Abstract
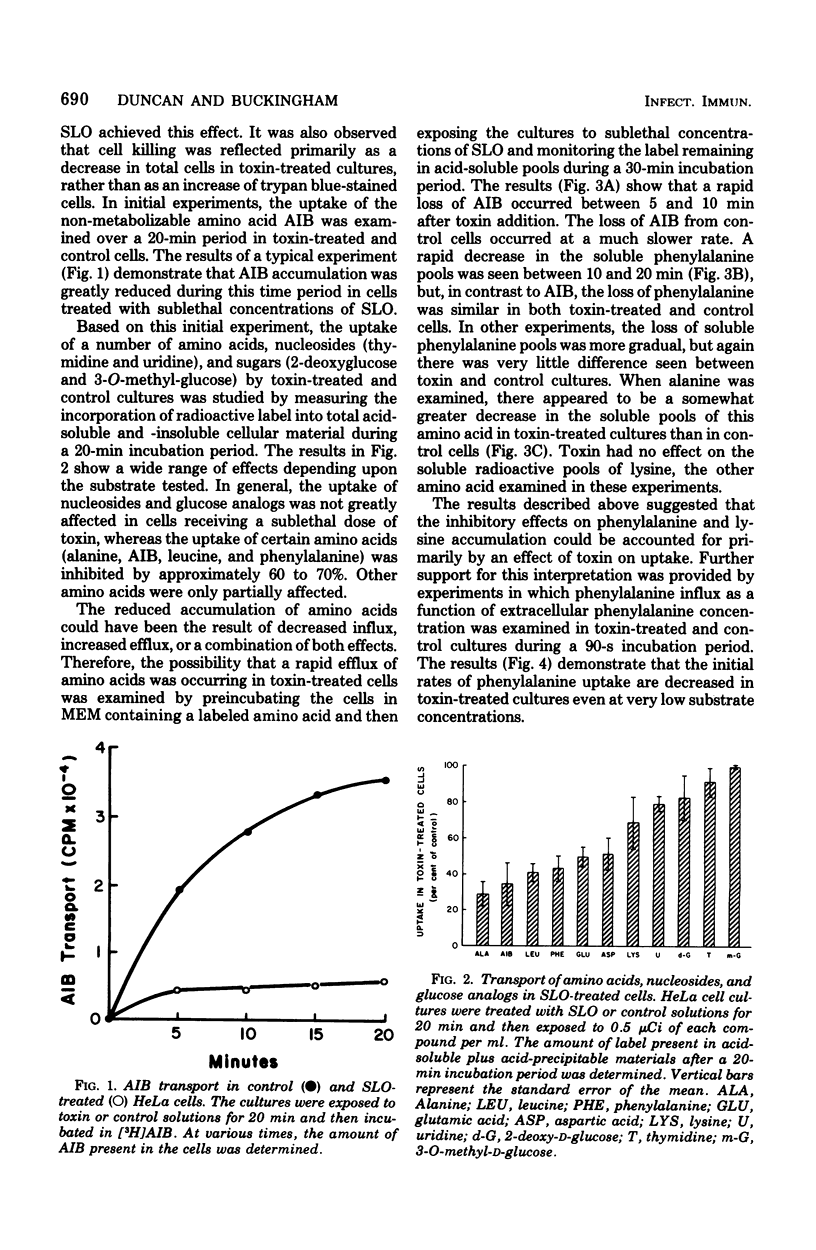
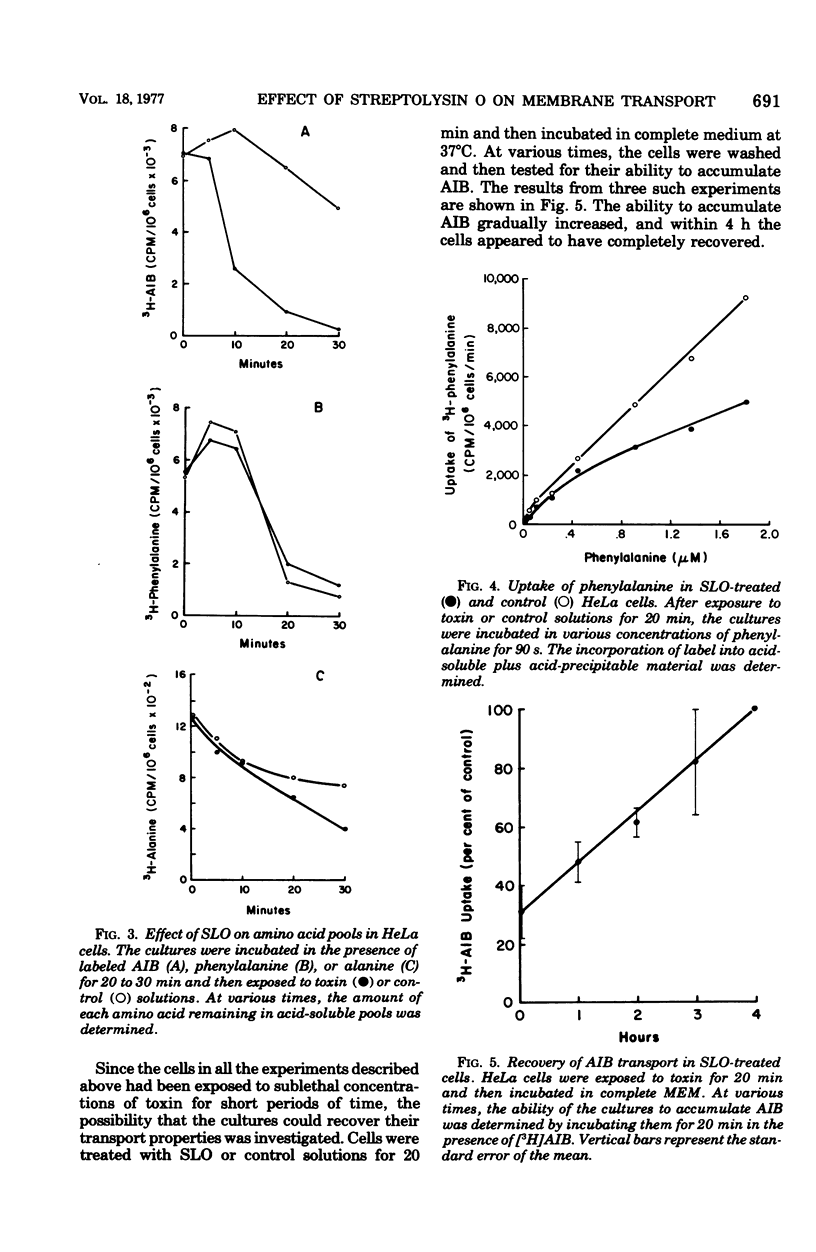
The transport of several amino acids, nucleosides, and glucose analogs was studies in HeLa cells treated with sublethal concentrations of streptolysin O. A differential effect of toxin on the various solutes tested was observed. The uptake of the neutral amino acids alanine, alpha-aminoisobutyric acid, leucine, and phenylalanine was reduced by 60 about to 70%. Less inhibition of transport was observed with acidic and basic amino acids, and the uptake of nucleosides and glucose analogs was reduced by 20% or less. The decreased uptake of alpha-aminoisobutyric acid could be explained by the inability of toxin-treated cells to retain this amino acid. The altered transport of phenylalanine and lysine, however, appeared to be due to decreased initial rates of uptake rather than to the loss of these amino acids from intracellular pools in toxin-treated cells. After treatment with sublethal concentrations of streptolysin O, the cells recovered their ability to transport and accumulate alpha-aminoisobutyric acid in about 4 h. The data suggest that the alterations in membrane transport observed in toxin-treated cells are due to an effect of streptolysin O on specific transport systems, rather than to the production of holes or pores in the membrane.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen B. R., Amirault H. J. Decreased E-rosette formation following streptolysin-O treatment. Proc Soc Exp Biol Med. 1976 Dec;153(3):405–407. doi: 10.3181/00379727-153-39556. [DOI] [PubMed] [Google Scholar]
- Andersen B. R., Cone R. Inhibition of human lymphocyte blast transformation by streptolysin O. J Lab Clin Med. 1974 Aug;84(2):241–248. [PubMed] [Google Scholar]
