Abstract
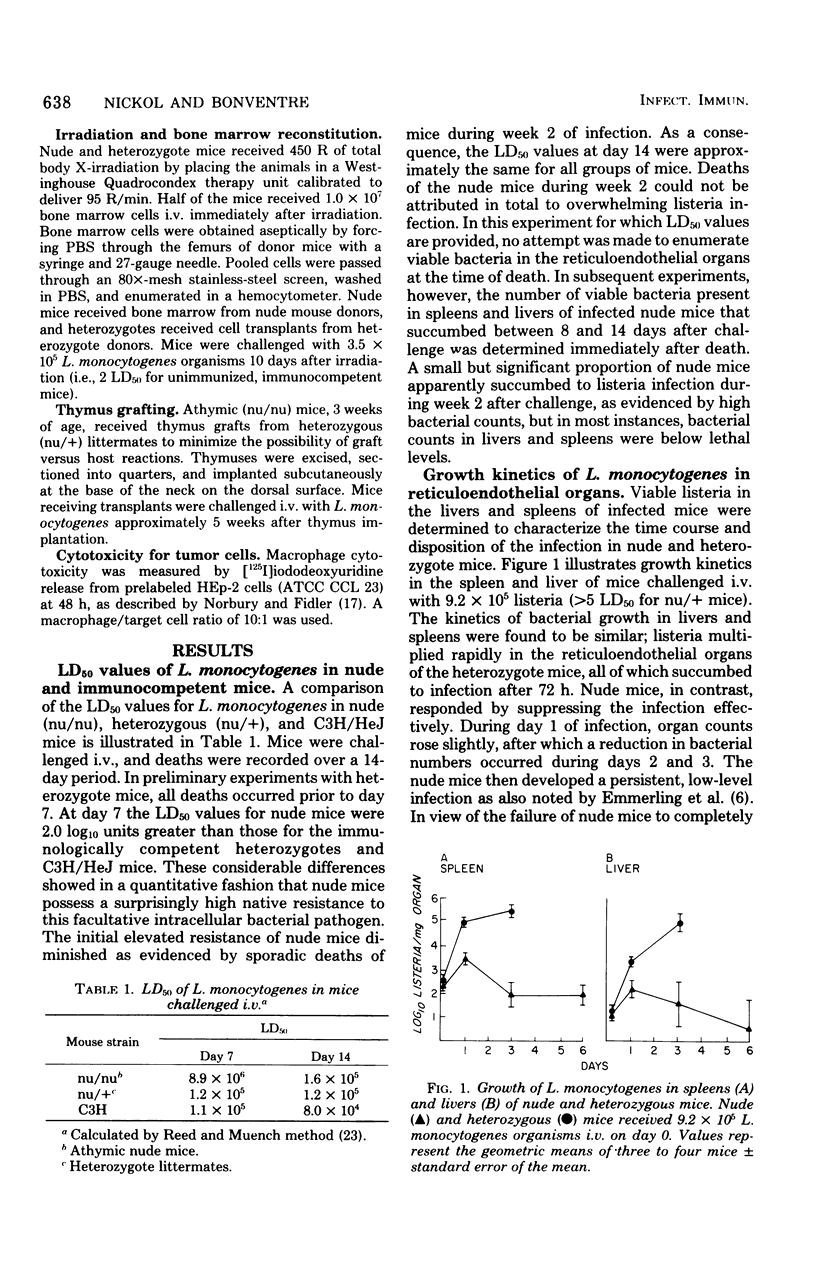
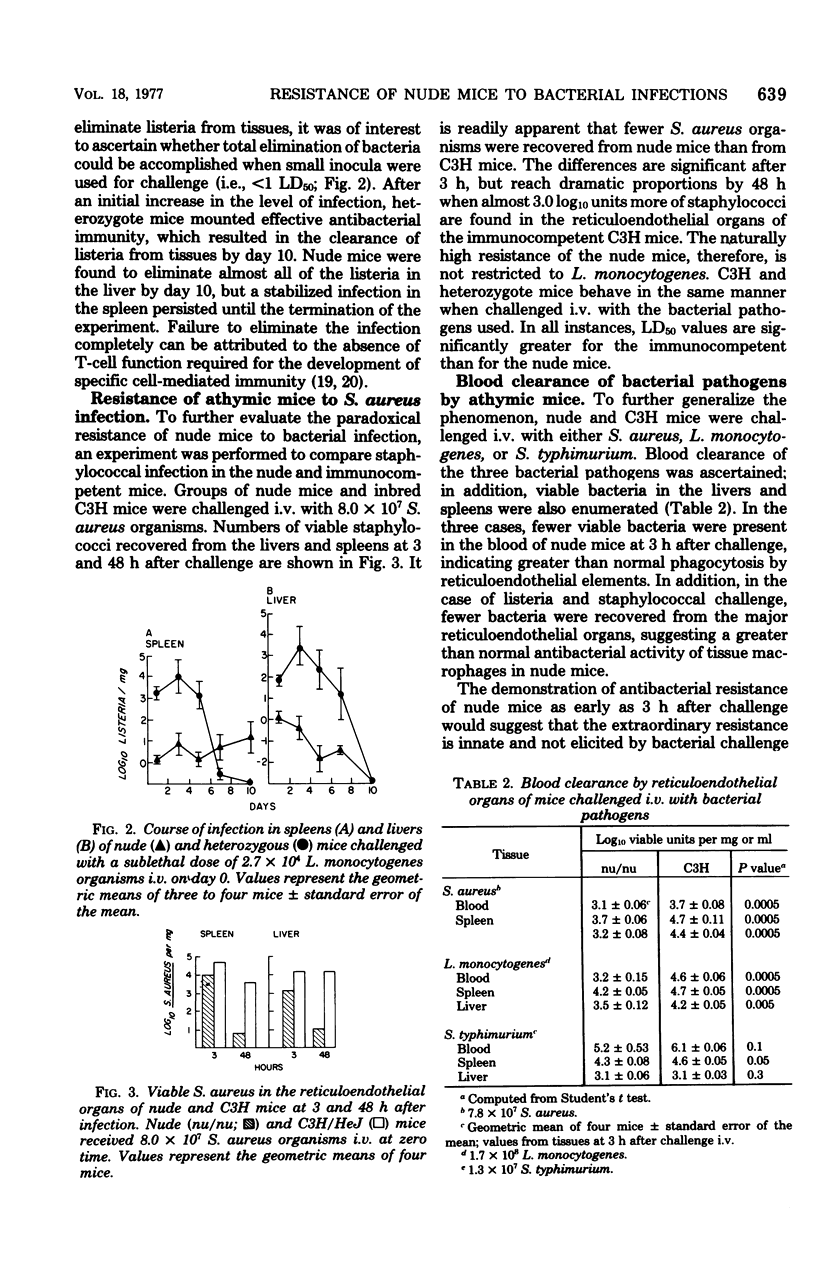
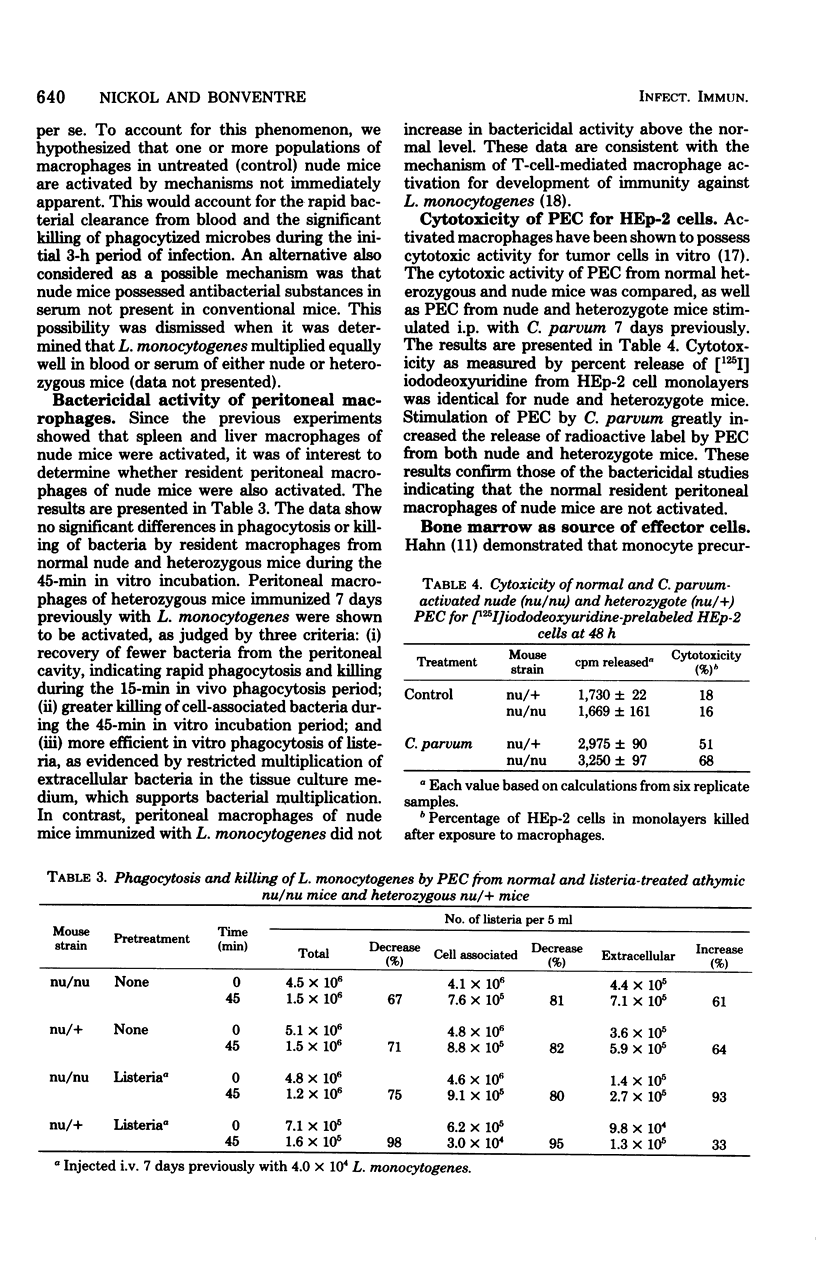
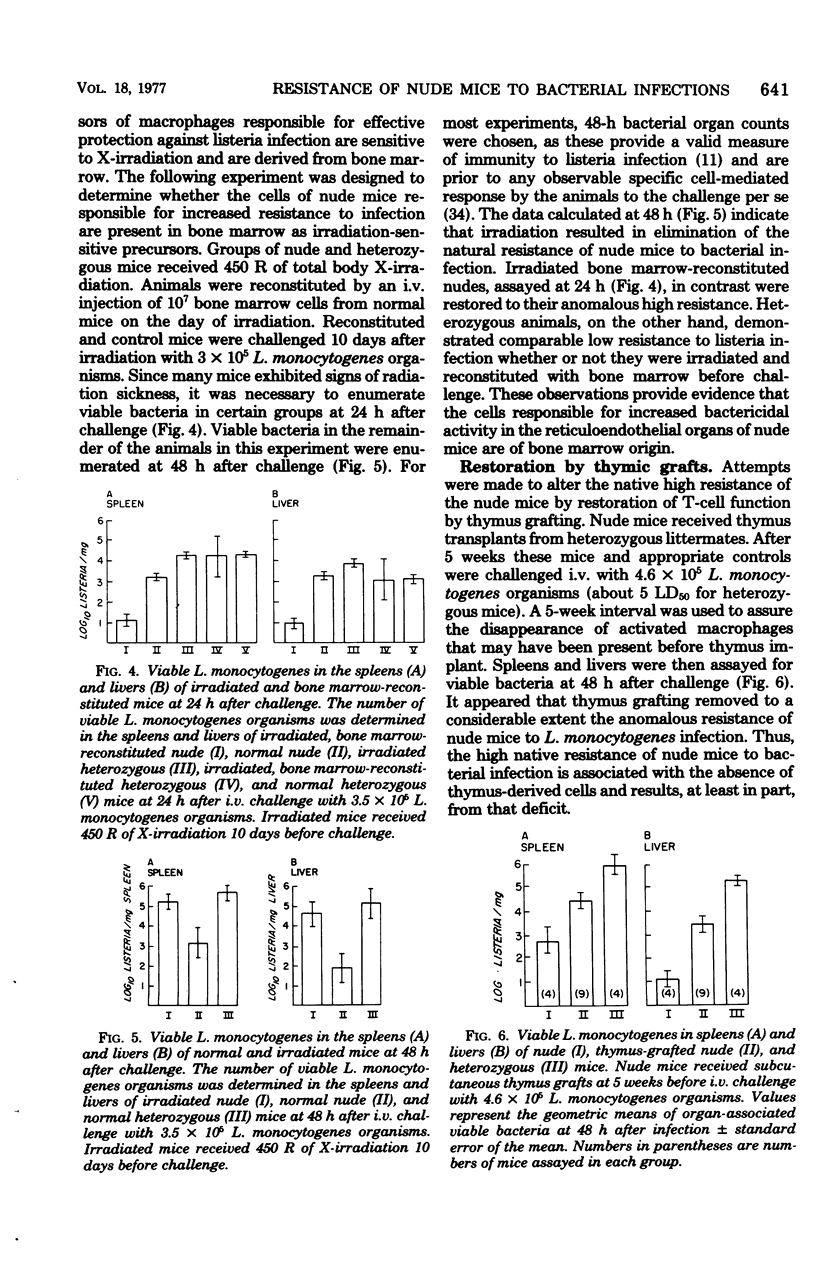
Congenitally athymic (nude) mice exhibited an anomalous high resistance against infections with the facultative intracellular parasite Listeria monocytogenes and other bacterial pathogens. Protection against lethal infection was demonstrated to result from the presence of naturally occurring activated macrophages in the reticuloendothelial organs of the nude mice. This was exemplified after intravenous challenge by enhanced bacterial clearance from the blood and augmented bacterial killing in the spleens and livers of nude mice as compared with immunologically competent control mice. Resident peritoneal macrophages of nude mice were not activated in terms of phagocytic, bactericidal, or tumoricidal potential. The development of activated fixed tissue macrophages appears to arise as a result of the T-lymphocyte deficiency since thymus implantation abrogated the enhanced resistance of nude mice. Antibiotic elimination of intestinal bacteria also modified resistance to bacterial infection, indicating a role of environmental factors on macrophage activation. Several possible mechanisms leading to macrophage activation and heightened resistance to infection in nude mice are offered.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENACERRAF B. Influence of irradiation on resistance to infection. Bacteriol Rev. 1960 Mar;24(1):35–40. doi: 10.1128/br.24.1.35-40.