Abstract
Bombyx mori L. (Lepidoptera: Bombycidae) nucleopolyhedrovirus (BmNPV) is a highly pathogenic virus in the sericultural industry, often causing severe damage leading to large economic losses. The immune mechanisms of B. mori against this virus remain obscure. Previous studies had demonstrated Bmlipase-1, BmNox and Bmserine protease-2 showing antiviral activity in vitro , but data on the transcription levels of these proteins in different resistant strains were not reported. In order to determine the resistance level of the four different strains (P50, A35, A40, A53) and gain a better understanding of the mechanism of resistance to BmNPV in B. mori , the relative expression level of the genes coding the three antiviral proteins in larval haemolymph and midgut of different B. mori strains resistant to BmNPV was determined. The results showed that these genes expressed significantly higher in the resistant strains compared to the susceptible strain, and the differential expression levels were consistent with the LC50 values in different strains. The transcription level of the target genes almost all up-regulated in the larvae midgut and down-regulated in the haemolymph. The results indicate the correlation of these genes to BmNPV resistance in B. mori.
Keywords: Bombyx mori nucleopolyhedrovirus , Bmlipase-1, BmNox, Bmserine protease-2, expression analysis
Introduction
The silkworm, Bombyx mori L. (Lepidoptera: Bombycidae), is a commercially important insect for production of silk and recombinant proteins and is also a good model of the Lepidoptera ( Goldsmith et al. 2005 ). Bombyx mori nucleopolyhedrovirus (BmNPV) is a primary pathogen of the domestic silkworm and causes large economic losses ( Miao et al. 2005 ). Most silkworm strains are highly susceptible to BmNPV, while only a few exhibit high resistance to BmNPV ( Bao et al. 2009 ). The heredity of silkworm resistance to BmNPV is relatively complicated because it is controlled both by major dominant genes and multiple micro-effective genes ( Yao et al. 2003 ). In insects, the midgut is involved in defense responses against pathogens, meanwhile it has been shown that BmNPV could invade the fat body and haemocytes of both susceptible and resistant strains through the injection route, but viral proliferation in the fat body and haemocytes of the resistant strain was notably slowed ( Bao et al. 2010 ).
A number of studies have been conducted on insect resistance to NPV. Bombyx mori serine protease-2, lipase-1, and alkaline trypsin protein purified from the digestive juice of B. mori larvae showed strong antiviral activity to BmNPV in vitro ( Ponnuvel et al. 2003 ; Nakazawa et al. 2004; Ponnuvel et al. 2012 ). Soluble B. mori NADPH oxidoreductase (BmNox) isolated from silkworm larvae gut juice was also confirmed to have antiviral activity to BmNPV ( Selot et al. 2007 ; Selot et al. 2010 ). Recently, comparative gene expression techniques, such as differential display, cDNA microarray assay, and two-dimensional gel electrophoresis (2-DE), have become routine to examine changes in gene expression. Using the fluorescent differential display (FDD) technique, Bmsop2 and Bms3a were identified to be related to BmNPV resistance in silkworm ( Xu et al. 2005 ; Xu et al. 2008 ). A gene encoding arginine kinase in B. mori has been identified differentially expressed in the midgut of B. mori resistant and susceptible strains to BmNPV by 2-DE ( Kang et al. 2011 ). But, the transcription level of the mentioned genes in different resistant strains was not confirmed accurately.
In this study, the expression of several previously reported antiviral genes in the haemolymph and midgut of silkworm strains differing in virus resistance was quantified using realtime quantitative PCR to confirm their relevance to antiviral activity.
Materials and Methods
Silkworm strains and virus
Long-established silkworm strains, including a low resistant strain P50 (a standard reference strain), a highly resistant strain A35, and strains A40 and A53, which were previously uncharacterized for viral resistance, were maintained in the Key Laboratory of Sericulture, Anhui Agricultural University, Hefei, China. The first three instars of larvae (P50, A35, A40, and A53) were reared on fresh mulberry leaves at 26 ± 1°C, 75 ± 5% of relative humidity, with a 12:12 L:D photoperiod, and the last two instars were reared on fresh mulberry leaves at 24 ± 1°C under the same relative humidity and photoperiod as before.