- Andersen B. R., Van Epps D. E. Suppression of chemotatic activity of human neutrophils by streptolysin O. J Infect Dis. 1972 Apr;125(4):353–359. doi: 10.1093/infdis/125.4.353. [DOI] [PubMed] [Google Scholar]
- Christensen H. N., de Cespedes C., Handlogten M. E., Ronquist G. Energization of amino acid transport, studied for the Ehrlich ascites tumor cell. Biochim Biophys Acta. 1973 Dec 28;300(4):487–522. doi: 10.1016/0304-4157(73)90017-8. [DOI] [PubMed] [Google Scholar]
- Duncan J. L. Characteristics of streptolysin O hemolysis: kinetics of hemoglobin and 86rubidium release. Infect Immun. 1974 Jun;9(6):1022–1027. doi: 10.1128/iai.9.6.1022-1027.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. L., Schlegel R. Effect of streptolysin O on erythrocyte membranes, liposomes, and lipid dispersions. A protein-cholesterol interaction. J Cell Biol. 1975 Oct;67(1):160–174. doi: 10.1083/jcb.67.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg I. Mechanisms of cell and tissue injury induced by group A streptococci: relation to poststreptococcal sequelae. J Infect Dis. 1972 Sep;126(3):294–340. doi: 10.1093/infdis/126.3.294. [DOI] [PubMed] [Google Scholar]
- OXENDER D. L., CHRISTENSEN H. N. DISTINCT MEDIATING SYSTEMS FOR THE TRANSPORT OF NEUTRAL AMINO ACIDS BY THE EHRLICH CELL. J Biol Chem. 1963 Nov;238:3686–3699. [PubMed] [Google Scholar]
- Ofek I., Bergner-Rabinowitz S., Ginsburg I. Oxygen-stable hemolysins of group A streptococci. 8. Leukotoxic and antiphagocytic effects of streptolysins S and O. Infect Immun. 1972 Oct;6(4):459–464. doi: 10.1128/iai.6.4.459-464.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxender D. L., Lee M., Moore P. A., Cecchini G. Neutral amino acid transport systems of tissue culture cells. J Biol Chem. 1977 Apr 25;252(8):2675–2679. [PubMed] [Google Scholar]
