Abstract
Background: Annexin A1 was investigated as prognostic factor because of its apparent association with tumorigenesis. The aim of this study was to investigate the expression of annexin A1 in lung cancer patients, and analysed the relationship with respect to the clinico-pathological features and assessed whether annexin A1 as a potential serum marker for lung cancer. Methods: Expression of annexin A1 was examined using immunohistochemistry, in-situ hybridization, western blot and enzyme-linked immunosorbent assay. Sensitivities and specificities for annexin A1 serum test were determined using receiver operator characteristic curve and cutoff was defined based on 95% and 85% sensitivities. Results: Lung cancer tissues exhibited higher expression of annexin A1 than the normal tissues (P < 0.05) and the serum annexin A1 of lung cancer patients also exhibited higher level than control groups (P < 0.05). Moreover, increased serum annexin A1 was significantly associated with the pathological grade and clinical stage of lung cancer patients (P < 0.05). Using receiver operator characteristic curve analysis, the cutoffs for distinguishing lung cancer from normal and benign groups were 4.77 and 4.84 ng/ml respectively. The sensitivities of annexin A1 for distinguishing lung cancer from normal and benign groups were 98.9% and 97.4%, and specificities were 88.3% and 66.4%. Conclusions: Up-regulation of serum annexin A1 was associated with pathological grade and clinical stage of lung cancer patients, which indicated that it could be considered molecular biomarker for diagnosis and prognosis of lung cancer.
Keywords: Lung cancer, serum, annexin A1, biomarker, diagnosis
Introduction
In the past decade, there have been considerable improvements in the way that human tumors are researched and explored. Knowledge of cancer at the molecular level has therefore increased greatly, and some achievements have led to a important shift towards using molecular diagnosis and targeted therapies for cancer [1]. One of the major challenges in lung cancer research is the identification of stable biomarkers, which can be routinely measured in easily accessible samples. In the world, lung cancer is one of the leading causes of disease deaths. It is commonly understood that an early detection of cancer leads to a better chance for reduced mortality and morbidity. Thus, identification and validation of diagnostic and prognostic biomarkers is tremendously important to improve the clinical outcome of lung cancer treatments [2]. In patients with lung cancer, several serum components have been proposed as markers of the prognosis of disease and of clinical diagnosis. Some of them seem to be sensitive and specific to early diagnosis and prognosis [3]. Identification of new molecular markers may lead to important clinical applications such as early diagnosis, prognosis, and drug targeting. However, lung cancer, the leading cause of cancer-related deaths, still lacks reliable molecular markers so far [2].
Previously, we has identified the increase of annexin A1 expression in the NCI-A549 and NCI-H446 lung cancer cells compared with the 16 HBE human bronchial epithelial cell lines, and also identified up-regulation of annexin A1 in lung cancer tissues was associated with poor post-surgical survival time and lymphatic metastasis of lung cancer patients [4]. Annexin A1 is an intracellular protein that can bind calcium and phospholipid, have several important functions in cell proliferation, such as apoptotic regulation, apoptotic cell phagocytosis, and carcinogenesis [5]. Besides, annexin A1 expression has been shown to be up-regulated in several cancers such as pancreatic, hepatocellular carcinoma, lung cancer as well as in several types of breast cancers [4,6,7]. However, compared with tissues of lung cancer, its serum level and significance in lung cancer patients remains unclearly. In this study, we further measured the level of annexin A1 in the serum of lung cancer patients, normal control and benign groups as well as to assess the clinical value of annexin A1 as serum diagnostic tool and its potential usefulness as a clinical marker for lung cancer.
Material and methods
Patient population
From January 2010 to February 2013, total 268 primary lung cancer patients were enrolled into the study (Gansu Provincial People’s Hospital, Lanzhou, China; Department of oncology, Lanzhou University Second Hospital, Lanzhou, China; Second Affiliated Hospital, Xi’an Jiaotong University, Xi’an, China). The serum samples were collected before prior radiotherapy, surgery and chemotherapy. Eighty two of 268 lung cancer patients underwent surgical resection and matched fresh cancer and adjacent normal tissues were also collected from these patients. All patients were divided according to the tumor-node-metastasis (TNM) classification of the International Association for The Study of Lung Cancer [8]. The tumors were histologically subtyped and graded according to the third edition of the World Health Organization guidelines. Male and female patients were included, with an age range of 41 to 84 years (median, 60.9 years). Clinical characteristics were retrieved from the clinical records available and were assessed retrospectively (Table 1). From January 2010 to February 2013, the serum of 256 healthy examined peoples (a healthy physical examination) were collected as normal control, including 143 men and 113 women whose mean (±SD) age was 64±8.21 years. The benign lung disease group comprised 221 patients (65±3.1 years) hospitalized in just mentioned hospital, those patients had benign lung disease of known aetiology (chronic obstructive lung disease, acute infectious diseases, tuberculosis, asthma and diffuse noninfectious interstitial diseases). Written and informed consent was obtained individually from all the participants prior to inclusion and commencement of the study. This study was conducted in accordance with the guidelines of the Ethics Committee of just mentioned hospital, by which it was approved.
Table 1.
Clinico-pathological features of lung cancer cases (N=268)
| Group | Characteristics | Number (%) |
|---|---|---|
| Sex | ||
| Male | 197 (73.8%) | |
| Female | 71 (26.2%) | |
| Age | ||
| > 60 | 138 (51.8%) | |
| ≥ 60 | 130 (48.2%) | |
| Smoking | ||
| Severe | 130 (48.5%) | |
| Moderate | 4 (1.3%) | |
| Slight | 14 (5.1%) | |
| Never | 120 (45.1%) | |
| Histology | ||
| LAC | 83 (31.2%) | |
| LSCC | 146 (54.6%) | |
| SCLC | 39 (14.2%) | |
| Pathologic grade | ||
| Poorly differentiated | 99 (37.1%) | |
| Moderately differentiated | 84 (31.4%) | |
| Well-differentiated | 53 (19.9%) | |
| Undifferentiated | 32 (11.6%) | |
| Clinical staging | ||
| I-II | 71 (26.5%) | |
| III | 97 (36.5%) | |
| IV | 68 (25.4%) | |
| Unavailable | 32 (11.6%) | |
| Pleural invasion | ||
| absent | 72 (26.4%) | |
| Present | 196(73.6%) | |
| Lymphatic invasion | ||
| Positive | 159 (59.32%) | |
| Negative | 78 (29.02%) | |
| Unavailable | 31 (11.66%) |
LAC, adenocarcinoma of the lung; LSCC, squamous cell carcinoma of the lung; SCLC, small cell lung cancer.
