Abstract
Many studies have pointed to vulnerability to stress and stress-related pathologies at different timepoints during an individual’s lifespan. These sensitive windows are usually during periods of neural development, such as embryogenesis, infancy, and adolescence. It is critical to understand how neural circuitry may change as an individual ages in ways that could affect susceptibility to stress. Here we compare two stages in Drosophila melanogaster: sexual immaturity and sexual maturity. We used the genetic resources available in Drosophila to manipulate pre- and post-synaptic dopamine signaling in sexually immature and mature animals that were then assayed for heart rate and locomotor behavior in response to starvation and oxidative stress. Our results show significant differences in the stress response for sexually immature and mature animals for heart rate, periods of high mobility, mean velocity, and several other parameters of locomotor behavior. Our data show that dopamine neurons are differentially recruited into the stress response circuitry for sexually immature and mature individuals. By observing behaviors that have been previously shown in mammalian models to be affected by stress and altered in models of affective disorders, we provide a genetically tractable model for development and maintenance of the stress response circuitry during sexual maturation.
Keywords: age, behavior, starvation, paraquat, locomotion, heart rate, oxidative stress
Introduction
Several factors influence an individual’s stress response. In the companion paper, we illustrated the importance of sex in the stress response; this is critical since many of the health consequences of a high stress load are sexually dimorphic (reviewed in Dedovic et al., 2009). These studies utilized transgenic animals to either decrease dopamine synthesis in subsets of neurons or to decrease dopamine receptor expression in male and female Drosophila that were then assayed for several behavioral parameters following stress exposure. The data from this study showed several differences in the behavioral responses of males and females from these genotypes, resulting in the conclusion that different subsets of dopamine neurons are recruited into the stress response circuitry depending upon the sex of the animal. Numerous studies have also shown that the age at the time of the stress exposure affects the response, resulting in differences in development of stress-related pathologies (Lupien et al., 2009). Previous studies from our lab have shown that mutant Drosophila lines with defects in specific areas of the brain known to be important for higher order functions respond differently to stress depending on whether they are sexually immature or sexually mature (Neckameyer and Matsuo, 2008). Additionally, exposure to starvation or oxidative stress differentially alters tyrosine hydroxylase (the rate limiting enzyme for dopamine synthesis) activity depending on age (Neckameyer and Weinstein, 2005). Similarly, animals with decreased dopamine synthesis also response differently to starvation and oxidative stress compared with controls, in an age-dependent manner (Neckameyer and Weinstein, 2005).
In this study, we focus on two developmental windows: sexually immature and sexually mature (adult). Many studies have suggested that adolescence is a particularly vulnerable time for stress exposure, since the adolescent brain must maintain plasticity to develop into a sexually mature organ. Although plasticity does decrease with increasing age, neurogenesis still occurs in specific areas of the adult brain that differ from the major areas of neurogenesis observed during adolescence (Sowell et al., 1999). Impaired neurogenesis in the adult brain has been linked to an increase in anxiety-like behaviors in rats (Revest et al., 2009), and is negatively impacted by stress and potentially enhanced by treatment with antidepressants (Perera et al., 2008). Differences in circuitry and plasticity in individuals from diverse age groups may help to explain why individuals respond differently to psychoactive drugs, which are often used to treat pathologies associated with high stress loads (Safer, 2011). Together, these data make a strong argument for the importance of studying circuitry differences between sexually immature and mature animals.
Differences in the behavioral responses to starvation or oxidative stress were assessed in sexually immature and mature transgenic animals with targeted knockdown of either specific subsets of central dopamine neurons or individual dopamine receptors. These stressors were specifically chosen for their correlation with the development of affective disorders (Roseboom et al., 2001, Marazziti et al., 2012) and alterations of tyrosine hydroxylase (the rate limiting enzyme for dopamine synthesis in Drosophila (Neckameyer and Weinstein, 2005) and rats (Philipp and Pirke, 1987, Kuter et al., 2007)). Behavioral analyses included heart rate and in depth locomotor studies, revealing several parameters that were temporally altered in response to stress. Both of these behavioral parameters have been previously shown to be altered in response to stress and effective at displaying age differences in Drosophila (Neckameyer and Weinstein, 2005; Neckameyer and Matsuo, 2008). Additionally, both heart rate and locomotion are centrally regulated in Drosophila (Dulcis and Levine, 2003 and Strauss and Heisenberg, 1993).
We predicted that sexually immature and mature animals from transgenic lines with distinct alterations in dopamine signaling would display different output responses, measured by heart rate and locomotor patterns after exposure to starvation and oxidative stress. Our results demonstrate that dopamine neurons are differentially recruited into the stress response circuitry for sexually immature and mature animals, and provide a useful model to further identify and test factors that are involved in plasticity and maintenance of the stress response circuitry.
Materials and Methods
Fly Culture
Flies were maintained in glass pint bottles containing standard agar-cornmeal-yeast food at 25°C on a 12 hour light-dark cycle. Male and female progeny were collected immediately following eclosion and maintained separately in groups of <20 under identical conditions until needed for analysis.