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V., Lefford M. J., Mackaness G. B. The host response to Calmette-Guérin bacillus infection in mice. J Exp Med. 1969 May 1;129(5):1079–1107. doi: 10.1084/jem.129.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V., Mackaness G. B., Collins F. M. Mechanisms of acquired resistance in mouse typhoid. J Exp Med. 1966 Oct 1;124(4):585–600. doi: 10.1084/jem.124.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomford R., Christie G. H. Mechanisms of macrophage activation by corynebacterium parvum. II. In vivo experiments. Cell Immunol. 1975 May;17(1):150–155. doi: 10.1016/s0008-8749(75)80015-3. [DOI] [PubMed] [Google Scholar]
- Cheers C., Waller R. Activated macrophages in congenitally athymic "nude mice" and in lethally irradiate mice. J Immunol. 1975 Sep;115(3):844–847. [PubMed] [Google Scholar]
- Emmerling P., Finger H., Bockemühl J. Listeria monocytogenes infection in nude mice. Infect Immun. 1975 Aug;12(2):437–439. doi: 10.1128/iai.12.2.437-439.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauve R. M., Hevin B. Résistance paradoxale des souris thymoprives à l'infection par Listeria monocytogenes et Salmonella typhimurium et action immunostimulante d'un extrait bactérien phospholipidique (EBP). C R Acad Sci Hebd Seances Acad Sci D. 1974 Nov 4;279(19):1603–1605. [PubMed] [Google Scholar]