Immunohistochemistry (IHC)
The expression level of annexin A1 was determined using an S-P combination of IHC techniques (UltraSensitive S-P Rabbit, Product Code: SP9000, Zhongshan Jinqiao biotech company, Beijing, China), and the first antibody concentration consisted of a rabbit anti-human annexin A1 polyclonal antibody was diluted according to 1:100 dilution (Product Code: 55018-1-AP, ProteinTech Group, Inc., USA). The kit provided positive slices that served as the positive control, and an identical volume of PBS as a replacement to the primary antibody incubated in identical conditions was used as the negative control. Immunostaining was blindly evaluated according to a scoring method previously described [4]. At least ten randomly selected high-power fields and > 1,000 cells were counted for each section. Each specimen was scored according to the intensity of staining (intensity) and the area of staining (extent). The intensity was graded according to the following scale: 0, no staining; 1+, mild staining; 2+, moderate staining; 3+, intense staining. The extent was evaluated as follows: 0, no staining of cells in any microscopic fie-lds; 1+, < 30% of tissue stained positive; 2+, between 30% and 60% stained positive; 3+, > 60% stained positive. A combined staining score (intensity+extension) of ≤ 2, between 3 and 4, and between 5 and 6 were considered as low, moderate, and high expression levels, respectively.
In-situ hybridization (ISH)
The mRNA expression of annexin A1 was determined by ISH. The probe sequence of annexin A1 was 5’-TACAC CAAGT ACAGT AAGCA TGACA TGAAC AAAGT-3’. The probe was synthesized in a DNA synthesizing instrument (Bostere biotech company, Wuhan, China). ISH was strictly performed according to the ISH kit (Product Code: MK1152, Bostere biotech company, Wuhan, China). The negative controls included the following: (i) RNase treatment (20 mg/ml) hybridization and (ii) use of neither probes nor anti-Digoxigenin antibody; the controls exhibited no positive signals. The positive controls included the positive slices provided by the kit and the combined use of ISH and IHC. The mRNA expression of annexin A1 was independently evaluated by two pathologists (Wang JS and Li J). The mRNA of annexin A1 exhibited positive staining in the cytoplasm. A specific scoring method for ISH was performed according to a previously published report [9]. The scoring method was as follows: according to the signal intensity, the signals were divided into 4 groups, namely, absent (0), low (+), moderate (++), and high (+++). For statistical analysis, we grouped the patients as low (0, +), moderate (++), and high (+++).
Western blot analysis (WB)
The harvested cells were washed once with PBS, lysed with 2× sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) sample buffer (20 mM Tris, pH 8.0, 2% SDS, 2 mM dithiothreitol, 1 mM Na3VO4, 2 mM EDTA, and 20% glycerol), and boiled for 5 min. The protein concentration of each sample was determined using a Micro-BCA protein assay. In all samples, 30 μg of the total cellular protein was loaded on a 10% SDS-PAGE gel and electrophoretically separated. The proteins were transferred to polyvinylidene difluoride membranes. The membranes were blocked for 2 h at 37°C in 20 mM Tris, pH 8.0, 150 mM NaCl, and 0.05% Tween 20 (TBST) containing either 5% BSA or 5% nonfat dried milk. The membranes was incubated with anti-Hsp90-beta antibody (for immunoblotting with anti-annexin A1 was 1:400) overnight at 4°C. The primary antibody was detected using horseradish peroxidase-conjugated secondary antibodies, and after three washes with TBST, positive signals were visualized using the enhanced chemiluminescence method. All experiments were performed for three separate times.
Enzyme-linked immunosorbent assay (ELISA)
Serum samples were obtained immediately before therapy start, after having obtained an informed consent. Collected blood was allowed to clot and was centrifuged within 3 days (3000 rpm for 10-15 minutes) and were harvested and frozen at -70°C. The numbering of all of the samples was done double blinded. Any serum sample demonstrating hemolysis was considered invalid and excluded from the study. Serum level of annexin A1 were measured by sandwich-type ELISA that was originally developed using rabbit anti–human annexin A1 antibodies. The assay was performed following the directions given by the manufacturer (Annexin A1 product Code: CK-E91728H, Xitang biotech company, Shanghai, China). In brief, for detection of annexin A1 in serum, 96-well flexible microtiter plates (Xitang biotech company, Shanghai, China) were coated with diluted serum samples in PBS (pH 7.4) containing 1% BSA. The wells were incubated for 1 h with 10 ng/ml of monoclonal anti-annexin A1 antibody at 37°C, followed by reaction with avidin-conjugated peroxidase (Dako Cytomation) using a Substrate Reagent (R&D Systems) at 37°C for 15 min. The color reaction was terminated by addition of 50μl 2N sulfuric acid and the intensity was determined by a photometer at a wavelength of 450 nm. Standard curve was drawn for each plate using concentration of the standard sample and corresponding OD value of each well.
Statistical analysis
The SPSS 21.0 software package was used to perform the statistical analysis (SPSS Institute, version 21.0, Chicago, USA). The expression of mRNA and protein of annexin A1 between normal and cancerous tissues was analyzed using the χ2 and Fisher’s exact. The serum annexin A1 was evaluated using the Student’s T-test, One-WAY ANOVA and Kruskal Wallis Test. Receiver operating characteristic (ROC) curves were constructed to evaluate the performance of serum annexin A1 for differentiating lung cancer patients. All tests were two-sided, and P-values < 0.05 were considered to be statistically significant.