Fly Strains
All stocks were obtained from the Bloomington Stock Center (Indiana University, Bloomington) unless otherwise noted. w1118 is the parental strain for the Drosophila tyrosine hydroxylase (DTH) RNAi transgenic line. y[1] v[1]; P{y[+t7.7]=CaryP}attP2 (pattP2) is the parental line for the dopamine receptor transgenics and was a gift from Excelixis (Harvard University, Boston, MA, USA). y1 v1; P{y+t7.7 v+t1.8 = TRiP.HM04077}attP2 carries a dsRNA transgene for the D1 dopamine receptor DopR under UAS control. y1 v1; P{y+t7.7 v+t1.8 = TRiP.JF02043}attP2 carries a dsRNA transgene for the D1 dopamine receptor DopR2 under UAS control. y1 v1; P{y+t7.7 v+t1.8 = TRiP.JF02025}attP2 carries a dsRNA transgene for the D2 dopamine receptor D2R under UAS control. pP{w+mW.hs = GawB}elavC155 (elavC155) is a pan-neuronal Gal4. Gal4 is one part of the Gal4/UAS system, which is used to target gene expression in Drosophila (see Duffy, 2002). DTH-Gal4 line drives expression in the majority of dopaminergic neurons (generated by Serge Birman, Developmental Biology Institute of Marseille, Marseille, France). P{w+mW.hs = GawB}23y was obtained from the University of Glasgow, UK (www.fly-trap.org). P{w+mW.hs =GawB}103y, P{w+mW.hs = GawB}201y, P{w+mW.hs = GawB}drlPGAL8 (referred to by stock number 4669), and P{w+mW.hs = GawB}ey OK107 (referred to as stock number 854) are Gal4 lines with diverse expression of Gal4 in the central nervous system with each containing a distinct pattern of overlap with dopaminergic neurons established by determining overlap between Gal4 and an anti-tyrosine hydroxylase antibody (see companion paper for more detail). The DTH RNAi line used (THK) was generated as previously described (Neckameyer and Bhatt, 2012) and used to decrease dopamine levels since tyrosine hydroxylase is the rate limiting step for dopamine synthesis.
Stress Paradigms
Male and female progeny from crosses designed to decrease dopamine synthesis in select subsets of dopamine neurons or to decrease dopamine receptor expression were collected into separate vials. On either the same day as collection (sexually immature, 1 day) or after 5 days (sexually mature), animals were placed in vials containing either 2% yeast, 5% sucrose dissolved in 1mL deionized water (control for stress), water only (starvation stress), or 2% yeast, 5% sucrose, 30mM methyl viologen dichloride hydrate (paraquat) (Sigma-Aldrich St. Louis, MO, USA) dissolved in 1mL deionized water (oxidative stress) on a 2.1cm glass fiber filter circle (Fisher Scientific Waltham, MA, USA). Animals were maintained under these conditions for 24 hr at 25°C on a 12 hour light-dark cycle prior to behavioral analyses.
Heart Rate
Animals were anesthetized using FlyNap (Carolina Biological Supply), placed dorsal side up on a piece of sticky tape with the wings extended, and viewed under 200X magnification in a temperature-controlled room as previously described (Neckameyer and Matsuo, 2008). n = 40 individuals for each population (sexually immature and mature males and females), for each stress (starvation and oxidative stress) and control for stress condition.
Locomotion
The EthoVision XT tracking system (Noldus Information Technology Leesburg, VA, USA) was used to assess locomotor behaviors. Flies were individually aspirated into a 60 mm diameter petri dish in which the bottom portion of the dish was painted white to increase color contrast and reduce glare from overhead lighting. The white paint also reduced any effects that could result from differences in eyesight amongst the genotypes since the animals would not be able to see outside of the dish. However, we do not believe that eyesight is a concern in these experiments since all of the animals assayed had red eyes. It has been previously shown that lack of red eye pigment results in visual abnormalities (Wu and Wong, 1977). Arenas consisted of a 30 mm diameter center zone and a 15 mm diameter outer zone around the outer edge of the petri dish to allow for determination of time spent in the center of the arena.
Tracking videos were 20 minutes long. After an initial 5 minute set up period, the following 2 minutes was designated as exploratory locomotion, followed by an 8 minute period during which no tracking occurred. The last 5 minutes were designated as adapted, or basal, locomotion. Threshold velocities for the degree of mobility (stopping, walking, hopping/flying) were determined as previously described in the companion paper. For the targeted decreases in dopamine expression, < 3.0mm/s was the threshold to stop, and > 3.1mm/s was the threshold to start walking. For the dopamine receptor knockdowns, < 3.2mm/s was the threshold to stop and > 3.3mm/s was the threshold to start walking. For all experiments, > 35mm/s was the threshold for high mobility, which would include very fast walking, hopping, and flying attempts. In our analysis 0 mm/s, the velocity which would correspond to absolutely no movement, was not used as the threshold for stopped because the flies are constantly making slight vibrations with their wings that are too small to be seen, yet are detected as having a velocity by the software we used. n = 45 individuals for each population (sexually immature and mature males and females) for each condition (control, starvation stress, and oxidative stress).
Statistical Analysis
All statistical analyses were performed using SPSS for Windows from IBM Corporation (Armonk, NY, USA). All analyses are ANOVAs, 3-way ANOVA (Genotype X Age X Stress), 2-way ANOVA (Age X Stress), ANOVA (Stress). For all ANOVAs, p < 0.05 was considered to be significant. ANOVAs with p > 0.05 for the corrected model were considered not significant. A 95% confidence interval was used for all analyses. All error bars are standard error of the mean.