- Gordon H. A., Pesti L. The gnotobiotic animal as a tool in the study of host microbial relationships. Bacteriol Rev. 1971 Dec;35(4):390–429. doi: 10.1128/br.35.4.390-429.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy-Grand D., Griscelli C., Vassalli P. Peyer's patches, gut IgA plasma cells and thymic function: study in nude mice bearing thymic grafts. J Immunol. 1975 Aug;115(2):361–364. [PubMed] [Google Scholar]
- Hahn H. Requirement for a bone marrow-derived component in the expression of cell-mediated antibacterial immunity. Infect Immun. 1975 May;11(5):949–954. doi: 10.1128/iai.11.5.949-954.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKIN C., BENACERRAF B. In vitro studies on the interaction between mouse peritoneal macrophages and strains of Salmonella and Escherichia coli. J Exp Med. 1960 Aug 1;112:403–417. doi: 10.1084/jem.112.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamanna C. Needs for illuminating the microbiology of the lumen. Am J Clin Nutr. 1972 Dec;25(12):1488–1494. doi: 10.1093/ajcn/25.12.1488. [DOI] [PubMed] [Google Scholar]
- Meltzer M. S. Tumoricidal responses in vitro of peritoneal macrophages from conventionally housed and germ-free nude mice. Cell Immunol. 1976 Mar 1;22(1):176–181. doi: 10.1016/0008-8749(76)90018-6. [DOI] [PubMed] [Google Scholar]
- Norbury K. C., Fidler I. J. In vitro tumor cell destruction by syngeneic mouse macrophoages: methods for assaying cytotoxicity. J Immunol Methods. 1975 Apr;7(1):109–122. doi: 10.1016/0022-1759(75)90136-2. [DOI] [PubMed] [Google Scholar]