Results
Increased expression of annexin A1 in matched lung cancer tissues than adjacent-cancer normal tissues
The mRNA and protein expression level of annexin A1 were determined using ISH and IHC respectively. High expression of annexin A1 protein was observed in 32 (37.5%) of the 82 lung cancer tissues, whereas was observed in 13 (16.7%) of the 82 normal lung tissues respectively (P < 0.0005). The mRNA of annexin A1 was also higher expressed in 82 lung cancer tissues (33.3%) than normal lung tissues (12.5%) respectively (P < 0.0005). Up-regulated mRNA and protein expression of annexin A1 were found in the lung cancer tissues (Table 2, Figure 1A-D). High mRNA and protein expression of annexin A1 were observed in 27 (33.3%) and 32 (37.5%) of the 82 lung cancer tissues, whereas were lowly expressed in 10 (12.5%) and 13 (16.7%) of the 82 normal lung tissues respectively (P < 0.0005). The results further indicated that mRNA expression of annexin A1 was consistent with protein expression (P < 0.0005) (Table 2, Figure 2A-D).
Table 2.
Differential mRNA and protein expression of annexin A1 between the lung cancer tissues and adjacent-cancer normal tissues (N=82)
| Groups | N | Expression of annexin A1 | |||||
|
| |||||||
| - (%) | + (%) | + + (%) | χ2 value | P value | |||
|
| |||||||
|---|---|---|---|---|---|---|---|
| mRNA | |||||||
| Normal | 82 | 51 (62.5) | 21 (25) | 10 (12.5) | 35.68 | < 0.0005 | |
| Cancerous | 82 | 10 (12.5) | 45 (54.2) | 27 (33.3) | |||
| Protein | |||||||
| Normal | 82 | 45 (54.2) | 24 (29.2) | 13 (16.7) | 25.19 | < 0.0005 | |
| Cancerous | 82 | 13 (16.7) | 37 (45.8) | 32 (37.5) | |||
|
| |||||||
| Kappa value | P value | ||||||
|
| |||||||
| Normal | |||||||
| mRNA | 82 | 51 (62.5) | 21 (25) | 10 (12.5) | 1.112 | < 0.0005 | |
| Protein | 82 | 45 (54.2) | 24 (29.2) | 13 (16.7) | |||
| Cancerous | |||||||
| mRNA | 82 | 10 (12.5) | 45 (54.2) | 27 (33.3) | 0.865 | < 0.0005 | |
| Protein | 82 | 13 (16.7) | 37 (45.8) | 32 (37.5) | |||
Figure 1.
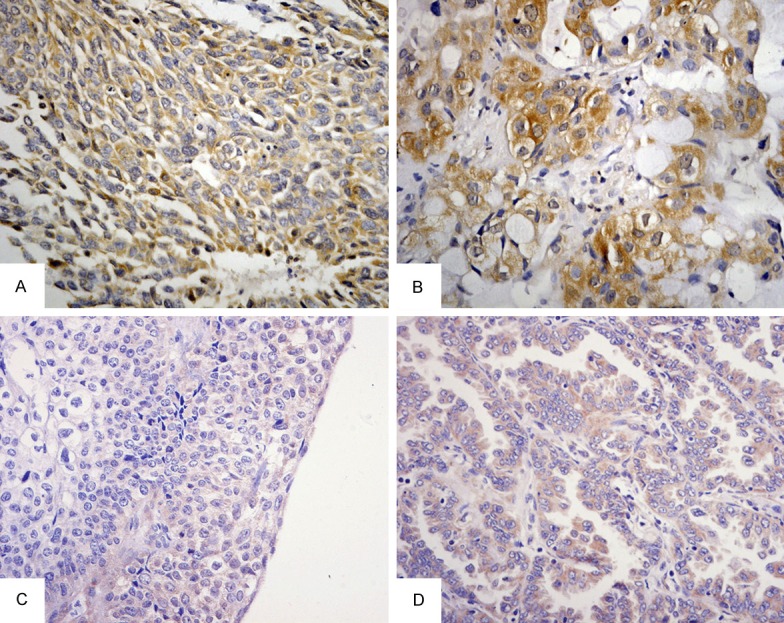
IHC and ISH of annexin A1 in lung cancer and adjacent-cancer normal tissues (×400). A. high staining of annexin A1 in poorly differential LSCC; B. high staining of annexin A1 in poorly differential LAC; C. Low staining of annexin A1 mRNA in poorly differential LSCC; D. Low staining of annexin A1 mRNA in well differentiated LAC. LSCC, squamous cell carcinoma of the lung; LAC, adenocarcinoma of the lung.
Figure 2.
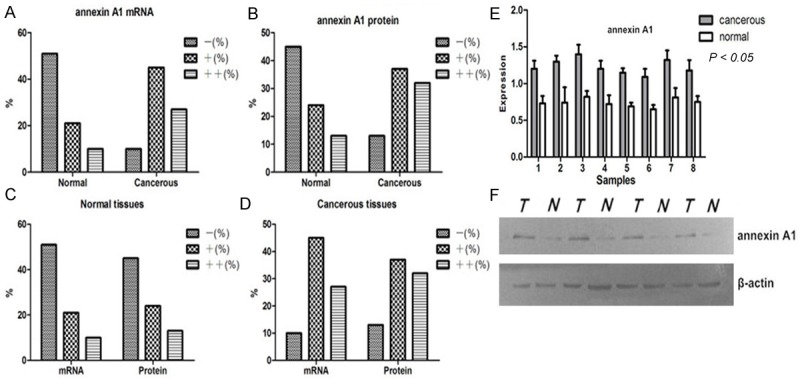
Expression rates of annexin A1 in lung cancer and adjacent-cancer normal tissues. A, B. up-regulated mRNA and protein expression of annexin A1 were found in the lung cancer tissues; C, D. mRNA expression of annexin A1 was consistent with protein expression; E, F. western blotting shows high expression of annexin A1 in cancer tissues than adjacent normal tissues (P < 0.05). Normal, adjacent-cancer normal tissues; cancerous, lung cancer tissues; N=normal tissues; T=tumor tissues.