Results
Behavior
In the companion paper we identified six different Gal4 lines which targeted expression in distinct subsets of dopamine neurons, and used them to express a tyrosine hydroxylase RNAi (THK). elavC155/w1118 was used as a negative control and elavC155 was used to express THK pan-neuronally, resulting in eight lines, each with reduced levels of dopamine synthesis in distinct populations of dopamine neurons. TH-Gal4 expresses in the majority, but not all, of tyrosine hydroxylase-expressing neurons. 23y, 201y, and 4669 all show scattered expression throughout the anterior and posterior of the brain with some areas of potential overlap. 103y shows sparse expression only within the anterior. 854 shows expression only within the PPM 1 neurons, and potentially overlaps with 23y, 201y, or 4669 within this cluster (see companion paper for more detail). Sexually immature and mature males and females from these eight genotypes were exposed to starvation or oxidative stress for 24 hours prior to behavioral analysis. Total distance moved, time spent in the center zone, mean velocity, walking duration, frequency of walking bouts, and mean duration of walking bouts all provide information about the animals’ exploration of their environment. Stopped duration, frequency of stops, mean duration of stopped bouts, time spent highly mobile, frequency of bouts of high mobility, and mean duration of bouts of high mobility describe the quality of the animals’ movement. These analyses provide evidence for how sexual maturity, sex, and type of stress can interact to differentially modulate several behaviors. However, we specifically focused here on genotype interactions with age and stress. Since each genotype contains a distinct pattern of dopaminergic circuitry, these interactions provide evidence of differential age-dependent recruitment of DA neurons in stress responses. The results of a 3-way ANOVA investigating the interaction between genotype, age, and stress for these locomotor parameters and heart rate are shown in Table 1.
Table 1. Genotype X Age X Stress Interactions Following Manipulation of Presynaptic Dopamine Neurons.
Sexually immature and mature males and females were assayed for heart rate and locomotion following a 24 hour exposure to starvation or oxidative stress. Exploratory and basal locomotion refer to the first 2 and last 5 minutes of a 15 minute period, respectively.
| Sex | Stress | Locomotor Phase | Distance Moved | In Zone Duration | Mean Velocity |
|---|---|---|---|---|---|
| Female | Starve | Exploratory | * | ns | * |
| Basal | ** | ns | * | ||
| Paraquat | Exploratory | *** | ns | *** | |
| Basal | *** | ns | ns | ||
| Male | Starve | Exploratory | ns | ns | ns |
| Basal | ns | ns | ns | ||
| Paraquat | Exploratory | ns | ns | ns | |
| Basal | *** | ns | *** |
| Sex | Stress | Locomotor Phase | Walking Duration | Walking Frequency | Walking Mean |
|---|---|---|---|---|---|
| Female | Starve | Exploratory | * | ** | * |
| Basal | * | *** | ns | ||
| Paraquat | Exploratory | ** | *** | *** | |
| Basal | ns | *** | ns | ||
| Male | Starve | Exploratory | ns | ns | ns |
| Basal | ns | ns | ns | ||
| Paraquat | Exploratory | ns | *** | ** | |
| Basal | ** | ** | ns |
| Sex | Stress | Locomotor Phase | Stopped Duration | Stopped Frequency | Stopped Mean |
|---|---|---|---|---|---|
| Female | Starve | Exploratory | * | ** | *** |
| Basal | * | *** | ns | ||
| Paraquat | Exploratory | ** | *** | ** | |
| Basal | ns | *** | ns | ||
| Male | Starve | Exploratory | ns | ns | ns |
| Basal | ns | ns | ns | ||
| Paraquat | Exploratory | ns | *** | ns | |
| Basal | ** | ** | ns |
| Sex | Stress | Locomotor Phase | Highly Mobile Duration | Highly Mobile Frequency | Highly Mobile Mean |
|---|---|---|---|---|---|
| Female | Starve | Exploratory | *** | *** | ns |
| Basal | * | *** | ns | ||
| Paraquat | Exploratory | *** | *** | ns | |
| Basal | ns | ns | ns | ||
| Male | Starve | Exploratory | ns | ns | * |
| Basal | ns | ** | ns | ||
| Paraquat | Exploratory | ns | ns | ns | |
| Basal | ** | * | ns |
Mean for walking, stopped, and highly mobile is a calculation of the mean duration of each bout. n = 40 individuals for each population (for heart rate) and n = 45 individuals for each population (for locomotion). 3-way ANOVA (Genotype X Sex X Stress),
p < 0.05,
p < 0.01,
p < 0.001.
Further analysis of the behaviors revealed the genotypes that displayed significant temporal dimorphisms in the stress response, as indicated by a significant 2-way interaction between age and stress within a given genotype. A select subset of these analyses is shown here. For these analyses, only the genotypes that displayed a significant age X stress interaction for the behavior are shown graphically. For females assayed for heart rate following a 24 hour exposure to oxidative stress, significant 2-way interactions (Age X Stress) were seen for elavC155/w1118 (samples 1 and 2, p = 0.000), elavC155/THK (samples 3 and 4, p = 0.000), 23/THK (samples 7 and 8, p = 0.000), 103y/THK (samples 9 and 10, p = 0.000), 201y/THK (samples 11 and 12, p = 0.011), 4669/THK (samples 13 and 14, p = 0.001), and 854/THK (samples 15 and 16, p = 0.025). It was observed that the control animals (elavC155/w1118) had temporally dimorphic responses to stress in some instances, yet differences between sexually immature and mature animals in the genotypes with altered dopamine expression were different in direction and extent. Within these genotypes, elavC155/w1118 and 854/THK sexually immature animals decreased their heart rate in response to oxidative stress (samples 1 and 15, p = 0.000 for both), and sexually mature animals showed no significant effect (sample 2, p = 0.917 and sample 16, p = 0.897, respectively). elavC155/THK sexually immature animals decreased their heart rate in response to oxidative stress (sample 3, p = 0.000), while sexually mature animals increased theirs (sample 4, p = 0.000). 23y/THK and 201y/THK sexually mature animals decreased their heart rate in response to oxidative stress (sample 8, p = 0.00 and sample 12, p = 0.004, respectively), while sexually immature animals displayed no significant effect (sample 7, p = 0.207 and sample 11, p = 0.44, respectively). 103y/THK sexually immature and mature animals increased their heart rate in response to oxidative stress (samples 9 and 10, p = 0.000 for both). 4669/THK sexually immature animals increased their heart rate in response to oxidative stress (sample 13, p = 0.000) and sexually mature animals showed no significant effect (sample 14, p = 0.222) (Figure 1).