BmNPV T3 strain ( Gomi et al. 1999 ) was maintained in our laboratory. The occlusion body (OB) of T3 strain was obtained from the haemolymph of infected larvae and was purified by repeated and differential centrifugation following the previously published protocol ( Rahman et al. 2004 ). The concentration of the virus (OB/mL) was determined using a haemocytometer (Hujiang Medical Instruments, ( www.shhjylqx.cn ). The suspension in sterile distilled water was prepared to contain 1×108 OB/mL. The desired concentrations of OB suspension were prepared by serial dilution in sterile distilled water.
Silkworm strain resistance level bioassays
The fourth instar B. mori larvae were used in oral infection assays to determine the infectivity of BmNPV. Fresh mulberry leaves were cut into 1 cm × 1 cm pieces, and 30 µL of BmNPV T3 strain with different concentrations (1×108 OB/mL, 1×107 OB/mL, 1×106 OB/mL, 1×105 OB/mL, and 1×104 OB/mL) was spread on each piece. Every larva was fed with a piece of aired mulberry leaf, then was transferred to fresh mulberry leaves and reared at 24 ± 1°C after the small piece was eaten, and the mortality was investigated every 24 hours. Every 30 larvae were used in each of the three independent experiments. The infectivity of BmNPV was determined by probit analysis with IBM SPSS Statistics 20 (IBM, ( www.ibm.com ).
Experimental treatments of silkworm
Thirty fifth instar molt larvae chosen from each strain were divided into three groups and starved overnight. All the 120 larvae were infected with 500 OB of BmNPV T3 strain per larva orally. The 120 control larvae were fed with 5 µL sterile distilled water instead of OB. The infected and control groups were reared in different rooms under the same condition.
Preparation of haemolymph and midgut tissues of silkworm larvae
Based on the reported time course of viral proliferation and expression of NPV responsive genes in the fat body and haemocytes of susceptible and resistant strains ( Bao et al. 2010 ), at 48 hr the caudal horns of the larvae were cut and the haemolymph was collected using microcentrifuge tubes (Kirgen, ( www.kirgen.com ) that contained a small aliquot of phenylthiocarbamide to protect the haemolymph from oxidation. 500 µL of haemolymph was mixed with an equal volume of TRIzol Reagent (Invitrogen, Life Technologies, ( www.lifetechnologies.com ) then homogenized using a pellet pestle motor (Kontes, ( www.kontes.cz ) and stored at −80°C for later use. Midguts were taken from the horn cut larvae and dissected in PBS (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, and 1.4 mM KH2PO4) prepared with DEPC-reated H2O (Sangon, ( www.sangon.com ). Each tube contained 100 mg wet weight midgut, was mixed with 1000 µL TRIzol reagent, and was then homogenized using a pellet pestle motor and stored at −80°C for later use.
RNA isolation and cDNA synthesis
Total RNA was extracted from B. mori haemolymph and midgut using TRIzol reagent according to the manufacturer’s instructions. The ratios of A260/280 and the concentrations for the RNA samples were determined using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, ( www.thermofisher.com ).
Total RNA samples were treated with RT reagent kit with gDNA Eraser (TaKaRa, ( www.takara.com.cn ) to remove genomic DNA and synthesize the first strand cDNA according to the manufacturer’s instructions. Briefly, 2.0 µL 5×g DNA Eraser buffer, 1.0 L gDNA Eraser, and 1.0 µg total RNA were ixed in a 200 µL PCR tube and then RNase Free dH2O was added up to 10 µL, and the solution was then incubated at room temperature for 5 minutes. 4.0 µL 5× PrimeScript buffer, 1.0 µL PrimeScript RT Enzyme Mix I, and 1.0 µL RT Primer Mix was then added to the previous tube, then RNase Free dH2O was added up to 20 µL, and the solution was then incubated at 37°C for 15 minutes followed by 85°C for 5 seconds and stored at −20°C for later use.
