Abstract
Transplantation of peripheral blood mononuclear cells (PBMNCs) is a promising therapeutic approach for the treatment of hindlimb ischemia. However, insufficient angiogenesis in ischemic hindlimb after cell transplantation reduces the importance and practicality of this approach. Previously, we demonstrated using mouse models that hypoxic preconditioning augmented the cellular functions of rodent PBMNCs, such as increased cell adhesion capacity and accelerated neovascularization in ischemic hindlimb. To test the clinical application of this therapeutic strategy in this study, we investigated whether the protocol of hypoxic preconditioning, which was established in a condition of 2% O2 for 24 h, can be made available for human PBMNCs (hPBMNCs). In addition, we grafted preconditioned hPBMNCs in a hindlimb ischemia mouse model. Hypoxic preconditioning enhanced cell adhesion capacity and oxidative stress resistance in hPBMNCs. We also observed an up-regulation of platelet endothelial cell adhesion molecule-1 (PECAM-1) in hPBMNCs by hypoxic preconditioning. Furthermore, preconditioned hPBMNCs significantly recovered limb blood flow in ischemic mice after transplantation. These results indicate that our established preconditioning protocol is available for hPBMNCs to effectively reinforce multiple cellular functions. Taken together with our series of study, we believe that this simple but powerful therapeutic strategy will be helpful in curing patients with severe hindlimb ischemia.
Keywords: Hindlimb ischemia, cell-based therapy, human peripheral blood mononuclear cells, hypoxic preconditioning, therapeutic angiogenesis
Introduction
Therapeutic angiogenesis, which can be induced by cell transplantation and/or growth factor delivery, is one of the most promising therapeutic approaches to treat severe vascular diseases, such as coronary and peripheral artery diseases [1]. In this therapeutic approach, the choice of transplanted cells/growth factors and the delivery method of these molecules are important to achieve a better outcome. For example, in growth factor delivery, vascular endothelial growth factor (VEGF) administered to patients with peripheral artery disease (PAD) enhanced angiogenesis in ischemic areas, resulting in a dramatic recovery of blood flow [2]. However, extremely high doses of VEGF were required to induce therapeutic effects in clinical trials, which may possibly lead to abnormal angiogenesis in patients with tumors [3]. In the meantime, cell-based therapy can provide angiogenesis-associating cells or transplanted cell-derived angiogenic factors to ischemic tissues. For instance, bone marrow-derived cells can induce therapeutic angiogenesis and improve blood flow in ischemic tissues after transplantation [4]. However, low levels of cellular retention in ischemic tissues and incomplete therapeutic angiogenesis of transplanted cells still remain as common unsolved problems in cell-based therapy. Thus, an absolute protocol for therapeutic angiogenesis needs to be established in both therapeutic approaches.
Peripheral blood mononuclear cells (PBMNCs) are recognized as one of the most effective cells to induce therapeutic angiogenesis in ischemic tissues. Indeed, human PBMNCs (hPBMNCs) secrete VEGF in addition to other growth factors, and CD34+ endothelial progenitor cells in hPBMNCs can directly contribute to angiogenesis after transplantation [5-8]. Although bone marrow-derived cells are also known as a promising cellular tool for therapeutic angiogenesis in severe artery diseases, PBMNCs present an important advantage for patients because anesthesia is not required for cell isolation [7]. Furthermore, many researchers have demonstrated that hypoxic pretreatment increases the angiogenic potentials of PBMNCs through reinforcement of cellular functions [9,10]. This simple method, which contentiously exposes cells to low oxygen, is useful in enhancing specific properties of PBMNCs for therapeutic angiogenesis. However, effective and clinically suitable hypoxic conditions have not yet been established to enhance cellular functions of PBMNCs. In previous studies, we demonstrated that a culture condition of “2% O2”, corresponding to relatively mild hypoxia, augmented multiple cellular functions, such as cell adhesion capacity, resistance to oxidative stress, and VEGF secretion [10-12], whereas stricter oxygen levels (< 2%) have been used as a condition of hypoxia [13,14]. Also, we recently reported that autologous transplantation of preconditioned PBMNCs resulted in new vessel formation and blood flow recovery in pre-clinical studies using a middle animal model of hindlimb ischemia [15].