Figure 1.
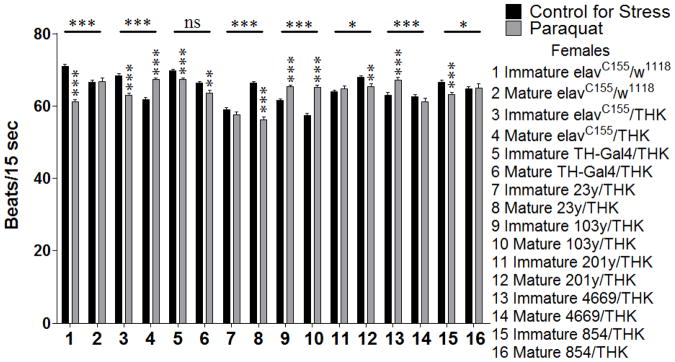
Sexually immature and mature females were assayed for heart rate following a 24 hour exposure to oxidative stress. n = 40 individuals for each population (males and females with either control for stress or oxidative (paraquat) stress conditions for each genotype). Individual heart rates were the average of 5 intervals of 15 seconds each with 15 seconds between each interval. 2-Way ANOVA (Sex X Stress) displayed on lines above the corresponding bars, ANOVA (Stress) displayed directly above the bars, * p < 0.05, ** p < 0.01, *** p < 0.001.
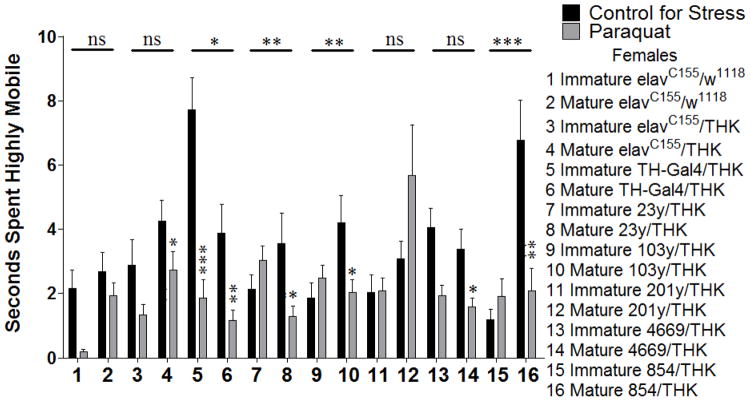
Females assayed for time spent highly mobile during exploratory locomotion following a 24 hour exposure to oxidative stress significant 2-way interactions (Age X Stress) for TH-Gal4/THK (samples 5 and 6, p = 0.037), 23y/THK (samples 7 and 8, p = 0.009), 103y/THK (samples 9 and 10, p = 0.008), and 854/THK (samples 15 and 16, p = 0.001). TH-Gal4/THK sexually immature and mature animals both decreased their time spent highly mobile in response to oxidative stress, but to varying degrees (sample 5, p = 0.000 and sample 6, p = 0.005, respectively). 23y/THK, 103y/THK, and 854/THK sexually mature animals decreased their time spent highly mobile in response to oxidative stress (sample 8, p = 0.023 for 23y/THK; sample 10, p = 0.022 for 103y/THK; sample 16, p = 0.002 for 854/THK), while sexually immature animals showed no significant effect (sample 7, p = 0.202 for 23y/THK; sample 9, p = 0.171 for 103y/THK; sample 15, p = 0.201 for 854/THK) (Figure 2).
Figure 2.
Sexually immature and mature females were assayed for time spent highly mobile during exploratory locomotion following a 24 hour exposure to oxidative stress. n = 45 (for each population). 2-way ANOVA (Sex X Stress) displayed on lines above corresponding bars, ANOVA (Stress) displayed directly above bars * p < 0.05, ** p < 0.01, *** p < 0.001.
Manipulation of dopamine receptors
To further investigate temporal dimorphism of dopamine signaling in response to stress, RNAi lines for the three Drosophila dopamine receptors DopR (type 1), DopR2 (type 1), and D2R (type 2) were crossed with elavC155 to direct pan-neuronal expression, and were assayed for the behaviors described above. We expected that if dopamine neurons are differentially recruited into the stress response circuitry for sexually immature and mature populations, then these populations would respond differently to manipulation of the dopamine receptors. The progeny from a cross between elavC155 and pattP2 (parental line for the dopamine receptor transgenics) was used as a control. Sexually immature and mature animals with decreased dopamine receptor expression were assayed for locomotor behaviors following a 24 hour exposure to starvation or oxidative stress. Results for a 3-way interaction (Genotype X Age X Stress) for each of the different locomotor parameters are shown in Table 2. A significant effect for this analysis supports our hypothesis that different neurons are differentially recruited into the stress response for sexually immature and mature animals and suggests that this differentially recruitment may occur for both pre- and post-synaptic neurons.