PCR primer design
Three target genes, Bmlipase-1 (GenBank accession no. AB076385.1), Bmserine protease-2 (GenBank accession no. AB073673.1), and BmNox (GenBank accession no. EF025315.2), related to antiviral activity against BmNPV were analyzed using the following primers:
5'-CTGGAACAGCAACGGAAACT-3' (forward primer) and 5'-TCCGTTGTTGATGAGCCAGA-3' (reverse primer);
5'-CCAGGATTGTGGGTGGTTCT-3' (forward primer) and 5'-CAAAAGCGAGGGTGAACTGA-3' (reverse primer);
5'-CGACGAGCGACTAACCCAAA-3' (forward primer) and 5'-GAGCTCATACGGACTCAACCTG-3' (reverse primer).
As an internal control, the Bombyx mori ribosomal protein s3 ( Bmrps3 ) gene (GenBank accession no. AY769316.1) was also analyzed using the forward primer:
5'-CGATTCAACATTCCAGAGCA-3' and the reverse primer: 5'-GAACACCATAGCAAGCACGAC-3'. Primers were designed using Primer Premier 5.0 (Premier Biosoft, ( www.premierbiosoft.com ).
Expression analysis of antiviral genes by realtime PCR
Realtime quantitative PCR was carried out in a 25 µL reaction mix containing 12.5 µL of SYBR Premix Ex Taq (TaKaRa, ( www.takara.com.cn ), 1 µL of 1:5 diluted cDNA template, 1 µL of each of the primers (10 µM), and 9.5 µL ddH2O. The thermal cycling profile consisted of initial denaturation at 95°C for 30 sec and 40 cycles at 95°C for 5 sec, 60°C for 30 sec, and 72°C for 20 sec. Relative expression levels were calculated using the 2−∆∆Ct method, where ∆∆Ct=∆Ctsample−∆Ctreference following the previously published protocol ( Livak and Schmittgen 2001 ). PCR reactions were performed in 96-well plates with a Multicolor Realtime PCR Detection System (Bio-Rad, ( www.bio-rad.com ) using SYBR Green to detect dsDNA synthesis. PCR amplification was performed in duplicate wells.
Statistical analysis
The infectivity of BmNPV was determined by probit analysis with IBM SPSS Statistics 20 (larval mortality was set as the response frequency, the larvae number as the total observed, the BmNPV concentrations as the covariate(s), and the transform option was set as Log base 10). The relative expression level of the three target genes were analyzed by multiple ANOVA with SAS 9.3 (SAS Institute, ( www.sas.com ).
Results
BmNPV infectivity in different silkworm strains
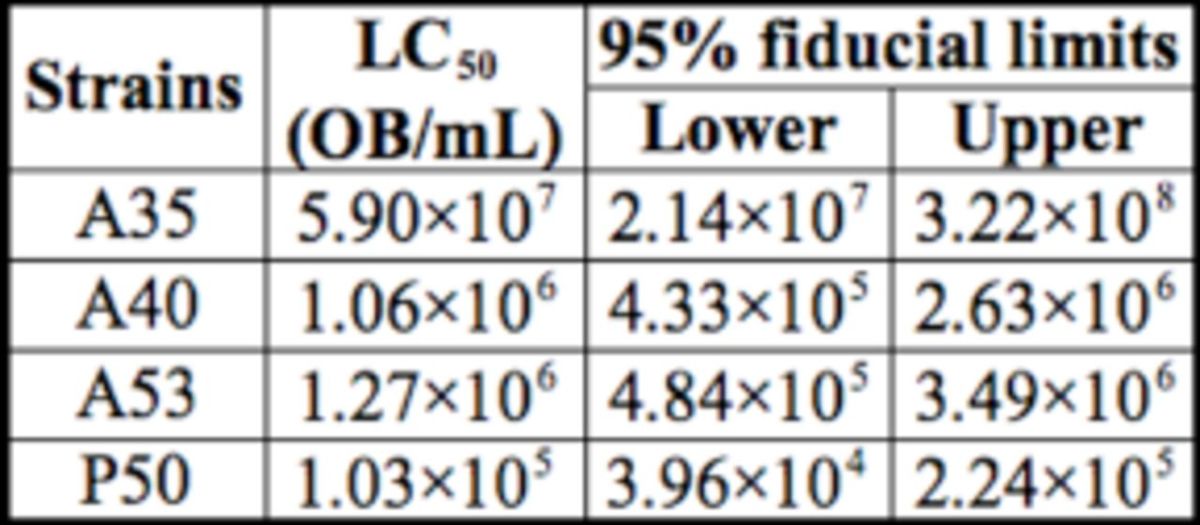
The LC50 value of A35 was about 50 fold greater than that of A40 and A53, and over 500 fold greater than that of P50. The value of A40 was a little higher than that of A53, but the difference was not significant ( Table 1 ).
