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Festa A, D’Agostino R, Jr, Mykkanen L, Tracy R, Howard BV, Haffner SM. Low-density lipoprotein particle size is inversely related to plasminogen activator inhibitor-1 levels. The Insulin Resistance Atherosclerosis Study. Arterioscler Thromb Vasc Biol. 1999;19:605–610. doi: 10.1161/01.atv.19.3.605. [DOI] [PubMed] [Google Scholar]
- 42.Biswas S, Ghoshal PK, Mandal SC, Mandal N. Association of low-density lipoprotein particle size and ratio of different lipoproteins and apolipoproteins with coronary heart disease. J Cardiol. 2008;52:118–126. doi: 10.1016/j.jjcc.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 43.Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, Faas FH, Linares E, Schaefer EJ, Schectman G, Wilt TJ, Wittes J. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999;341:410–418. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 44.Coronary Drug Project Collaborators. Clofibrate and niacin in coronary heart disease. JAMA. 1975;231:360–381. [PubMed] [Google Scholar]
- 45.Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, Forder P, Pillai A, Davis T, Glasziou P, Drury P, Kesaniemi YA, Sullivan D, Hunt D, Colman P, d’Emden M, Whiting M, Ehnholm C, Laakso M. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 46.Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 47.HPS2-THRIVE Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J. 2013;34(17):1279–91. doi: 10.1093/eurheartj/eht055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.