Coincidence rate of mRNA and protein expressions for annexin A1
We performed Western blot to test the expressions of A1 in matched lung cancer tissues and adjacent-cancer normal tissues and to verify their differential expressions trend. Equal protein loading was indicated by a parallel β-actin blot experiment. The results displayed that annexin A1 was up-regulated in cancerous tissues compared with normal tissues (P < 0.05) (Figure 2E and 2F).To assess the expression trend of three methods (IHC, ISH and Western blot), nonparametric test was performed. The results also shown that coincidence rate of annexin A1 expression using three different methods was 87.8% and indicated three methods have a consistent trend.
Increased serum level of annexin A1 in lung cancer patients than benign and normal control groups
Serum level of annexin A1 was detected by enzyme-linked immunosorbent assay in a series of 268 specimens of lung cancer patients, a series of 221 specimens of benign lung diseases and a series of 256 specimens of healthy examined peoples. The results shown serum level of annexin A1 was higher in lung cancer patients (5.61±0.47 ng/ml) than in benign lung diseases group (4.01±0.58 ng/ml) and healthy examined peoples (3.63±1.01 ng/ml) (P < 0.0005) (Table 3, Figure 3A).
Table 3.
Correlation between the serum level of annexin A1 and clinicopathologic factors of lung cancer patients
| Groups | N | Level of annexin A1 | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| M±SD (ng/ml) | df | F | P | |||
| Resource | ||||||
| Normal | 256 | 3.63±1.01 | 2 | 494.73 | < 0.0005 | |
| Benign | 221 | 4.01±0.58 | ||||
| Cancerous | 268 | 5.61±0.47● | ||||
| Histology | ||||||
| LAC | 86 | 5.68±0.48 | 2 | 163.16 | 0.551 | |
| LSCC | 144 | 5.53±0.45 | ||||
| SCLC | 38 | 5.78±0.44 | ||||
| Pathologic grade | ||||||
| Undifferentiated | 32 | 5.78±0.44▲ | 3 | 256.58 | < 0.0005 | |
| Poorly | 98 | 5.99±0.27▲ | ||||
| Moderate | 84 | 5.40±0.22 | ||||
| Well | 54 | 5.09±0.35 | ||||
| Lymphatic invasion | ||||||
| N0 | 80 | 5.28±0.47▲▲ | 2 | 465.11 | < 0.0005 | |
| N1-N2 | 98 | 5.56±0.34 | ||||
| N3 | 58 | 6±0.26 | ||||
| Unavailable | 32 | 5.62±0.17 | ||||
| TNM stage | ||||||
| I-II | 72 | 5.17±0.35* | 2 | |||
| III-IV | 164 | 5.75±0.28 | 231.65 | 0.028 | ||
| Unavailable | 32 | 5.36±0.59 | ||||
| Pleural invasion | ||||||
| absent | 72 | 5.26±0.36 | 2 | 198.75 | 0.781 | |
| Present | 196 | 5.63±0.18 | ||||
LAC, adenocarcinoma of the lung; LSCC, squamous cell carcinoma of the lung; SCLC, small cell lung cancer;
cancerous compared with benign and normal using Kruskal Wallis Test;
undifferentiated and poorly compared with moderate and well;
N0 compared with N1-N3;
I-II compared with III-IV;
M±SD, mean±standard deviation; df, degree of freedom.
Figure 3.
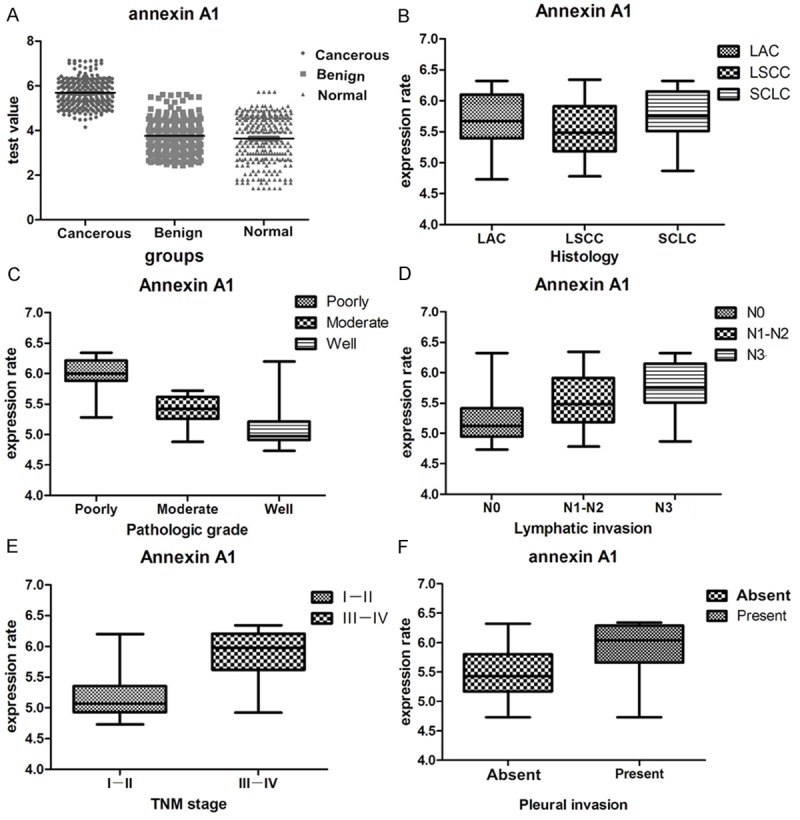
Correlation between serum level of annexin A1 and clinicopathologic factors of lung cancer patients. A. Increased serum level of annexin A1 in lung cancer patients than benign and normal control groups; B. serum level of annexin A1 was not significant difference between different histological types; C. undifferentiated and poorly undifferentiated patients revealed up-regulated level of annexin A1 than moderate and well differentiation; D. Lymphatic invasion patients (N1-N3) revealed increased level of annexin A1 than non-lymphatic invasion; E. Patients of III IV stage had higher annexin A1 value than both the I and II stage; F. Serum level of annexin A1 was not significant difference about pleural invasion. LAC, adenocarcinoma of the lung; LSCC, squamous cell carcinoma of the lung; SCLC, small cell lung cancer; N, node stage (TNM classification).