In the present study, we investigated whether our hypoxic preconditioning protocol is appropriate for hPBMNCs and whether the procedure effectively reinforces the cellular functions of hPBMNCs. We also examined, using a xenograft model, whether preconditioned hPBMNCs could improve blood flow after transplantation into ischemic hindlimb.
Materials and methods
Ethics statement
All experiments performed using human subjects were approved by the Medical Ethics Committee of Yamaguchi University School of Medicine (MECCYUSM; No. H23-44-4). Informed consent to collect blood samples was written and obtained from patients according to the MECCYUSM guidance. The study was conducted in accordance with the Declaration of Helsinki.
Animals
Male SCID mice (CB17/Icr-Prkedc scid/CrlCrlj; 2-3 months old; Charles River Laboratories Japan, Yokohama, Kanagawa, Japan) were used for cell transplantation experiments. All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC; No. 31-078) of Yamaguchi University.
Isolation and hypoxic preconditioning of human PBMNCs
Human blood collected from the basilic vein was separated into serum and cell components. Human PBMNCs (hPBMNCs) were isolated (Ficoll-Paque PREMIUM; GE Healthcare, Little Chalfont, UK) and suspended in GT-T503 medium (Takara Bio, Otsu, Shiga, Japan) with 10% fetal bovine serum (FBS) at a density of 5 × 106 cells/mL. Then, hPBMNCs were cultured in hypoxic (33°C in 2% O2 and 5% CO2 for 24 h; hypoxia) or normoxic conditions (37°C in 20% O2 and 5% CO2 for 24 h; normoxia).
Cell adhesion assay
Following 24 h of culture in normoxic or hypoxic conditions, hPBMNCs were plated onto fibronectin-coated cell culture dishes and cultivated under normal cell culture conditions (37°C in 20% O2 and 5% CO2). After 24 h, cells were immersed in 4’,6-diamidino-2-phenylindole dihydrochloride (DAPI) solution for 10 min to visualize cell nuclei [10].
Cell survival assay
Cell viability was determined by trypan blue dye exclusion, and the cell survival rate was calculated as the percentage of surviving cells in the culture at 24 h after plating [10].
Assay for oxidative stress resistance
To examined whether hypoxic preconditioning affects the resistance of hPBMNCs to oxidative stress, hPBMNCs cultured hypoxically or normoxically were exposed to growth medium containing H2O2 (100 μM) for 24 h. Preconditioned or non-preconditioned hPBMNCs (2 × 105 cells) were cultivated in 20% O2 at 37°C for 24 h. The total number of cells was counted using an auto-cell counter and live/dead cells were evaluated by an incorporation of trypan blue [10].
Assay for VEGF production in human PBMNCs
Human PBMNCs (5 × 106 cells/mL) were cultivated in the following culture conditions: (1) 20% O2, 12 h; (2) 20% O2, 24 h; (3) 5% O2, 12 h; (4) 5% O2, 24 h; (5) 2% O2, 12 h; and (6) 2% O2, 24 h. After 12 h or 24 h of culture under each condition, the concentration of human VEGF in the conditioned media of hPBMNCs was measured with enzyme-linked immunosorbent assay (ELISA; Human VEGF Quantikine immunoassay kit; R&D systems, Minneapolis, MN, USA).
Immunocytochemistry
After 24 h of culture in normoxic or hypoxic conditions, hPBMNCs were plated onto fibronectin-coated dishes and cultivated in normal cell culture conditions. Cells were gently washed with PBS to remove unattached cells from the dish 24 h after plating and then fixed with 4% paraformaldehyde for 20 min. To evaluate the adhesion capacity of PECAM-1+ cells in hPBMNCs, we stained hPBMNCs with goat anti-human CD31 antibody (1:50; eBioscience, San Diego, CA, USA). Alexa Flour 488-conjugated goat anti-mouse IgG (1:500; Invitrogen, San Diego, CA, USA) was used as a secondary antibody. Five microscopic fields (200× magnification) per well were randomly chosen, and the number of PECAM-1+ cells was counted.