- North R. J. Nature of "memory" in T-cell-mediated antibacterial immunity: anamnestic production of mediator T cells. Infect Immun. 1975 Oct;12(4):754–760. doi: 10.1128/iai.12.4.754-760.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. T cell dependence of macrophage activation and mobilization during infection with Mycobacterium tuberculosis. Infect Immun. 1974 Jul;10(1):66–71. doi: 10.1128/iai.10.1.66-71.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantelouris E. M., Flisch P. A. Responses of athymic ("nude") mice to sheep red blood cells. Eur J Immunol. 1972 Jun;2(3):236–239. doi: 10.1002/eji.1830020308. [DOI] [PubMed] [Google Scholar]
- Rao G. R., Rawls W. E., Perey D. Y., Tompkins W. A. Macrophage activation in congenitally athymic mice raised under conventional or germ-free conditions. J Reticuloendothel Soc. 1977 Jan;21(1):13–20. [PubMed] [Google Scholar]
- Rocklin R. E., MacDermott R. P., Chess L., Schlossman S. F., David J. R. Studies on mediator production by highly purified human T and B lymphocytes. J Exp Med. 1974 Nov 1;140(5):1303–1316. doi: 10.1084/jem.140.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T. J., Balish E., Manning D. D. The role of thymus-dependent cell-mediated immunity in resistance to experimental disseminated candidiasis. J Reticuloendothel Soc. 1976 Oct;20(4):291–298. [PubMed] [Google Scholar]
- Rygaard J., Povlsen C. O. Heterotransplantation of a human malignant tumour to "Nude" mice. Acta Pathol Microbiol Scand. 1969;77(4):758–760. doi: 10.1111/j.1699-0463.1969.tb04520.x. [DOI] [PubMed] [Google Scholar]