Table 2. Genotype X Age X Stress Interactions Following Manipulation of Postsynaptic Dopamine Signaling.
Sexually immature and mature males and females were assayed for heart rate and locomotion following a 24 hour exposure to starvation or oxidative stress. Exploratory and basal locomotion refer to the first 2 and last 5 minutes of a 15 minute period, respectively.
| Sex | Stress | Locomotor Phase | Distance Moved | In Zone Duration | Mean Velocity |
|---|---|---|---|---|---|
| Female | Starve | Exploratory | ** | ns | *** |
| Basal | *** | ns | *** | ||
| Paraquat | Exploratory | *** | ns | *** | |
| Basal | *** | ns | *** | ||
| Male | Starve | Exploratory | ns | ns | ns |
| Basal | ns | ns | ns | ||
| Paraquat | Exploratory | ns | ns | ns | |
| Basal | * | ns | ns |
| Sex | Stress | Locomotor Phase | Walking Duration | Walking Frequency | Walking Mean |
|---|---|---|---|---|---|
| Female | Starve | Exploratory | * | *** | *** |
| Basal | *** | *** | ns | ||
| Paraquat | Exploratory | *** | *** | *** | |
| Basal | *** | *** | ns | ||
| Male | Starve | Exploratory | *** | ns | ns |
| Basal | ns | ns | ns | ||
| Paraquat | Exploratory | ns | ns | ns | |
| Basal | ns | ns | ns |
| Sex | Stress | Locomotor Phase | Stopped Duration | Stopped Frequency | Stopped Mean |
|---|---|---|---|---|---|
| Female | Starve | Exploratory | * | *** | ns |
| Basal | *** | *** | ns | ||
| Paraquat | Exploratory | *** | *** | ns | |
| Basal | *** | *** | ns | ||
| Male | Starve | Exploratory | *** | ns | ns |
| Basal | ns | ns | ns | ||
| Paraquat | Exploratory | ns | ns | ns | |
| Basal | ns | ns | ns |
| Sex | Stress | Locomotor Phase | Highly Mobile Duration | Highly Mobile Frequency | Highly Mobile Mean |
|---|---|---|---|---|---|
| Female | Starve | Exploratory | ** | * | ns |
| Basal | *** | ns | ns | ||
| Paraquat | Exploratory | *** | *** | ns | |
| Basal | *** | *** | ns | ||
| Male | Starve | Exploratory | ** | ** | ns |
| Basal | ns | *** | ns | ||
| Paraquat | Exploratory | ** | ns | ns | |
| Basal | ns | ** | ns |
Mean for walking, stopped, and highly mobile is a calculation of the mean duration of each bout. n = 40 individuals for each population (for heart rate) n = 45 individuals for each population (for locomotion). 3-way ANOVA (Genotype X Sex X Stress),
p < 0.05,
p < 0.01,
p < 0.001.
A subset of these analyses depicting those behaviors with temporally dimorphic effects is shown here. Males assayed for heart rate following a 24 hour exposure to starvation stress displayed significant 2-way interactions (Age X Stress) for elavC155/pattP2 (sample 1 and 2, p = 0.000), elavC155/dsDopR2 (samples 5 and 6, p = 0.031), and elavC155/dsD2R (samples 7 and 8, p = 0.000). In several cases, the control animals (elavC155/pattP2) had temporally dimorphic responses to stress, however differences were seen in direction and extent of responses in the sexually immature and mature animals from the other genotypes. Although elavC155/dsDopR2 showed a significant 2-way interaction, the corrected model was not significant (samples 5 and 6, p = 0.091). elavC155/pattP2 and elavC155/dsD2R sexually immature animals increased their heart rate in response to starvation stress (sample 1, p = 0.015 and sample 7, p = 0.004, respectively), while sexually mature animals decreased theirs (samples 2 and 8, p = 0.000 for both) (Figure 3).
Figure 3.
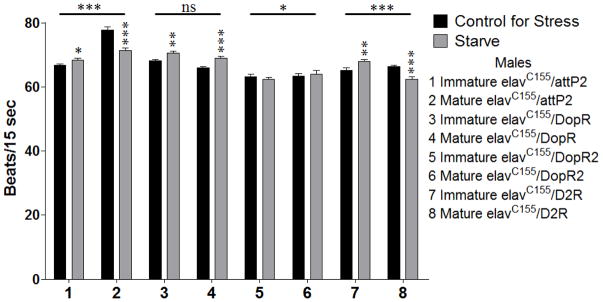
Sexually immature and mature males were assayed for heart rate as above following a 24 hour exposure to starvation stress. n = 40 (for each population). 2-way ANOVA (Sex X Stress) displayed on lines above the corresponding bars, ANOVA (Stress) displayed directly above the bars * p < 0.05, ** p < 0.01, *** p < 0.001.