Hindlimb ischemic model and cell transplantation
Mice were anesthetized, and the left femoral artery, popliteal artery, and branches in the left hindlimb were removed to induce ischemia. Human PBMNCs were collected from four different healthy volunteers (Cases 1-4) and cultured under hypoxic or normoxic conditions for 24 h. Then, hPBMNCs were injected intramuscularly into the ischemic regions (1 × 106 cells × 4 points) at post-operative day 0 [16]. As a control, 10 μL of PBS was injected into the ischemic regions. Mice were divided into the following four groups: PBS (injection of PBS); fresh (injection of isolated hPBMNCs); normoxia (injection of normoxically cultured hPBMNCs); and hypoxia (injection of hypoxically preconditioned hPBMNCs).
Measurement of blood flow in ischemic hindlimb
Blood flow in ischemic hindlimb was measured using a Laser Doppler perfusion imaging system (PeriScan System; Perimed AB, Stockholm, Sweden) at day 21 after cell transplantation [16]. Both intact (right) and ischemic (left) hindlimb were scanned, and the average perfusion score in the left hindlimb was normalized by that in the right hindlimb (n = 4-5). All procedures were performed under anesthesia.
Statistical analysis
All data are expressed as means ± standard error. Differences between mean values of multiple groups were evaluated with one-way ANOVA analysis with Fisher’s PLSD post-hoc test. Comparisons between two groups were made with the Student’s t-test. P values of < 0.05 or < 0.01 were considered significant. All analyses were performed with the SPSS software (IBM, Chicago, IL, USA).
Results
Hypoxic preconditioning reinforced the adhesion capacity of human PBMNCs
We investigated whether hypoxic preconditioning would reinforce cellular functions of hPBMNCs as well as PBMNCs from small/middle sized animals [10,15]. Initially, we tested that the cell adhesion capacity of hPBMNCs could be affected by hypoxic preconditioning. Human PBMNCs, which were cultivated in hypoxic (Hypoxia; 2% O2, 33°C) or normoxic (Normoxia; 20% O2, 33°C) conditions for 24 h, were plated onto cell culture dishes and further incubated in normoxic conditions for 24 h. After removal of floating (unattached) cells, the number of attached cells on the dishes was counted and compared between the normoxia and hypoxia groups. Attached hPBMNCs in hypoxia were twice as much in number as normoxia (p < 0.05; Figure 1A), indicating that hypoxic preconditioning reinforced the cell adhesion capacity of hPBMNCs.
Figure 1.
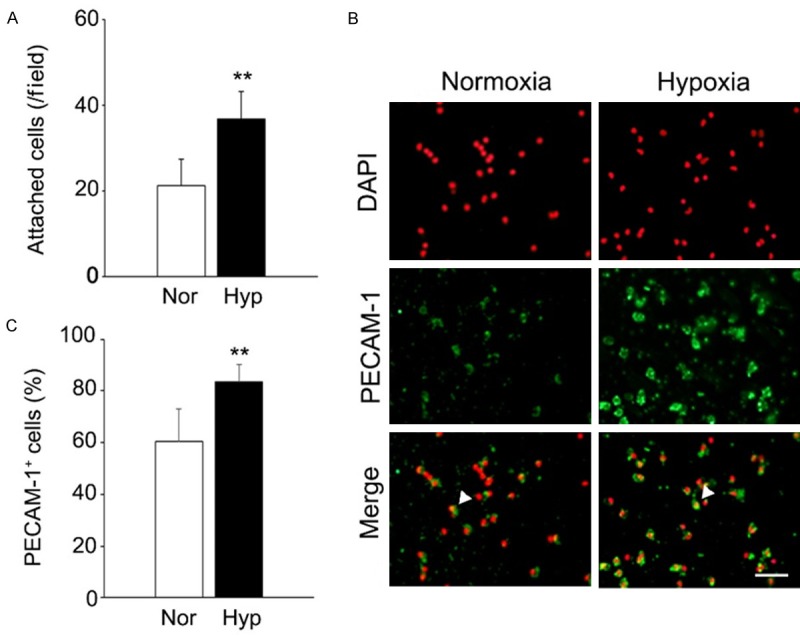
Hypoxic preconditioning augments the cell adhesion capacity of human PBMNCs and up-regulates the expression of cell adhesion molecule. A. The cell adhesion capacity of human PBMNCs can be reinforced by hypoxic preconditioning. The number of attached hPBMNCs, which were pre-cultured in normoxic or hypoxic conditions for 24 h, on cell culture dishes was counted one day after plating. n = 3. B. Immunocytochemistry for PECAM-1 was performed on attached hPBMNCs. Arrowhead represents PECAM-1+ cells. Scale bar: 50 μm. C. PECAM-1+ cells in attached hPBMNCs were significantly increased in the hypoxia group. The percentage of PECAM-1+ cells in attached hPBMNCs was calculated by the occupation of PECAM-1+ cells in total nuclei. n = 6. **p < 0.05 vs. normoxia. Nor: Normoxia. Hyp: Hypoxia.