- Sher N. A., Chaparas S. D., Greenberg L. E., Merchant E. B., Vickers J. H. Response of congenitally athymic (nude) mice to infection with Mycobacterium bovis (strain BCG). J Natl Cancer Inst. 1975 Jun;54(6):1419–1426. doi: 10.1093/jnci/54.6.1419. [DOI] [PubMed] [Google Scholar]
- Ueda K., Yamazaki S., Someya S. Experimental mycobacterial infection in congenitally athymic "nude" mice. J Reticuloendothel Soc. 1976 Feb;19(2):77–90. [PubMed] [Google Scholar]
- Ueda K., Yamazaki S., Someya S. Studies on tubercle bacillus infection in germ-free mice. J Reticuloendothel Soc. 1972 Nov;12(5):545–563. [PubMed] [Google Scholar]
- Winkelstein J. A., Swift A. J. Host defense against the pneumococcus in T-lymphocyte-deficient, nude mice. Infect Immun. 1975 Nov;12(5):1222–1223. doi: 10.1128/iai.12.5.1222-1223.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Sonozaki H., Cohen S. The production of migration inhibition factor by B and T cells of the guinea pig. J Exp Med. 1973 Oct 1;138(4):784–797. doi: 10.1084/jem.138.4.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Blanden R. V., Langman R. E. Early appearance of sensitized lymphocytes in mice infected with Listeria monocytogenes. J Immunol. 1974 Feb;112(2):496–501. [PubMed] [Google Scholar]
- Zinkernagel R. M., Blanden R. V. Macrophage activation in mice lacking thymus-derived (T) cells. Experientia. 1975 May 15;31(5):591–593. doi: 10.1007/BF01932477. [DOI] [PubMed] [Google Scholar]