Table 1.
Concentration mortality of Bombyx mori larvae infected with BmNPV.
Realtime quantitative PCR analysis
In order to determine the expression differences of the antiviral genes, analysis of the larval haemolymph and midgut of different B. mori strains was examined by realtime quantitative PCR. In the larval haemolymph, the relative expression level of Bmlipase-1 in the A35 strain was notably up-regulated compared to the P50 strain ( P < 0.01) in both infected and control groups, and the A40 and A53 strains were also up-regulated compared to the P50 strain ( P < 0.05) ( Figure 1A ). The transcripts of BmNox and Bmserine protease-2 showed a similar situation in A35, A40, and A53 strains in both infected and control groups (Figures 2A, 3A). A40 and A53 did not show significant differences in the expression of Bmlipase-1 , BmNox control group, or Bmserine protease-2 infected group, and notably decreased compared to the A35 strain ( Figure 1A–C ).
Figure 1.
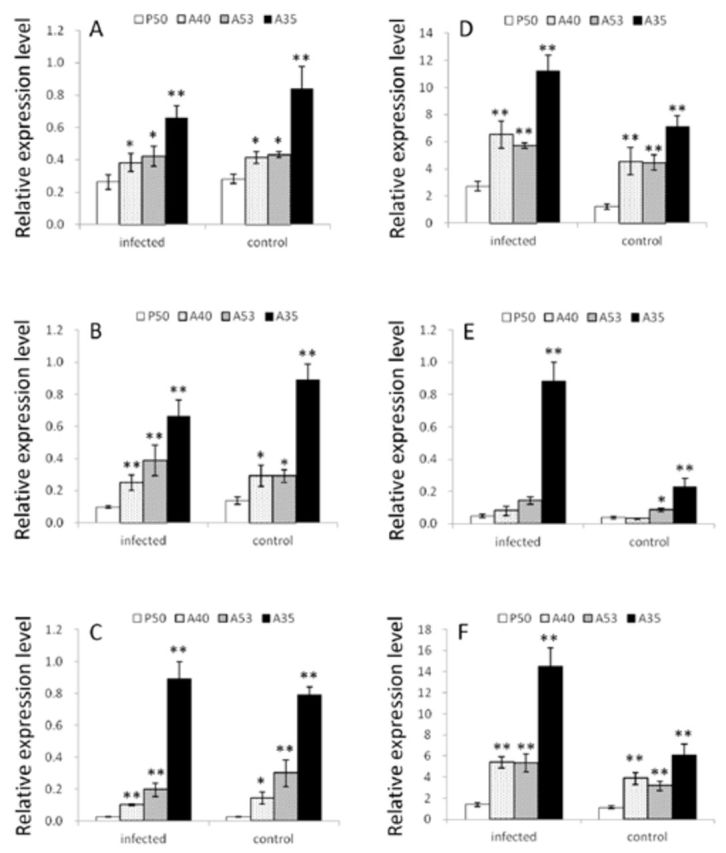
Relative expression level of antiviral genes in Bombyx mori hemolymph and midgut of different strains. A, B, and C refer to relative expression levels of Bmlipase-1 , BmNox and Bmserine protease-2 in hemolymph, respectively. D, E, and F refer to the same, but in the midgut. The bars from left to right indicate P50, A40, A53, and A35 respectively. *, P < 0.05; **, P < 0.01 (ANOVA and LSD aposteriori test). Error bars ± SEM. High quality figures are available online.
