Abstract
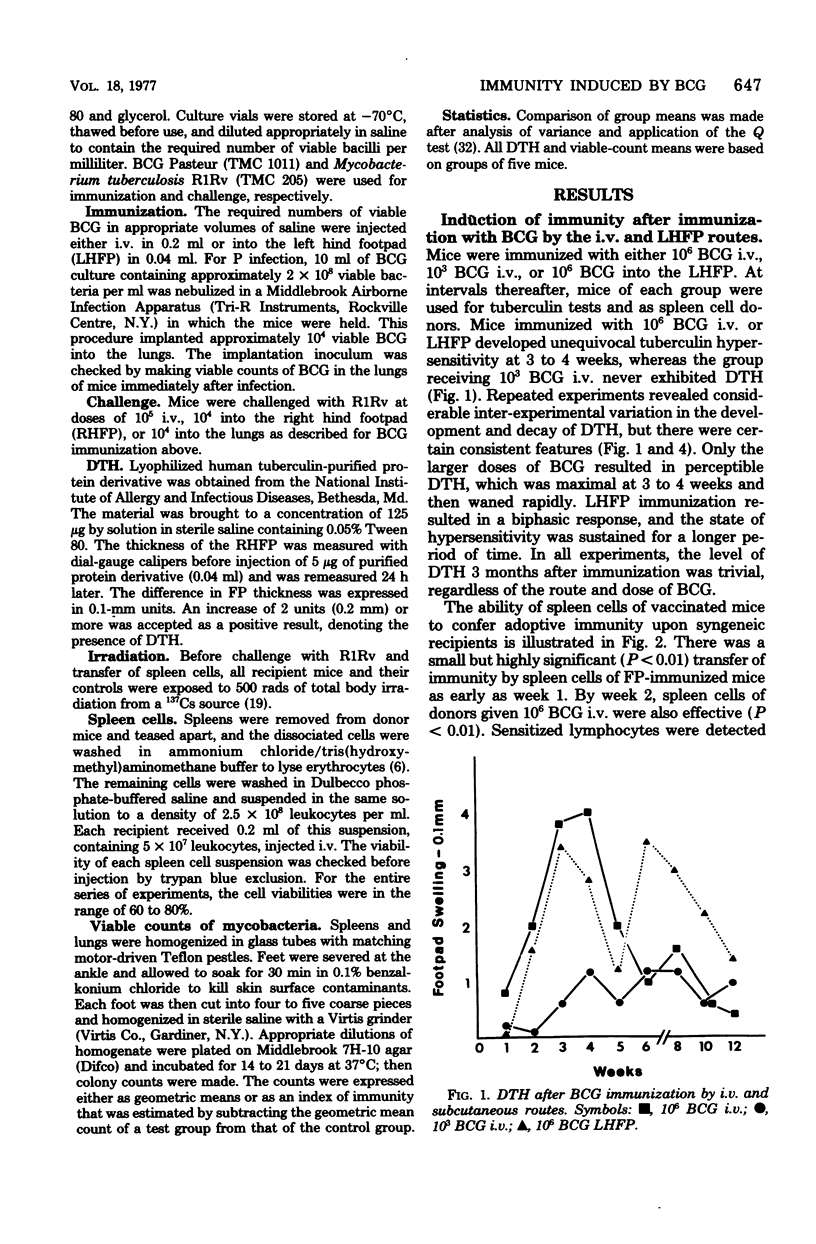
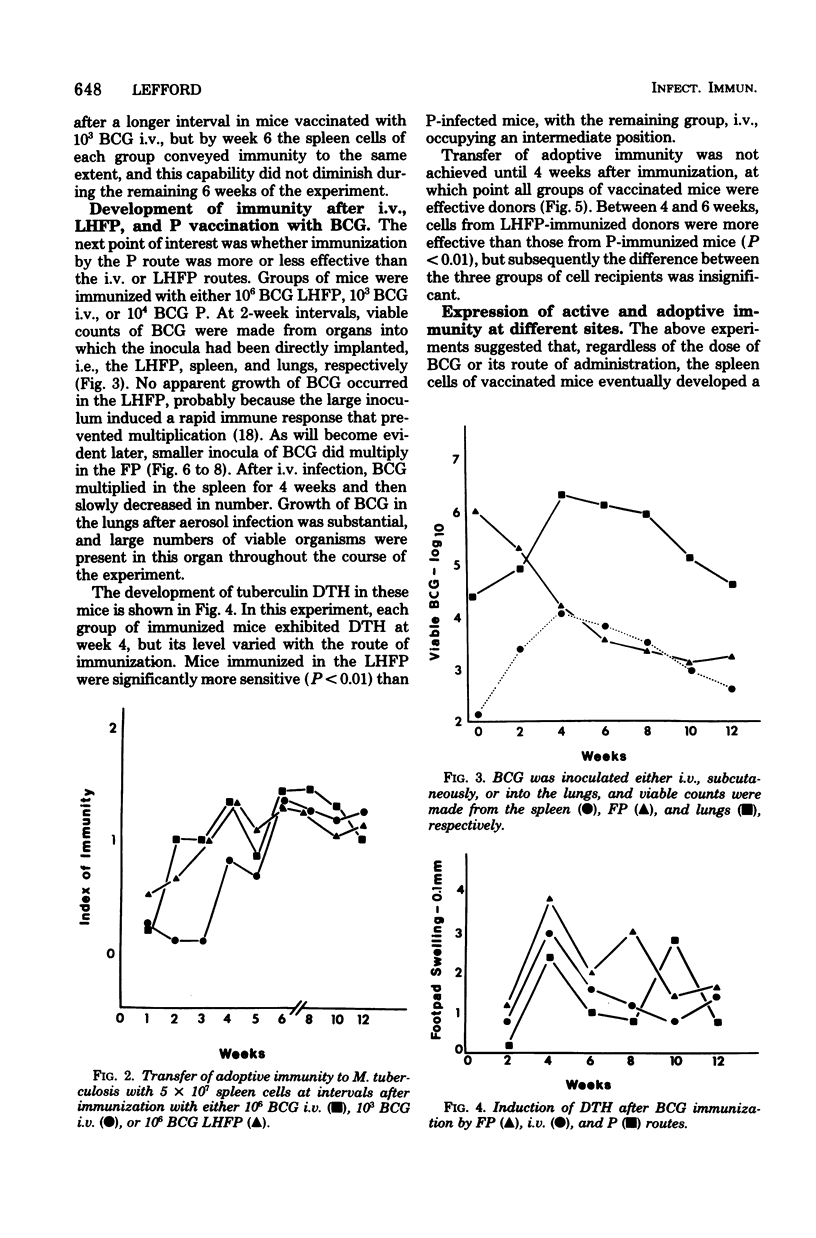
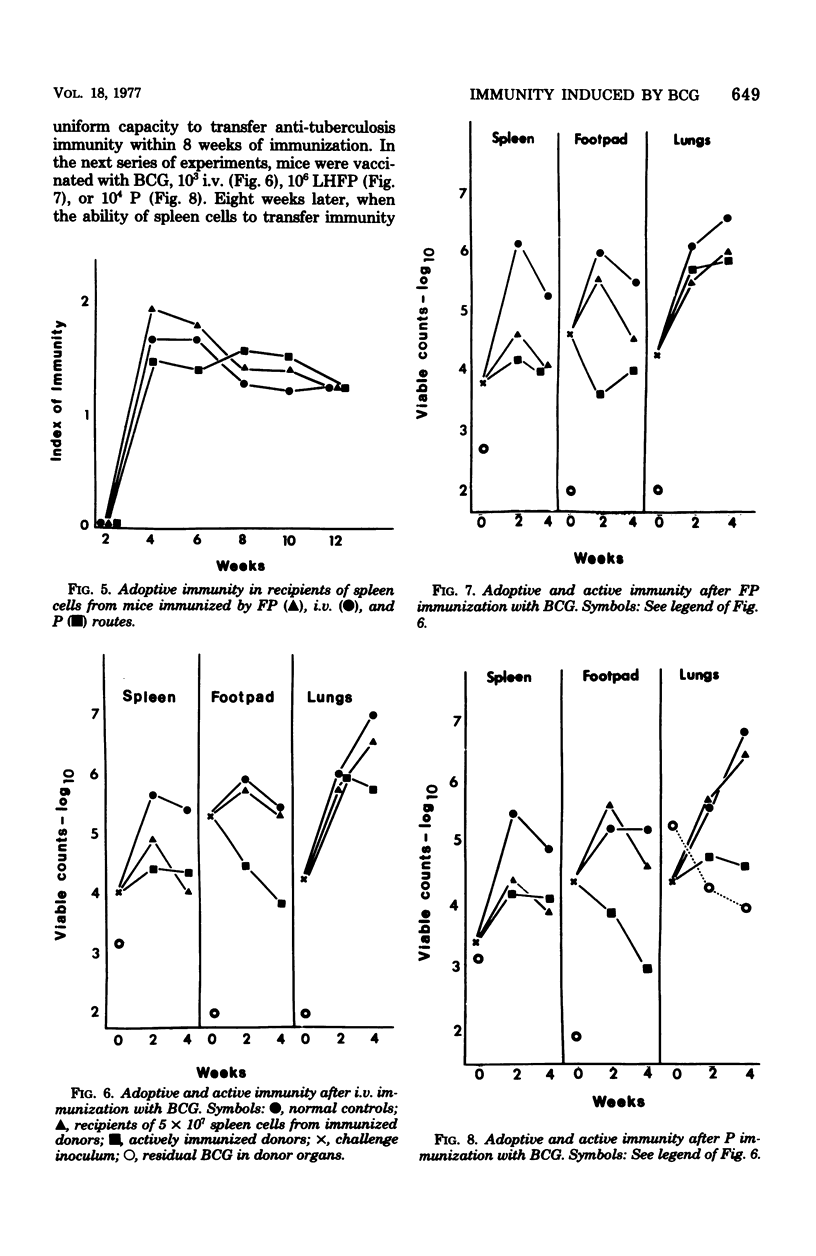
The induction and expression of immunity to Mycobacterium tuberculosis after BCG immunization by intravenous, subcutaneous, and pulmonary routes has been investigated in mice. The speed with which protective immunity was engendered was a function of inoculum size; the immunization route was a less influential factor. Tuberculin hypersensitivity varied both with the inoculim size and immunization route, being least after pulmonary immunization. Once immunity was established, a steady state ensued in which the number of sensitized lymphocytes in the spleen was similar, regardless of the route or dose of vaccination. Actively immunized animals controlled intravenous and subcutaneous challenge infections, regardless of the method of vaccination. However, pulmonary challenge was resisted most efficiently by mice immunized by the pulmonary route. Adoptive immunity was well expressed in the spleen only, but the lungs were no more deficient in this regard than the footpad. It is suggested that enhanced immunity in the lungs depends on a population of resident sensitized lymphocytes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anacker R. L., Barclay W. R., Brehmer W., Goode G., List R. H., Ribi E., Tarmina D. F. Effectiveness of cell walls of Mycobacterium bovis strain BCG administered by various routes and in different adjuvants in protecting mice against airborne infection with Mycobacterium tuberculosis strain H37Rv. Am Rev Respir Dis. 1969 Feb;99(2):242–248. doi: 10.1164/arrd.1969.99.2.242. [DOI] [PubMed] [Google Scholar]
- Anacker R. L., Barclay W. R., Brehmer W., Larson C. L., Ribi E. Duration of immunity to tuberculosis in mice vaccinated intravenously with oil-treated cell walls of Mycobacterium bovis strain BCG. J Immunol. 1967 Jun;98(6):1265–1273. [PubMed] [Google Scholar]