Correlation between the serum level of annexin A1 and clinicopathologic factors
Mean values of various clinical and biochemical parameters measured in different group are given in Table 3. Different group showed significant differences with respect to the following parameters: undifferentiated (5.78±0.44 ng/ml) and poorly undifferentiated patients (5.99±0.27 ng/ml) revealed up-regulated level of annexin A1 than moderate and well differentiation (P < 0.0005); lymphatic invasion patients (N1-N3) revealed increased level of annexin A1 than non-lymphatic invasion (5.28±0.47 ng/ml) (P < 0.0005); patients of III-IV stage (5.75±0.28 ng/ml) had higher annexin A1 (p=0.028) value than both the I and II stage (5.17±0.35 ng/ml) (Figure 3B-F).
Cut-off of serum annexin A1 for differentiating lung cancer patient from benign lung disease patient and normal people
The cut-off for serum annexin A1 was selected based on receiver operating characteristic (ROC) curve analysis [10]. The sensitivity and specificity for the outcome under study were plotted, thus generating an ROC curve. The minimum value that was tested by enzyme-linked immunosorbent assay was 1.41 ng/ml and located in the healthy control group and the maximum was 4.77 ng/ml, which distributed in the lung cancer group. It shown that the values of lung cancer and healthy groups intersected near by value of 4-5 ng/ml as well as lung cancer and benign groups, which indicated that their thresholds located in the segmented of 5-6 ng/ml, and 95% confidence interval were 0.282 to 0.534 and 0.635 to 1.455 (Table 4, Figure 4A, 4B, 4D and 4E).
Table 4.
NLR and PLR of annexin A1 serum level in cancerous, benign and normal groups
| NLR and PLR of serum annexin A1 for differentiating lung cancer patients from normal people | ||||
|
| ||||
| Interval | Positive | Negative | Likelihood ratio | 95% CI |
|
| ||||
| 1-2 | 0 | 25 | 0.000 | 0.000 to 0.312 |
| 2-3 | 0 | 60 | 0.000 | 0.000 to 0.128 |
| 3-4 | 0 | 55 | 0.000 | 0.000 to 0.140 |
| 4-5 | 41 | 101 | 0.388 | 0.282 to 0.534 |
| 5-6 | 156 | 15 | 9.934 | 6.017 to 16.402 |
| 6-7 | 71 | 0 | ∞ | 8.446 to ∞ |
| Total | 268 | 256 | ||
|
| ||||
| NLR and PLR of serum annexin A1 for differentiating lung cancer patients from benign lung disease patient | ||||
|
| ||||
|---|---|---|---|---|
| Interval | Positive | Negative | Likelihood ratio | 95% CI |
|
| ||||
| 1.5-2.0 | 0 | 6 | 0.000 | 0.000 to 1.494 |
| 2.0-2.5 | 0 | 17 | 0.000 | 0.000 to 0.400 |
| 2.5-3.0 | 0 | 16 | 0.000 | 0.000 to 0.426 |
| 3.0-3.5 | 0 | 36 | 0.000 | 0.000 to 0.185 |
| 3.5-4.0 | 0 | 27 | 0.000 | 0.000 to 0.248 |
| 4.0-4.5 | 0 | 20 | 0.000 | 0.000 to 0.338 |
| 4.5-5.0 | 41 | 35 | 0.962 | 0.635 to 1.455 |
| 5.0-5.5 | 76 | 53 | 1.177 | 0.870 to 1.592 |
| 5.5-6.0 | 80 | 7 | 9.382 | 4.424 to 19.894 |
| 6.0-6.5 | 71 | 4 | 14.571 | 5.407 to 39.265 |
| Total | 268 | 221 | ||
NLR, negative likelihood ratio; PLR, positive likelihood ratio; 95% CI, 95% confidence interval.
Figure 4.
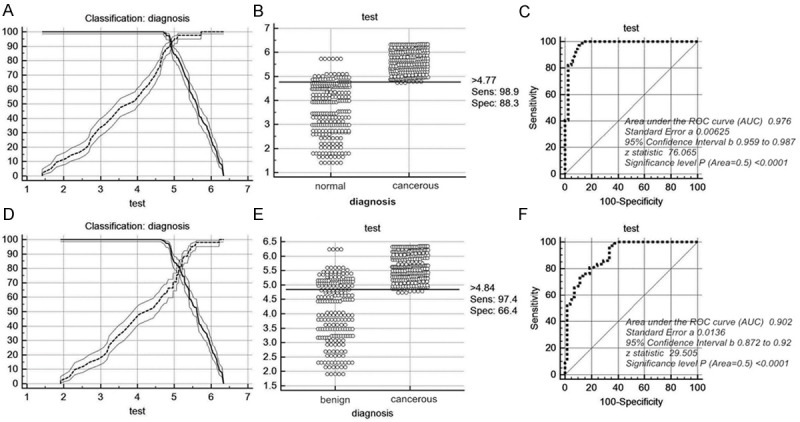
Cut-off selection of serum annexin A1 by ROC curve analysis. A-C. Selection of cut-off for serum value of annexin A1 in distinguishing lung cancer patients from normal group; receiver operating characteristic curves for annexin A1 [area under the curve (auc): 0.976]; D-F. Selection of cut-off for serum value of annexin A1 in distinguishing lung cancer patients from benign group; receiver operating characteristic curves for annexin A1 [area under the curve (auc): 0.902]. ROC, receiver operating characteristic curve; Sens, sensitivity; Spec, specificity.