Previous studies reported that hypoxic culture for seven days increased the number of cells expressing platelet endothelial cell adhesion molecule-1 (PECAM-1; also known as CD31) in hPBMNCs [9]. In addition, hypoxia up regulated the phosphorylation of PECAM-1 in human umbilical vascular endothelial cells (HUVECs) [17]. Hence, we hypothesized that hypoxic pretreatment would enhance the expression of PECAM-1 in hPBMNCs, resulting in higher adhesion of hPBMNCs. To test this hypothesis, immunocytochemistry was performed for PECAM-1 in attached hPBMNCs. The percentage of PECAM-1+ cells in attached cells was higher in the hypoxia group compared with the normoxia group (p < 0.05; Figure 1B, 1C), indicating that hypoxic preconditioning increased the expression of PECAM-1 in hPBMNCs, possibly enhancing cell adhesion as well.
Hypoxic preconditioning augmented the resistance capacity of hPBMNCs to oxidative stress
We next investigated whether hypoxic preconditioning would also influence the resistant capacity of hPBMNCs to oxidative stress. Human PBMNCs were cultivated in hypoxic or normoxic conditions for 24 h, and cell survival was compared between each group. The cell survival rate was significantly higher in the hypoxia group compared with the normoxia group (p < 0.01; Figure 2A). Then, we performed an oxidative stress tolerance test to examine whether hPBMNCs could achieve stress resistance with hypoxic pretreatment. Human PBMNCs were cultivated in each oxygen condition, and the same number of cells was exposed to H2O2 in normal cell culture conditions (37°C, 20% O2). After 24 h, oxidative stress caused the death of hPBMNCs in normoxia (survival rate was changed from 67.8 ± 7.1% to 41.6 ± 4.4%). In contrast, preconditioned hPBMNCs exhibited higher cell survival than normoxically cultured hPBMNCs in response to oxidative stress (46.6 ± 4.4% vs. 62.1 ± 8.2%; p < 0.05), although there is no significant difference in survival rate among each group without H2O2 stimulation (67.8 ± 7.1% vs. 69.7 ± 10.1%; p = 0.805) (Figure 2B). These observations indicated that hypoxic preconditioning resulted in oxidative stress resistance of hPBMNCs as well as an increase in cell adhesion capacity.
Figure 2.
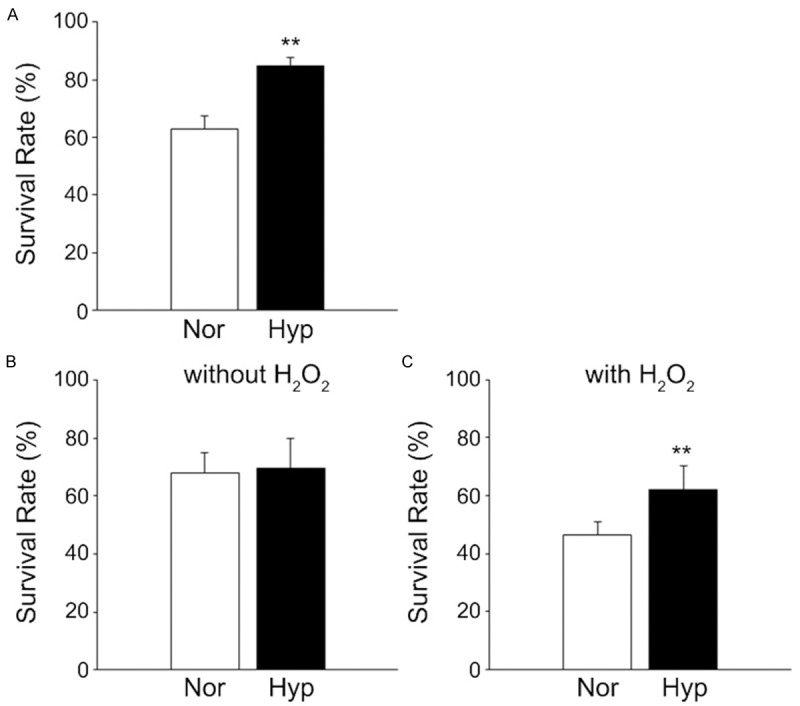