- Plagemann P. G., Erbe J. Temperature-dependent changes in activation energies of the transport systems for nucleosides, choline and deoxyglucose of cultured Novikoff rat hepatoma cells and effects of cytochalasin B and lipid solvents. J Membr Biol. 1975;25(3-4):381–396. doi: 10.1007/BF01868585. [DOI] [PubMed] [Google Scholar]
- Prigent D., Alouf J. E. Interaction of steptolysin O with sterols. Biochim Biophys Acta. 1976 Aug 16;443(2):288–300. doi: 10.1016/0005-2736(76)90511-3. [DOI] [PubMed] [Google Scholar]
- Thelestam M., Möllby R. Cytotoxic effects on the plasma membrane of human diploid fibroblasts--a comparative study of leakage tests. Med Biol. 1976 Feb;54(1):39–49. [PubMed] [Google Scholar]
- Thelestam M., Möllby R. Determination of toxin-induced leakage of different-size nucleotides through the plasma membrane of human diploid fibroblasts. Infect Immun. 1975 Apr;11(4):640–648. doi: 10.1128/iai.11.4.640-648.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelestam M., Möllby R. Sensitive assay for detection of toxin-induced damage to the cytoplasmic membrane of human diploid fibroblasts. Infect Immun. 1975 Aug;12(2):225–232. doi: 10.1128/iai.12.2.225-232.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G. B., Houslay M. D., Metcalfe J. C., Birdsall N. J. Cholesterol is excluded from the phospholipid annulus surrounding an active calcium transport protein. Nature. 1975 Jun 26;255(5511):684–687. doi: 10.1038/255684a0. [DOI] [PubMed] [Google Scholar]
- Wilkinson P. C. Inhibition of leukocyte locomotion and chemotaxis by lipid-specific bacterial toxins. Nature. 1975 Jun 5;255(5508):485–487. doi: 10.1038/255485a0. [DOI] [PubMed] [Google Scholar]