Accuracy of parameters for predicting lung cancer by ROC analysis
When compared lung cancer patients with healthy people using serum annexin A1, the threshold was 4.77 ng/ml while the sensitivity and specificity were 98.9% and 88.3% respectively, which indicated that this threshold could be used to discriminate lung cancer patients from healthy people. When compared lung cancer patients with benign lung disease patients, the value of 4.84 ng/ml could be used to discriminate lung cancer from benign lung disease patients. Furthermore, the sensitivity and specificity were 97.4% and 66.4% respectively, which meant that ability of annexin A1 was higher in discriminating lung cancer from healthy people than benign lung disease patients (Table 5). Figure 4C and 4F depicts receiver operating characteristic (ROC) curves for annexin A1 when cancer group were compared with benign and healthy groups. To discriminate lung cancer from healthy people, the area under the curve (AUC) of annexin A1 was 0.976, Standard error was 0.00625, 95% confidence interval was 0.959-0.987, Z value was 76.065 (P < 0.0001) while to discriminate lung cancer from benign lung patients, the area under the curve (AUC) was 0.902, Standard error was 0.0136, 95% confidence interval was 0.872-0.92, Z value was 29.505 (P < 0.0001). The results displayed that serum annexin A1 contributes not only to the differentiated diagnosis of lung cancer patients and benign lung patients, but also to the screening of lung cancer, there for promoting early diagnosis of lung cancer as well.
Table 5.
Cut-off score of annexin A1 serum level for differentiating lung cancer patients from normal and benign groups
| Cut-off score for serum annexin A1 for differentiating lung cancer patient from normal people | ||||
|
| ||||
| Criterion | Sensitivity | 95% CI | Specificity | 95% CI |
|
| ||||
| > 4.71 | 100.00 | 98.6-100.0 | 85.94 | 81.1-90.0 |
| > 4.73 | 98.88 | 96.8-99.8 | 85.94 | 81.1-90.0 |
| > 4.77* | 98.88 | 96.8-99.8 | 88.28 | 83.7-92.0 |
| > 4.87 | 95.90 | 92.8-97.9 | 89.45 | 85.0-92.9 |
| > 4.99 | 84.70 | 79.8-88.8 | 94.14 | 90.5-96.7 |
| > 5.73 | 40.67 | 34.7-46.8 | 100.00 | 98.6-100.0 |
|
| ||||
|---|---|---|---|---|
| Cut-off score for serum annexin A1 for differentiating lung cancer patient from benign lung disease patient | ||||
|
| ||||
| Criterion | Sensitivity | 95% CI | Specificity | 95% CI |
|
| ||||
| > 4.64 | 100.00 | 98.6-100.0 | 60.00 | 53.2-66.5 |
| > 4.73 | 98.88 | 96.8-99.8 | 62.73 | 56.0-69.1 |
| > 4.78 | 97.39 | 94.7-98.9 | 62.73 | 56.0-69.1 |
| > 4.84* | 97.39 | 94.7-98.9 | 66.36 | 59.7-72.6 |
| > 5.06 | 83.58 | 78.6-87.8 | 75.45 | 69.2-81.0 |
| > 5.22 | 74.63 | 69.0-79.7 | 86.36 | 81.1-90.6 |
| > 5.45 | 58.58 | 52.4-64.5 | 95.00 | 91.2-97.5 |
| > 6.34 | 0.00 | 0.0-1.4 | 100.00 | 98.3-100.0 |
95% CI, 95% confidence;
threshold of serum annexin A1 for differentiating lung cancer patient from benign lung disease patient and normal people.
Discussion
Lung cancer is the most common cancer and the number one cancer killer in the world because it is often diagnosed at an advanced stage, which has lost best time for surgery and medication [11], so early diagnosis is the most important determinant of survival. Surgery is the diagnostic criterion standard and definitive treatment for lung cancer, but should be avoided in cases of benign growths. Needle biopsy can establish a specific benign or malignant diagnosis, but is invasive, potentially risky, and sometimes nondiagnostic [11]. Therefore, it is clinically important to develop new techniques for noninvasively diagnosing lung cancer with high accuracy. Over the past many years, carcinoembryonic antigen (CEA), neuron-specific enolase (NSE), cytokeratin 19 fragment (CYFRA21-1) and squamous cell carcinoma antigen (SCC) have been used as biomarkers for the diagnosis and management of patients with lung cancer [12,13]. However, specific marker of lung cancer is extremely absent, thus researchers never stopped exploring new valuable biomarkers for diagnosis and prognostic in lung cancer [14]. Blood is an easily accessible and rich body fluid. Furthermore, blood plasma contains specific proteins that provide potential circulating biomarkers. Annexin A1 is a calcium-dependent phospholipid-linked protein, involved in anti-inflammatory effects, regulation of cellular differentiation, proliferation and apoptosis [15]. The functions of annexin A1 in tumor growth and development have been examined in various types of cancer, but are not clearly understood, since annexin A1 expression is differentially expressed in different cancer types, including esophageal cancer, pancreatic cancer, skin squamous cell carcinoma, colon cancer, cervical cancer, oral squamous cell carcinoma, prostate cancer, breast cancer and laryngeal squamous cell carcinoma [5]. At present the research of annexin A1 expression pattern in lung cancer is confine to basic research in vitro and the serum expression features of lung cancer patients is merely understood . To uncover the serum expression of annexin A1 and its correction with lung cancer, this study was performed.