Hypoxic preconditioning renders human PBMNCs resistant to oxidative stress. To test the oxidative stress resistance of human cells, hPBMNCs were cultured in hypoxic or normoxic conditions and then exposed to oxidative stress for 24 h. A. Hypoxic preconditioning increases cell survival of hPBMNCs in normal cell culture conditions. Live/dead cells were evaluated by trypan blue incorporation to the nucleus at 24 h in hypoxic or normoxic cultures. B. The resistance capacity of human PBMNCs to oxidative stress was reinforced by hypoxic pretreatment. Preconditioned hPBMNCs were exposed to H2O2 for 24 h, and cell survival was evaluated. n = 4. **p < 0.05 vs. normoxia. Nor: Normoxia. Hyp: Hypoxia.
The combination of hypoxia (2% O2) and 24 hours in culture is optimal to augment stress resistance and VEGF production in human PBMNCs
To optimize the protocol of hPBMNC preconditioning, cells were cultivated in different oxygen levels after various incubation periods. Oxygen levels were set at 2% (hypoxia), 5% (low OX), 10% (low OX), and 20% (normoxia), and incubation times were 12 or 24 h for each oxygen level. Initially, we investigated the various culture conditions to determine the ones at which hPBMNCs would yield high resistance to oxidative stress. Human PBMNCs were cultivated in six combinations (see Materials and Methods) and then exposed to H2O2 for 24 h. Low OX (5% and 10% O2) cultures did not influence the survival of hPBMNCs regardless of culture period. In the case of hypoxia, interestingly, the survival of hPBMNCs cultured for 12 h did not change, but in contrast, the survival of hPBMNCs cultured for 24 h in hypoxia did improve (Figure 3A, 3B). Similarly, we also found that up-regulation of VEGF production was induced only in hypoxia for 24 h but not in low OX or normoxia (Figure 3C, 3D). These results suggest that cultivation in “2% O2 for 24 h” is the optimized combined protocol to sufficiently augment cellular functions in hPBMNCs.
Figure 3.
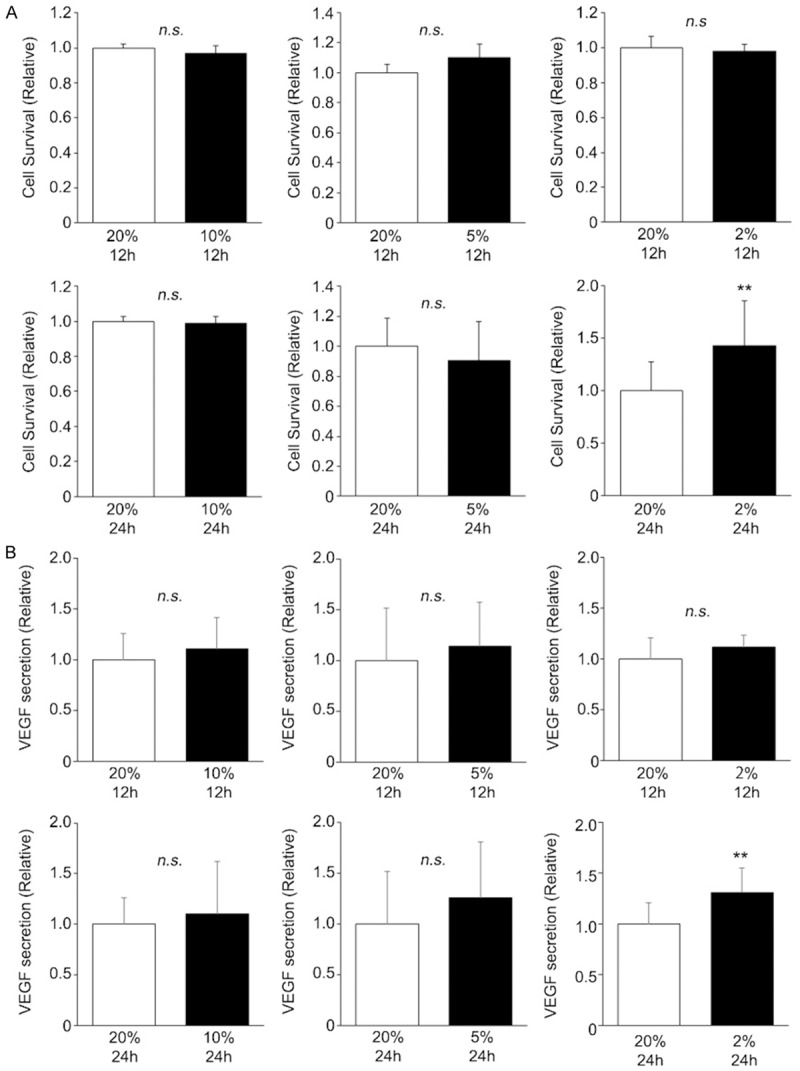
Combination of hypoxia and 24 h cultivation is suitable to augment cellular functions of hPBMNCs. To optimize cell culture conditions for clinical application of hPBMNCs, cells were cultured in different conditions. Then cell survival (A) and VEGF secretion (B) were evaluated in each experimental set, and optimal culture conditions were determined. For both parameters, a combination of hypoxic conditions and culture for 24 h resulted in significantly higher values than other cell culture conditions, whereas all other combinations showed no significant differences. n = 4. **p < 0.05 vs. 20%, 12 h or 20%, 24 h. n.s.: no significant difference.