- Anacker R. L., Ribi E., Tarmina D. F., Fadness L., Mann R. E. Relationship of footpad sensitivity to purified protein derivatives and resistance to airborne infection with Mycobacterium tuberculosis of mice vaccinated with mycobacterial cell walls. J Bacteriol. 1969 Oct;100(1):51–57. doi: 10.1128/jb.100.1.51-57.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURRELL R. G., RHEINS M. S. Production of anti-lung substances in rabbits by homologous tuberculous tissue antigens. Am Rev Tuberc. 1958 Aug;78(2):259–267. doi: 10.1164/artpd.1958.78.2.259. [DOI] [PubMed] [Google Scholar]
- Barclay W. R., Busey W. M., Dalgard D. W., Good R. C., Janicki B. W., Kasik J. E., Ribi E., Ulrich C. E., Wolinsky E. Protection of monkeys against airborne tuberculosis by aerosol vaccination with bacillus Calmette-Guerin. Am Rev Respir Dis. 1973 Mar;107(3):351–358. doi: 10.1164/arrd.1973.107.3.351. [DOI] [PubMed] [Google Scholar]
- Blanden R. V., Lefford M. J., Mackaness G. B. The host response to Calmette-Guérin bacillus infection in mice. J Exp Med. 1969 May 1;129(5):1079–1107. doi: 10.1084/jem.129.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle W. An extension of the 51Cr-release assay for the estimation of mouse cytotoxins. Transplantation. 1968 Sep;6(6):761–764. doi: 10.1097/00007890-196809000-00002. [DOI] [PubMed] [Google Scholar]
- Cantey J. R., Hand W. L. Cell-mediated immunity after bacterial infection of the lower respiratory tract. J Clin Invest. 1974 Nov;54(5):1125–1134. doi: 10.1172/JCI107856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cate C. C., Burrell R. Lung antigen induced cell-mediated immune injury in chronic respiratory diseases. Am Rev Respir Dis. 1974 Jan;109(1):114–123. doi: 10.1164/arrd.1974.109.1.114. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Miller T. E. Growth of a drug-resistant strain of Mycobacterium bovis (BCG) in normal and immunized mice. J Infect Dis. 1969 Nov;120(5):517–533. doi: 10.1093/infdis/120.5.517. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Wayne L. G., v Montalbine The effect of cultural conditions on the distribution of Mycobacterium tuberculosis in the spleens and lungs of specific pathogen-free mice. Am Rev Respir Dis. 1974 Aug;110(2):147–156. doi: 10.1164/arrd.1974.110.2.147. [DOI] [PubMed] [Google Scholar]