In the histological level, the results showed that annexin A1 mRNA and protein exhibited higher expression in all histological types of lung cancer, and in particular in poorly differentiated lung cancer. Thus, we may infer that higher annexin A1 expression is potentially effective in development of lung cancer. Moreover, the high expression of annexin A1 was also shown to be intimately involved in tumor cell lymph node invasion, larger tumor size and high TNM stage according to this study. These findings are in concordance with previous reports showing that a higher level of annexin A1 in cancer is associated with a poor clinical outcome compared with patients who express lower levels of annexin A1 [15-18], suggesting that increased expression of annexin A1 in the cytoplasm of the tumor cells may contribute to the progression of cancer. Besides, the results also indicated that mRNA expression of annexin A1 was consistent with its protein expression, which is important for an utility of biomarker. In order to assess annexin A1 expression trend of three different methods (IHC, ISH and Western blot), nonparametric test was performed. The results shown coincidence rate of annexin A1 expression using three different methods was 87.8%, and indicated this is a consistent trend. Via the coincidence rate test, we may infer that the level of transcription and translation of annexin A1 is more stable relatively, which is very important and useful aspect for testing stability of tumor biomarkers, and strengthen the credibility of high expression about annexin A1 in lung cancer cells. Obviously, the credibility of evidence in both levels of mRNA and protein expression will surpass single expression level. So combined detection of mRNA and protein for the same molecule should be better than each one of them, which will bring about a more accurate results. Generally, ISH represents a powerful and sensitive method for examining gene expression in cells in a manner analogous to IHC which is used to localize and identify cells containing a particular protein or labile antigens such as tumor biomarkers. Paraffin embedding allows a better preservation of tissue morphology than freezing procedure. This is of particular importance since simultaneous detection of mRNAs and proteins in the same cell populations on adjacent sections requires conditions where (a) cell morphology is highly retained in a same manner on adjacent sections, and (b) reactivity of nucleic acids and antigens is preserved [19]. Now IHC and fluorescence in situ hybridization have emerged as the most viable assays for evaluation of HER-2/neu in routine clinical practice. However, each of these methods has its advantages and disadvantages [20]. So the combined test of mRNA and protein in cancer tissues has important practical value.
In recent years, a large number of biomarkers for lung cancer have been examined in serum, such as CEA, NSE, CYFRA21-1 and so on [21,22]. In our study, the serum level of annexin A1 was analyzed in patients with lung cancer and in control groups. Median level of annexin A1 was found to be significantly higher in patients with lung cancer, compared with control groups. The serological detection of annexin A1 displayed a significant association between the high serum level and an increased risk and aggression for lung cancer. In addition, lung cancer with the high serum level of annexin A1 is more likely to show an aggressive phenotype being exemplified by poorly undifferentiated lung cancer and lymphatic metastasis. These results seem to suggest a possibility that the serum level of annexin A1 could be risk factor for lung cancer and to provide a new insight into understanding of the association between annexin A1 and lung cancer risk. Because tumor-related protein may secrete into the peripheral circulation of patients with lung cancer, it may be detected by protein analysis such as ELISA and electrochemiluminescence assay and so on. Peripheral serum tumor markers are non-invasive diagnostic tools for indentifying lung cancer, and are commonly used for the screening for lung cancer. Cutoff for annexin A1 for the diagnosis of lung cancer at specificity was determined by ROC curve analysis. Logistic regression analysis, using ROC-derived cutoff value, was used to determine the sensitivity of annexin A1. At predefined specificity of 88.3% for distinguishing lung cancer from absence of disease (healthy subjects), the sensitivity was 98.9%. Namely, when distinguishing the lung cancer group from the healthy group, annexin A1 specificity was 88.3% and sensitivity was 98.9%. We adopted the cut-off level derived from the ROC analysis, corresponding to 4.77 ng/ml. The value showed the best compromise between sensitivity and specificity in predicting lung cancer. When benign lung disease patients were used as the controls, the ROC AUC yielded annexin A1 cutoff of 4.84 ng/ml, and demonstrating that this value might provide clinically meaningful cutoff in the diagnosis of lung cancer by providing an optimal sensitivity and specificity balance in lung patients. The current availability of a simple and reliable immunoassay for measuring serum annexin A1 concentration will facilitate further studies to establish the clinical usefulness of serum annexin A1 analysis for the management of patients with lung cancer. It will be important to conduct clinical studies to evaluate the value of serum annexin A1 concentration in predicting response to treatment, including chemotherapy.
However, the study also has several limitations. Fist, the study was conducted at a single center in China, which may limit generalizability of the findings to the wider population. Second, further experimental investigations involving a larger number of samples of lung cancer patients and molecular mechanism were required to reach a more definitive conclusion. Therefore, the preliminary results need to be confirmed by a prospective study including a large number of subject s as well as by the functional analysis of annexin A1 through in vitro studies in the future. As lung cancer is a highly malignant respiratory system tumor, a detailed understanding of the function and significance of annexin A1 will help to further elucidate the biological mechanisms of lung cancer and aid in the design of preventive treatment.
Conclusion
We have demonstrated that the expression of serum annexin A1 was up-regulated in lung cancer patients and these results indicated that higher expression of increased was potentially involved in the aggression and histology of lung cancer. Furthermore, the findings in this study indicated that serum annexin A1 concentration represents a novel biomarker for lung cancer, which has potential utility as a diagnostic, prognostic, and predictive tool. However, larger numbers of lung cancer subjects are required for prospective studies as well as for further studies that are warranted to investigate the potential mechanism of increased in lung cancer.
Acknowledgements
This study was supported by grants from the National Natural Scientific Foundation of China (NO: 81172234) and the Fundamental Research Funds for the Central Universities of China. We are grateful for the technical advice provided by Dr. Du Mengang (ChaoYing Biotech Company, Xi’an, Shaanxi, China), Li Jun (The fourth Military Medical University, Xi’an, Shaanxi, China).
Disclosure of conflict of interest
None to declare.