Females assayed for mean velocity during exploratory locomotion following a 24 hour exposure to oxidative stress displayed significant 2-way interactions (Age X Stress) for elavC155/dsDopR (samples 3 and 4, p = 0.000) and elavC155/dsD2R (samples 7 and 8, p = 0.000). elavC155/dsDopR sexually immature animals increased their mean velocity in response to oxidative stress (sample 3, p = 0.000) and sexually mature animals decreased theirs (sample 4, p = 0.000). elavC155/dsD2R sexually immature animals decreased their mean velocity in response to oxidative stress (sample 7, p = 0.004), while sexually mature animals increased their mean velocity (sample 8, p = 0.026) (Figure 4).
Figure 4.
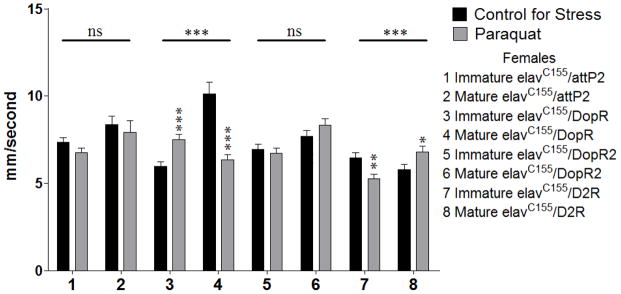
Sexually immature and mature females were assayed for mean velocity during exploratory locomotion following a 24 hour exposure to oxidative stress. n = 45 (for each population). 2-way ANOVA (Sex X Stress) displayed on lines above corresponding bars, ANOVA (Stress) displayed directly above bars * p < 0.05, ** p < 0.01, *** p < 0.001.
Discussion
In addition to the sexual dimorphism of the stress response circuitry discussed in the companion paper, earlier work from our lab suggested that the stress response circuitry is plastic and changes with age (Neckameyer and Matsuo, 2008). This is well supported by data indicating that the timing of a stressful exposure can determine how an individual will be affected in terms of the immediate behavioral response, and the pathologies that may result either shortly afterward or even years after the stress exposure. In these studies, we focused on two specific stages, sexually immature and sexually mature, because the transition between these periods has been shown to be particularly sensitive for the development of adult cognitive ability, and may contribute to the development of psychiatric disorders (reviewed in McCormick and Mathews, 2007). We specifically chose ages in flies that would correspond to mammalian adolescent (1 day old flies) or sexually mature adults (5 day old flies), since our previous studies have pointed to distinct differences between these two timepoints that suggest a sensitive window (Neckameyer and Matsuo, 2008). The analysis of the data in this paper used 3-way ANOVAs to assess interactions between genotype, age, and stress. Since each genotype has a different pattern of dopamine signaling, a significant interaction between these genotypes with age and sex suggests that different subsets of neurons are recruited into the dopamine stress response circuitry for sexually immature and mature populations.
Two stressors, starvation and oxidative stress, were used for these experiments to illustrate that temporal differences in the response circuitry are general and not limited to one specific stress. Additionally, these stressors have been previously linked to the development of psychiatric disorders. Starvation stress has been correlated with an increased incidence of depression and schizophrenia that is dependent on the timing of the stress exposure (see Roseboom et al., 2001 for review), and oxidative stress has been linked to schizophrenia and bipolar disorder (see Marazziti et al., 2012 for review). Previous work in rats has compared the effects of oxidative stress across different ages, showing that older animals display more behavioral changes in response to oxidative stress in elevated plus maze and open field measures of anxiety compared to younger animals (Chakraborti et al., 2008). Our work has also shown that these stressors produce sexually and temporally dimorphic behavioral effects in Drosophila (Neckameyer and Matuso, 2008 and companion paper). Together these data indicate that these stressors have been effective in producing age-dependent behavioral effects across a variety of species.
The behavioral parameters were also chosen to be effective at displaying age-dependent changes in behavior in response to stress, while keeping our Drosophila data relevant to what has been accomplished in mammalian studies. There is a large body of literature showing alterations in heart rate, exploration, centering time, stopping and highly mobile behaviors in response to stress in mammals, but very few of these studies have compared behavioral responses to stress across different ages. However, the different results that we obtained for adolescent and sexually mature animals with these behavioral parameters support immunohistochemical analyses that show that different populations of hippocampal neurons (Chen et al., 2006) and neurons in the paraventricular nucleus of the hypothalamus (Romeo et al., 2006) were responsive to restraint stress in juvenile compared to adult rats (Chen et al., 2006).
The Gal4 lines used in this study were carefully chosen based on areas of overlapping expression between Gal4 and tyrosine hydroxylase. The data on the precise dopaminergic neurons targeted by these Gal4 lines is shown in the companion paper. Here we reported the general area of overlap as well as a mention of the probable areas of overlap amongst these lines. While we found the pattern of dopamine neurons to be fairly consistent between individuals in terms of the placement and overall shape of the clusters, there was a degree of individual variability in the placement of a specific dopaminergic neuron within a given cluster that make it difficult to pinpoint the precise neuron that is targeted by a given line and therefore a precise neuron that is shared in common between two or more lines. This difficulty would provide a challenge to be overcome in attempting to assign individual neurons that are responsible for a given behavioral output in response to a specific stressor in a particular population of animals. However, the individual variability in specific neuron placement did not confound our study as we established that each of the Gal4 lines has a distinct and reproducible pattern of overlap with tyrosine hydroxylase expressing neurons that is consistent across the ages used for these studies. We were also unable to precisely determine whether the degree of expression of Gal4 was identical in all neurons for a given Gal4 line and whether or not the degree of expression changed with age. We did, however, observe robust expression in all neurons that were determined to overlap with tyrosine hydroxylase, indicating that dopamine synthesis is definitively reduced in these neurons.