In the larval midgut, Bmlipase-1 in A35, A40, and A53 strains all expressed differently compared to the P50 strain, and the changes were significantly high ( P < 0.01) in both infected and control groups ( Figure 1D ). For BmNox , the transcription level in A35 was significantly higher than the P50 strain ( P < 0.01), was not significant in the A53 infected group and A40 infected and control groups, and increased significantly in the A53 control group compared to the P50 strain ( P < 0.05) ( Figure 1E ). The relative expression levels of Bm-serine protease-2 in both infected and control populations of A35, A40, and A53 strains were significantly higher than those of the P50 strain ( P < 0.01) ( Figure 1F ).
We also analyzed the expression differences between the infected and control groups for all four strains and found that the relative expression level of the three target genes all decreased in the larval haemolymph after BmNPV infection, except BmNox in A53 and Bmserine protease-2 in A35, but the up and down regulation were not significant ( Figure 1A –C). In the larval midgut, the expression of the target genes up-regulated after BmNPV infection, and the changes were significant in A40, A53, and A35; the upregulation of Bmserine protease-2 was significant ( P < 0.01) in the P50 strain, but BmNox and Bmlipase-1 were not ( Figure 1D –F).
Discussion
Based on the previous studies, we carried out gene expression analysis to three identified proteins (Bmlipase-1, BmNox, Bmserine protease-2) related to BmNPV-resistance in silkworm on the transcription level.
Bmlipase-1, a protein first purified by Ponnuvel et al. (2003) from the digestive juice of silkworm larvae, which showed 56% homology with Drosophila melanogaster lipase and had lipase activity in vitro , is representative of such protein. It was reported that overexpressing the Bmlipase-1 gene in silkworm reduced the mortality of the transgenic line by approximately 33% compared to the nontransgenic line when the virus dose was 106 OB/larva ( Jiang et al. 2012 ). In the current study, the relative expression level of Bmlipase-1 in resistant strains increased significantly compared to the susceptible strain in the larval haemolymph and midgut ( Figure 1A , D). As expected, the transcription level of Bmlipase-1 between A40 and A53 did not have a significant difference, while the levels in A40 and A53 were down-regulated significantly compared to that in A35. These results corresponded to the LC50 values ( Table 1 ) and were similar to previous research ( Ponnuvel et al. 2003 ; Jiang et al. 2012 ). Du et al. (2012) identified four lipases directly involved in PBAN-stimulated sex pheromone biosynthesis in Bombyx mori using the DGE and RNAi approaches, but there is no evidence demonstrating the relevance between PBAN and viral resistance in insects.
B. mori NADPH oxidoreductase, a 26.5 kDa protein identified by Selot et al. (2007) displaying anti-BmNPV activity in BmN cell lines, was given such a name because of the homology with NADPH oxidoreductase and NADPH oxidase activity. Using western blotting and semi-quantitative expression, Selot et al. (2010) found this protein existed in the larval midgut but not in the fat body, silk gland, hemolymph, or hemocyte, and there was not any difference in the gut fluid of resistant and susceptible strains, with the exception that the anterior midgut of the susceptible strain showed a total absence of BmNox expression. In our study, BmNox expression level was determined much more accurately by qRT-PCR, and the existence at the transcription level of BmNox in larval haemolymph was confirmed, which was not in contradiction with the previous research, probably because of the mRNA transport from hemocyte to other tissues. Using qRT-PCR, it was determined that the BmNox relative expression level in the A35 strain was approximately 6.9 and 6.6 fold greater than that of the P50 strain in the infected and control groups of larval haemolymph respectively, and 18.1 and 7.4 fold great in larval midgut, respectively.