References
- 1.Sawyers CL. The cancer biomarker problem. Nature. 2008;452:548–552. doi: 10.1038/nature06913. [DOI] [PubMed] [Google Scholar]
- 2.Kim B, Lee HJ, Choi HY, Shin Y, Nam S, Seo G, Son DS, Jo J, Kim J, Lee J, Kim K, Lee S. Clinical validity of the lung cancer biomarkers identified by bioinformatics analysis of public expression data. Cancer Res. 2007;67:7431–7438. doi: 10.1158/0008-5472.CAN-07-0003. [DOI] [PubMed] [Google Scholar]
- 3.Tufman A, Huber RM. Biological markers in lung cancer: A clinician’s perspective. Cancer Biomark. 2010;6:123–135. doi: 10.3233/CBM-2009-0124. [DOI] [PubMed] [Google Scholar]
- 4.Biaoxue R, Xiling J, Shuanying Y, Wei Z, Xiguang C, Jinsui W, Min Z. Upregulation of Hsp90-beta and annexin A1 correlates with poor survival and lymphatic metastasis in lung cancer patients. J Exp Clin Cancer Res. 2012;31:70. doi: 10.1186/1756-9966-31-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim LHK, Pervaiz S. Annexin 1: the new face of an old molecule. FASEB J. 2007;21:968–975. doi: 10.1096/fj.06-7464rev. [DOI] [PubMed] [Google Scholar]
- 6.Bai XF, Ni XG, Zhao P, Liu SM, Wang HX, Guo B, Zhou LP, Liu F, Zhang JS, Wang K. Overexpression of annexin 1 in pancreatic cancer and its clinical significance. World J Gastroenterol. 2004;10:1466–1470. doi: 10.3748/wjg.v10.i10.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masaki T, Tokuda M, Ohnishi M, Watanabe S, Fujimura T, Miyamoto K, Itano T, Matsui H, Arima K, Shirai M, Maeba T, Sogawa K, Konishi R, Taniguchi K, Hatanaka Y, Hatase O, Nishioka M. Enhanced expression of the protein kinase substrate annexin in human hepatocellular carcinoma. Hepatology. 1996;24:72–81. doi: 10.1053/jhep.1996.v24.pm0008707286. [DOI] [PubMed] [Google Scholar]
- 8.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 9.Fiore E, Campani D, Muller I, Belardi V, Giustarini E, Rossi G, Pinchera A, Giani C. IGF-II mRNA expression in breast cancer: predictive value and relationship to other prognostic factors. Int J Biol Markers. 2010;25:150–156. doi: 10.1177/172460081002500305. [DOI] [PubMed] [Google Scholar]
- 10.Hanley JA. Receiver operating characteristic (ROC) methodology: the state of the art. Crit Rev Diagn Imaging. 1989;29:307–335. [PubMed] [Google Scholar]
- 11.Shen J, Liu Z, Todd NW, Zhang H, Liao J, Yu L, Guarnera MA, Li R, Cai L, Zhan M, Jiang F. Diagnosis of lung cancer in individuals with solitary pulmonary nodules by plasma microRNA biomarkers. BMC Cancer. 2011;11:374. doi: 10.1186/1471-2407-11-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cedres S, Nunez I, Longo M, Martinez P, Checa E, Torrejon D, Felip E. Serum tumor markers CEA, CYFRA21-1, and CA-125 are associated with worse prognosis in advanced non-small-cell lung cancer (NSCLC) Clin Lung Cancer. 2011;12:172–179. doi: 10.1016/j.cllc.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Yu D, Du K, Liu T, Chen G. Prognostic Value of Tumor Markers, NSE, CA125 and SCC, in Operable NSCLC Patients. Int J Mol Sci. 2013;14:11145–11156. doi: 10.3390/ijms140611145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sung HJ, Cho JY. Biomarkers for the lung cancer diagnosis and their advances in proteomics. BMB Rep. 2008;41:615–625. doi: 10.5483/bmbrep.2008.41.9.615. [DOI] [PubMed] [Google Scholar]
- 15.Sato Y, Kumamoto K, Saito K, Okayama H, Hayase S, Kofunato Y, Miyamoto K, Nakamura I, Ohki S, Koyama Y, Takenoshita S. Up-regulated Annexin A1 expression in gastrointestinal cancer is associated with cancer invasion and lymph node metastasis. Exp Ther Med. 2011;2:239–243. doi: 10.3892/etm.2011.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li CF, Shen KH, Huang LC, Huang HY, Wang YH, Wu TF. Annexin-I overexpression is associated with tumour progression and independently predicts inferior disease-specific and metastasis-free survival in urinary bladder urothelial carcinoma. Pathology. 2010;42:43–49. doi: 10.3109/00313020903434405. [DOI] [PubMed] [Google Scholar]
- 17.Su N, Xu XY, Chen H, Gao WC, Ruan CP, Wang Q, Sun YP. Increased expression of annexin A1 is correlated with K-ras mutation in colorectal cancer. Tohoku J Exp Med. 2010;222:243–250. doi: 10.1620/tjem.222.243. [DOI] [PubMed] [Google Scholar]
- 18.Liu YF, Zhang PF, Li MY, Li QQ, Chen ZC. Identification of annexin A1 as a proinvasive and prognostic factor for lung adenocarcinoma. Clin Exp Metastasis. 2011;28:413–425. doi: 10.1007/s10585-011-9380-1. [DOI] [PubMed] [Google Scholar]
- 19.Cloez-Tayarani I, Fillion G. The in situ hybridization and immunocytochemistry techniques for characterization of cells expressing specific mRNAs in paraffin-embedded brains. Brain Res Brain Res Protoc. 1997;1:195–202. doi: 10.1016/s1385-299x(96)00029-3. [DOI] [PubMed] [Google Scholar]
- 20.Schnitt SJ. Breast cancer in the 21st century: neu opportunities and neu challenges. Mod Pathol. 2001;14:213–218. doi: 10.1038/modpathol.3880288. [DOI] [PubMed] [Google Scholar]
- 21.Hanada S, Nishiyama N, Mizuguchi S, Yamano S, Kakehashi A, Wei M, Inoue H, Komatsu H, Chung K, Suehiro S, Wanibuchi H. Clinicopathological significance of combined analysis of cytokeratin19 expression and preoperative serum CYFRA21-1 levels in human lung squamous cell carcinoma. Osaka City Med J. 2013;59:35–44. [PubMed] [Google Scholar]
- 22.Cao X, Zhang L, Feng GR, Yang J, Wang RY, Li J, Zheng XM, Han YJ. Preoperative Cyfra21-1 and SCC-Ag serum titers predict survival in patients with stage II esophageal squamous cell carcinoma. J Transl Med. 2012;10:197. doi: 10.1186/1479-5876-10-197. [DOI] [PMC free article] [PubMed] [Google Scholar]