- Ellis S. T., Gowans J. L. The role of lymphocytes in antibody formation. V. Transfer of immunological memory to tetanus toxoid: the origin of plasma cells from small lymphocytes, stimulation of memory cells in vitro and the persistence of memory after cell-transfer. Proc R Soc Lond B Biol Sci. 1973 Mar 13;183(1071):125–139. doi: 10.1098/rspb.1973.0009. [DOI] [PubMed] [Google Scholar]
- GOWANS J. L., KNIGHT E. J. THE ROUTE OF RE-CIRCULATION OF LYMPHOCYTES IN THE RAT. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- Galindo B., Myrvik Q. N. Migratory response of granulomatous alveolar cells from BCG-sensitized rabbits. J Immunol. 1970 Jul;105(1):227–237. [PubMed] [Google Scholar]
- KAWATA H., MYRVIK Q. N., LEAKE E. S. DISSOCIATION OF TUBERCULIN HYPERSENSITIVITY AS MEDIATOR FOR AN ACCELERATED PULMONARY GRANULOMATOUS RESPONSE IN RABBITS. J Immunol. 1964 Sep;93:433–438. [PubMed] [Google Scholar]
- LARSON C. L., WICHT W. C. Studies of resistance to experimental tuberculosis in mice vaccinated with living attenuated tubercle bacilli and challenged with virulent organisms. Am Rev Respir Dis. 1962 Jun;85:833–846. doi: 10.1164/arrd.1962.85.6.833. [DOI] [PubMed] [Google Scholar]
- Lefford M. J., McGregor D. D., Mackaness G. B. Immune response to Mycobacterium tuberculosis in rats. Infect Immun. 1973 Aug;8(2):182–189. doi: 10.1128/iai.8.2.182-189.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J., McGregor D. D., Mackaness G. B. Properties of lymphocytes which confer adoptive immunity to tuberculosis in rats. Immunology. 1973 Oct;25(4):703–715. [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J. The effect of inoculum size on the immune response to BCG infection in mice. Immunology. 1971 Aug;21(2):369–381. [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J. Transfer of adoptive immunity to tuberculosis in mice. Infect Immun. 1975 Jun;11(6):1174–1181. doi: 10.1128/iai.11.6.1174-1181.