Although it was beyond the scope of this analysis to establish neurons or even subsets of neurons that are important for the behavioral output to stress for a given population, a few implications of important subsets were suggested by the data. Heart rate for both 103y/THK and 4669/THK sexually immature animals increased in response to oxidative stress. Although these two drivers do not direct expression in overlapping subsets of DA neurons, identification of the neuronal populations targeted by the two lines would be an interesting avenue for further study. For sexually mature individuals, no effects were observed in the elavC155/w1118 animals, whereas the elavC155/THK animals significantly increased their heart rate in response to oxidative stress. A similar response to that of the elavC155/THK animals was also observed for sexually mature 103y/THK animals, indicating that reduction of dopamine synthesis in the dopamine neurons targeted by 103y could be of importance. Both TH-Gal4/THK and 23y/THK showed similar effects that were opposite of that observed for the positive control animals, indicating that the neurons shared in common between TH-Gal4 and 23y, within the PPMa and PPMb clusters, could also be of importance. Similarly, in the analysis of time spent highly mobile following oxidative stress, TH-Gal4/THK, 4669/THK, and 854/THK sexually mature animals all displayed effects that were similar to those observed for the positive control sexually mature animals suggesting that the neurons shared in common between those three genotypes, within the PPMa cluster, could be of importance. While these subsets stood out during our analysis, all dopaminergic neurons targeted by a Gal4 line found to have significant differences between sexually immature and mature individuals would be of interest for further study on the maturation of the neural circuitry important for a specific behavioral response to stress.
In the heart rate analysis following manipulation of the dopamine receptors, decreasing levels of DopR was observed to have an opposite effect on sexually mature animals than that observed for the negative control animals, indicating the possibility that this receptor is of importance for this response. Also interesting, was the complete ablation of a heart rate response to starvation stress following decreases in DopR2, which suggests the possibility that this receptor is necessary for the heart rate response to starvation stress. In the analysis of mean velocity, both DopR and D2R knockdown animals were observed to have very different responses compared to the negative controls, whereas DopR2 did not appear to be involved in this particular response. Further analysis to verify roles for these neuronal subsets and receptor populations within the behaviors for which their importance was suggested would be an interesting avenue for future study and could provide valuable insights into differences between sexually immature and mature neuronal circuitry.
We noticed greater significance in the heart rate compared with the locomotor behaviors, which can be explained, at least in part, by the small variability that is seen with the heart rate analysis in Drosophila. This is similar to what was seen in our analysis of sexually dimorphic recruitment of dopamine neurons into the stress response circuitry. While we did not perform the 4-way analysis to directly compare genotype, sex, age, and stress, we did observe the following trends when considering these data in light of the companion paper focusing on sex differences. The results from the companion paper with 3-way ANOVAs for interaction between genotype, sex, and stress showed a greater number of instances of statistical significance as well as more p values < 0.001 with manipulations of pre-synaptic dopamine signaling compared to post-synaptic manipulations. In this study, using 3-way ANOVAs for interaction between genotype, age, and stress, the opposite was observed, with greater statistical significance observed with post-synaptic manipulations of dopamine signaling. A possible explanation may be that individuals do not progress from male to female or vice versa, whereas an individual would progress from sexually immature to sexually mature. Although we did not design our experiments to test the same individual when sexually immature and then again after it had matured, these experiments may be representative of changes that could occur within a single individual as it ages. It is possible that as an individual matures, the pre-synaptic neurons for a specific circuit are less plastic and the post-synaptic targets change to a greater extent.
The studies investigating the post-synaptic dopaminergic targets were limited by the usage of a single pan-neuronal Gal4 driver line, as opposed to the design for the presynaptic experiments, which utilized Gal4 lines which targeted expression in subsets of neurons. Without reliable antibodies for the different dopamine receptors, it was not feasible to identify areas of overlap between different Gal4 lines and these receptors, which would have been necessary to show that each Gal4 line was targeting a distinct population of post-synaptic dopamine neurons. Taking this limitation into account, the analyses on the post-synaptic dopaminergic neurons served directly to demonstrate that the dopamine circuitry differed for sexually immature and mature individuals rather than to specifically show that different post-synaptic targets are recruited for these populations. Taken together with the presynaptic data, however, the post-synaptic data support the conclusion that different dopamine neurons are recruited into the stress response circuitry for sexually immature and mature populations. Conversely, data showing that sexually immature and mature populations demonstrate the same behavioral changes in response to manipulations of dopamine receptors would not have supported our conclusions. This data was complicated further by previous work showing that similar to mammalian D2R, some D2R receptors in Drosophila functions as autoreceptors located on pre-synaptic neurons that are involved in the regulation of dopamine release (Vickrey and Venton, 2011).
While the inclusion of autoreceptors does complicate the dopaminergic stress response circuitry, taking this into account would not alter the implications of our analysis. While no statistical analysis was performed to directly compare males and females within this analysis, as the focus was to provide evidence for temporal dimorphisms, it is evident from Table 1 that sexual maturity had a greater influence on the behavioral response to stress for females compared to males. This may be due to sex differences in neuronal plasticity and adaptation. It would be expected that attainment of sexual maturation would be different for each sex, since it requires the development of distinct behaviors for males and females. A sexually mature and competent male fly must select an acceptable female, secrete the appropriate pheromones, and perform the correct sequence of courtship events to win her as a mate. Females need to be receptive to an acceptable male and then lay fertilized eggs in an optimal spot for survival of the offspring; a single copulation usually results in egg-laying over a two week period. It is also possible that the timing of maturation differs between males and females. Further studies would be required to determine whether the lack of change in males is adaptive or maladaptive or whether changes occur at a later age for males compared to females.