Preconditioned hPBMNCs recover blood flow in ischemic hindlimb
To test the therapeutic availability of the hypoxic preconditioning protocol, preconditioned hPBMNCs were transplanted in a hindlimb ischemia mouse model, and blood flow in ischemic hindlimb was evaluated using the Laser Doppler Imaging System. Human PBMNCs were isolated from four different healthy volunteers, and preconditioned (hypoxia group), normoxically cultured (normoxia group), or non-pre-incubated (fresh group) hPBMNCs (4 × 106 cells/mouse) were intramuscularly transplanted into the ischemic hindlimb. In all independent cases, transplantation of hPBMNCs showed an improvement of limb blood flow at day 21 even in the fresh and normoxia groups. Importantly, transplantation of preconditioned hPBMNCs resulted in higher blood flow than any other group in all experimental cases (Figure 4A). In addition, blood flow recovery was 1.3-fold higher in hypoxic conditions compared with the non-treated control (Figure 4B). Taken together, hypoxic preconditioning of hPBMNCs is therapeutically useful to induce blood flow recovery in ischemic hindlimb.
Figure 4.
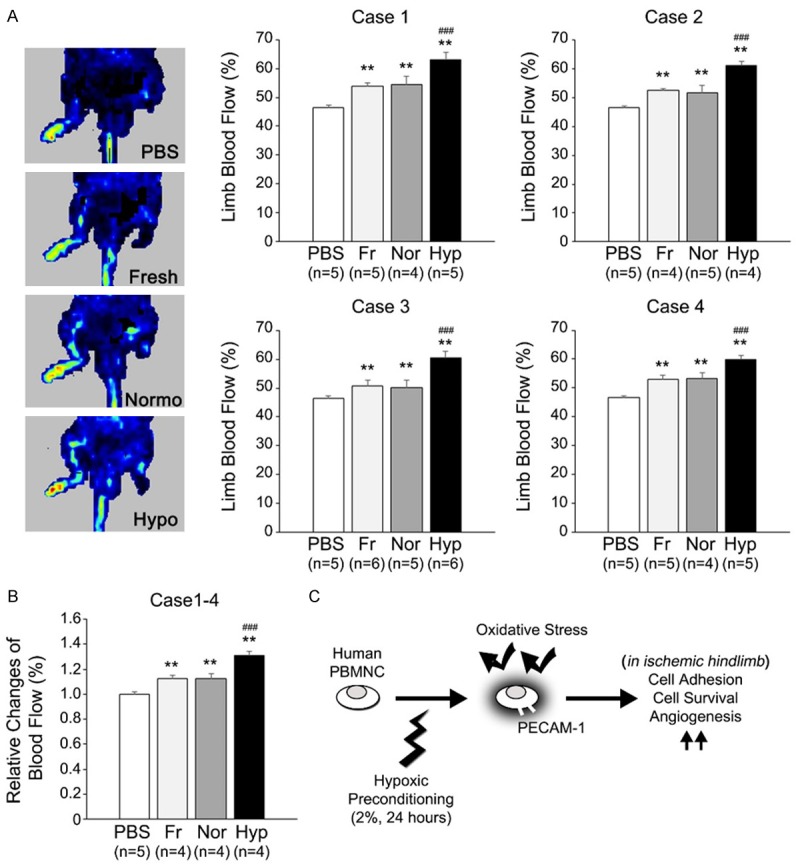
Hypoxically preconditioned hPBMNCs improve blood flow in hindlimb ischemia xenograft model. Human PBMNCs were hypoxically preconditioned for 24 h and then grafted into the ischemic right leg of the SCID mouse. In other experimental sets, freshly isolated hPBMNCs, normoxically cultured hPBMNCs, and PBS were injected into ischemic hindlimb. Blood flow was measured by the Laser Doppler Imaging System at day 21 after cell transplantation. (A) Human PBMNCs were prepared from four different healthy volunteers, and each hPBMNC line was transplanted into the ischemic leg (cases 1-4). Left panels indicate representative Laser Doppler images at day 21 after transplantation (case 4). Blood flow was significantly increased in hPBMNC-transplanted legs. Particularly, preconditioned hPBMNCs resulted in higher blood flow compared with any other group (A). Average change in blood flow in cases 1-4 is also shown to be higher in preconditioned hPBMNCs compared with any other group (B). Fr: Freshly isolated hPBMNCs. Nor: Normoxically cultured hPBMNCs. Hyp: Hypoxically preconditioned hPBMNCs. **p < 0.01 vs. PBS; ###p < 0.01 vs. Fr and Nor.