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIDDLEBROOK G. Immunological aspects of airborne infection: reactions to inhaled antigens. Bacteriol Rev. 1961 Sep;25:331–346. doi: 10.1128/br.25.3.331-346.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYRVIK Q. N., LEAKE E. S., OSHIMA S. A study of macrophages and epitheloid-like cells from granulomatous (BCG-induced) lungs of rabbits. J Immunol. 1962 Nov;89:745–751. [PubMed] [Google Scholar]
- Meyer T. J., Ribi E., Azuma I. Biologically active components from mycobacterial cell walls. V. Granuloma formation in mouse lungs and guinea pig skin. Cell Immunol. 1975 Mar;16(1):11–24. doi: 10.1016/0008-8749(75)90181-1. [DOI] [PubMed] [Google Scholar]
- Nash D. R., Holle B. Local and systemic cellular immune responses in guinea-pigs given antigen parenterally or directly into the lower respiratory tract. Clin Exp Immunol. 1973 Apr;13(4):573–583. [PMC free article] [PubMed] [Google Scholar]
- North R. J. T cell dependence of macrophage activation and mobilization during infection with Mycobacterium tuberculosis. Infect Immun. 1974 Jul;10(1):66–71. doi: 10.1128/iai.10.1.66-71.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribi E., Larson C., Wicht W., List R., Goode G. Effective nonliving vaccine against experimental tuberculosis in mice. J Bacteriol. 1966 Mar;91(3):975–983. doi: 10.1128/jb.91.3.975-983.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. B., Cunningham A. J., Lafferty K. J., Morris B. The role of the lymphatic system and lymphoid cells in the establishment of immunological memory. Aust J Exp Biol Med Sci. 1970 Feb;48(1):57–70. doi: 10.1038/icb.1970.6. [DOI] [PubMed] [Google Scholar]
- Spencer J. C., Waldman R. H., Johnson J. E., 3rd Local and systemic cell-mediated immunity after immunization of guinea pigs with live or killed m. tuberculosis by various routes. J Immunol. 1974 Apr;112(4):1322–1328. [PubMed] [Google Scholar]
- Truitt G. L., Mackaness G. B. Cell-mediated resistance to aerogenic infection of the lung. Am Rev Respir Dis. 1971 Dec;104(6):829–843. doi: 10.1164/arrd.1971.104.6.829. [DOI] [PubMed] [Google Scholar]