Bmserine protease-2 was a midgut-specific protease and could significantly reduce the infectivity of BmNPV ( Nakazawa et al. 2004 ). As reported by Qin et al. (2012) , it was expressed significantly high in BmNPV-resistant silkworms, but not in BmNPV-susceptible silkworms based on 2-DE and western blotting. Yao et al. (2008) also found that this protease only expressed in midgut, not in silk gland, fat body, hemocyte, or trachea of Bombyx mandarina . According to the data we obtained, the relative expression level of Bmserine protease-2 in larval haemolymph was much lower than that in midgut (data not shown). The data also told us that the relative expression level in resistant strains was significantly higher than in the susceptible strains ( Figure 1C , F). This situation was similar to that of Bmlipase-1 and corresponded to the LC50 values ( Table 1 ).
BmNPV infection could lead to upregulation of Bmlipase-1 , BmNox , and Bmserine protease-2 expression in the larval midgut ( Figure 1D –F) and down-regulate the expression in the haemolymph ( Figure 1A –C), suggesting midgut was the major immune barrier against BmNPV in B. mori . The decline of expression in the haemolymph possibly occurred because the viral infection damaged physical functions, resulting in the reduction of most of the gene expression.
Acknowledgments
The research was supported by grants from the National 863 plans projects of China (No. 2011AA100306), the National Natural Sciences Foundation of China (No. 10975002), and the International Cooperation Project of Anhui province (No. 11030603028).
References
- Bao YY, Tang XD, Lv ZY, Wang XY, Tian CH, Xu YP, Zhang CX . 2009. . Gene expression profiling of resistant and susceptible Bombyx mori strains reveals nucleopolyhedrovirus-associated variations in host gene transcript levels . Genomics 94 : 138 – 145 . [DOI] [PubMed] [Google Scholar]
- Bao YY, Lv ZY, Liu ZB, Xue J, Xu YP, Zhang CX . 2010. . Comparative analysis of Bombyx mori nucleopolyhedrovirus responsive genes in fat body and haemocyte of B. mori resistant and susceptible strains . Insect Molecular Biology 19 : 347 – 358 . [DOI] [PubMed] [Google Scholar]
- Goldsmith MR, Shimada T, Abe H . 2005. . The genetics and genomics of the silkworm, Bombyx mori . Annual Review of Entomology 50 : 71 – 100 . [DOI] [PubMed] [Google Scholar]
- Gomi S, Majima K, Maeda S . 1999. . Sequence analysis of the genome of Bombyx mori nucleopolyhedrovirus . Journal of General Virology 80 : 1323 – 1337 . [DOI] [PubMed] [Google Scholar]
- Jiang L, Wang GH, Cheng TC, Yang Q, Jin SK, Lu G, Wu FQ, Xiao Y, Xu HF, Xia QY . 2012. . Resistance to Bombyx mori nucleopolyhedrovirus via overexpression of an endogenous antiviral gene in transgenic silkworms . Archives of Virology 157 : 1323 – 1328 . [DOI] [PubMed] [Google Scholar]
- Kang LQ, Shi HF, Liu XY, Zhang CY, Yao Q, Wang Y, Chang C, Shi J, Cao J, Kong J, Chen KP . 2011. . Arginine kinase is highly expressed in a resistant strain of silkworm ( Bombyx mori , Lepidoptera): Implication of its role in resistance to Bombyx mori nucleopolyhedrovirus . Comparative Biochemistry and Physiology, Part B 158 : 230 – 234 . [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD . 2001. . Analysis of Relative Gene Expression Data Using RealTime Quantitative PCR and the 2-∆∆CT Method . Methods 25 : 402 – 408 . [DOI] [PubMed] [Google Scholar]