Conclusions
These data definitively show that the dopamine stress response circuitry differs for sexually immature and mature individuals. These results provide valuable insight into neuronal plasticity and disease susceptibility as individuals mature. Our conclusions from this analysis, along with our previous work, also point to the importance of studying both sexes and across a range of ages when establishing physiological models. Taken together, we can conclude that the dopamine stress response circuitry is different for both sexes, for the level of sexual maturity, and for each stressor and behavioral output.
References
- Chakraborti A, Gulati K, Ray A. Age related differences in stress-induced neurobehavioral responses in rats: modulation by antioxidants and nitergic agents. Behavioral Brain Research. 2008;194:86–91. doi: 10.1016/j.bbr.2008.06.027. [DOI] [PubMed] [Google Scholar]
- Chen Y, Fenoglio KA, Dubé CM, Grigoriadis DE, Baram TZ. Cellular and molecular mechanisms of hippocampal activation by acute stress are age-dependent. Molecular Psychiatry. 2006;11:992–1002. doi: 10.1038/sj.mp.4001863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedovic K, Wadiwalla M, Engert V, Pruessner JC. The role of sex and gender socialization in stress reactivity. Developmental Psychology. 2009;45:45–55. doi: 10.1037/a0014433. [DOI] [PubMed] [Google Scholar]
- Duffy JB. GAL4 system in Drosophila: a fly geneticists’s Swiss army knife. Genesis. 2002;34:1–15. doi: 10.1002/gene.10150. [DOI] [PubMed] [Google Scholar]
- Dulcis D, Levine RB. Innervation of the heart of the adult fruit fly, Drosophila melanogaster. Journal of Comparative Neurology. 2003;465:560–578. doi: 10.1002/cne.10869. [DOI] [PubMed] [Google Scholar]
- Kuter K, Smialowska M, Wierońska J, Zieba B, Warda J, Pietraszek M, Nowak P, Biedka I, Roczniak W, Konieczny J, Wolfarth S, Ossowska K. Toxic influence of subchronic paraquat administration on dopaminergic neurons in rats. Brain Research. 2007;1155:196–207. doi: 10.1016/j.brainres.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Marazziti D, Baroni S, Picchetti M, Landi P, Silvestri S, Vatteroni E, Catena Dell’Osso M. Psychiatric disorders and mitochondrial dysfunctions. European Review for Medical and Pharmacological Sciences. 2012;16:270–275. [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ. HPA functions in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacology, Biochemistry, and Behavior. 2007;86:220–233. doi: 10.1016/j.pbb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Neckameyer WS, Bhatt P. Neurotrophic actions of dopamine on the development of a serotonergic feeding circuit in Drosophila melanogaster. BMC Neuroscience. 2012;13:26. doi: 10.1186/1471-2202-13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckameyer WS, Matsuo H. Distinct neural circuits reflect sex, sexual maturity, and reproductive status in response to stress in Drosophila melanogaster. Neuroscience. 2008;156:841–856. doi: 10.1016/j.neuroscience.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Neckameyer W, Weinstein J. Stress affects dopaminergic signaling pathways in Drosophila melanogaster. Stress. 2005;8:117–132. doi: 10.1080/10253890500147381. [DOI] [PubMed] [Google Scholar]
- Perera TD, Park S, Nemirovskaya Y. Cognitive role of neurogenesis in depression and antidepressant treatment. Neuroscientist. 2008;14:326–338. doi: 10.1177/1073858408317242. [DOI] [PubMed] [Google Scholar]
- Philipp E, Pirke KM. Effects of starvation on hypothalamic tyrosine hydroxylase activity in adult male rats. Brain Research. 1987;413:53–59. doi: 10.1016/0006-8993(87)90153-3. [DOI] [PubMed] [Google Scholar]
- Revest JM, Dupret D, Koehl M, Funk-Reiter C, Grosjean N, Piazza PV, Abrous DN. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Molecular Psychiatry. 2009;14:959–967. doi: 10.1038/mp.2009.15. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, McEwen BS. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology. 2006;147:1664–1674. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- Rooseboom TJ, Painter RC, van Abeelen AFM, Veenendaal MVE, Rooij SR. Hungry in the womb: What are the consequences? Lessons from the Dutch famine. Muturitas. 2011;70:141–145. doi: 10.1016/j.maturitas.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Safer DJ. Age-grouped differences in adverse drug events from psychotropic medication. Journal of Child and Adolescent Psychopharmacology. 2011;21:299–309. doi: 10.1089/cap.2010.0152. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature Neuroscience. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Strauss R, Heisenberg M. A higher control center of locomotor behavior in the Drosophila brain. Journal of Neuroscience. 1993;13:1852–1861. doi: 10.1523/JNEUROSCI.13-05-01852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickrey TL, Venton BJ. Drosophila Dopamine2-like receptors function as autoreceptors. ACS Chemical Neuroscience. 2011;2:723–729. doi: 10.1021/cn200057k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CF, Wong F. Frequency characteristics in the visual system of Drosophila. Journal of General Physiology. 1977;69:705–724. doi: 10.1085/jgp.69.6.705. [DOI] [PMC free article] [PubMed] [Google Scholar]