- Du MF, Yin XM, Zhang SD, Zhu B, Song QS, An SH . 2012. . Identification of lipases involved in PBAN stimulated pheromone production in Bombyx mori using the DGE and RNAi approaches . PLoS ONE 7 : 2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao XX, Xu SJ, Li MH, Li MW, Huang JH, Dai FY, Marino SW, Mills DR, Zeng PY, Mita K, Jia SH, Zhang Y, Liu WB, Xiang H, Guo QH, Xu AY, Kong XY, Lin HX, Shi YZ, Lu G, Zhang XL, Huang W, Yasukochi Y, Sugasaki T, Shimada T, Nagaraju J, Xiang ZH, Wang SY, Goldsmith MR, Lu C, Zhao GP, Huang YP . 2005. . Simple sequence repeat-based consensus linkage map of Bombyx mori . Proceedings of the National Academy of Sciences USA 102 : 16303 – 16308 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa H, Tsuneishi E, Ponnuvel KM, Furukawa S, Asaoka A, Tanaka H, Ishibashi J, Yamakawa M . 2004. . Antiviral activity of a serine protease from the digestive juice of Bombyx mori larvae against nucleopolyhedrovirus . Virology 321 : 154 – 162 . [DOI] [PubMed] [Google Scholar]
- Ponnuvel KM, Nakazawa H, Furukawa S, Asaoka A, Ishibashi J, Tanaka H, Yamakawa M . 2003. . A Lipase Isolated from the Silkworm Bombyx mori Shows Antiviral Activity against Nucleopolyhedrovirus . Journal of Virology 77 : 10725 – 10729 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnuvel KM, Nithya K, Sirigineedi S, Awasthi AK, Yamakawa M . 2012. . In vitro antiviral activity of an alkaline trypsin from the digestive juice of Bombyx mori larvae against nucleopolyhedrovirus . Archives of Insect Biochemistry and Physiology 81 : 90 – 104 . [DOI] [PubMed] [Google Scholar]
- Yao Q, Lin MW, Wang Y, Wang WB, Lu J, Dong Y, Chen KP . 2003. . Screening of molecular markers for NPV resistance in Bombyx mori L. (Lep., Bombycidae) . Journal of Applied Entomology 127 : 134 – 136 . [Google Scholar]
- Qin LG, Xia HC, Shi HF, Zhou YJ, Chen L, Yao Q, Liu XY, Feng F, Yuan Y, Chen KP . 2012. . Comparative proteomic analysis reveals that caspase-1 and serine protease may be involved in silkworm resistance to Bombyx mori nuclear polyhedrosis virus . Journal of Proteomics 75 : 3630 – 3638 . [DOI] [PubMed] [Google Scholar]
- Rahman MM, Gopinathan KP . 2004. . Systemic and in vitro infection process of Bombyx mori nucleopolyhedrovirus . Virus Research101 : 109 – 118 . [DOI] [PubMed] [Google Scholar]
- Selot R, Kumar V, Sekhar SC, Kumar PG . 2010. . Molecular characterization and expression analysis of BmNOX in two strains of Bombyx mori with contrasting viral resistance phenotype . Archives of Insect Biochemistry and Physiology 73 : 163 – 175 . [DOI] [PubMed] [Google Scholar]
- Selot R, Kumar V, Shukla S, Chandrakuntal K, Brahmaraju M, Dandin SB, Laloraya M, Kumar PG . 2007. . Identification of a Soluble NADPH Oxidoreductase (BmNOX) with Antiviral Activites in the Gut Juice of Bombyx mori . Bioscience, Biotechnology, and Biochemistry 71 : 200 – 205 . [DOI] [PubMed] [Google Scholar]
- Xu IP, Chen KP, Liu MH, Yao Q, Gao GT, Zhao Y . 2008. . Identification and characterization of Bms3a in Bombyx mori L . African Journal of Biotechnology 7 : 3424 – 3430 . [Google Scholar]
- Xu JP, Chen KP, Yao Q, Liu MH, Gao GT, Zhao Y . 2005. . Identification and characterization of an NPV infection-related gene Bmsop2 in Bombyx mori L . Journal of Applied Entomology 129 : 425 – 431 . [Google Scholar]
- Yao HP, He FQ, Guo AQ, Cao CP, Lu XM, Wu XF . 2008. . Gene analysis of an antiviral protein SP-2 from Chinese wild silkworm, Bombyx mandarina Moore and its bioactivity assay . Science in China Series C: Life Sciences 51 : 879 – 884 . [DOI] [PubMed] [Google Scholar]