Discussion
In a series of our studies, we demonstrated that hypoxic preconditioning in “2% O2 for 24 h” could augment cellular functions of PBMNCs. Transplantation of preconditioned rodent PBMNCs resulted in neovascularization and recovery of blood flow in ischemic hindlimb of animal models [10,12,15]. These observations allowed us to progress in testing whether the same preconditioning protocol could be used in hPBMNCs to reinforce cellular functions in a manner similar to that demonstrated in rodent cells. We previously found that hypoxic preconditioning enhanced cell adhesion capacity through up-regulation of Integrin-αM in mouse PBMNCs, resulting in longer retention of PBMNCs in ischemic hindlimb [11]. In the present study, we found that hypoxic preconditioning increased the expression of PECAM-1 in hPBMNCs. Hypoxia up-regulates the expression of cell adhesion molecules, such as intracellular adhesion molecule (ICAM-1) in monocytes and endothelial cells [18,19], and accelerates the phosphorylation of PECAM-1 in HUVECs [17]. Taken together, it is possible that ex vivo hypoxic pretreatment simultaneously stimulates the expression of multiple cell adhesion molecules, such as integrins and PECAMs, to remain in ischemic tissues after transplantation. Meanwhile, another possibility is that hypoxia may increase the population of endothelial progenitor cells (EPCs) in hPBMNCs during pre-incubation, because one week of exposure in hypoxic conditions allows the differentiation of hPBMNCs into EPC-like cells [9]. Because PECAM-1+ (CD31+) cells in hPBMNCs possess angiogenic differentiation potentials to form new vessels in ischemic hindlimb [20,21], an increase in PECAM-1+ cells in hPBMNCs by hypoxic preconditioning possibly accelerates therapeutic angiogenesis after transplantation. However, we have no evidences to support the idea that short-term exposure to hypoxic conditions induces differentiation of hPBMNCs into EPCs. In any case, an increase in PECAM-1 expression in hPBMNCs by hypoxic preconditioning surely contributes to therapeutic angiogenesis in direct or indirect manners.
In this study, we also optimized the condition of hypoxic pretreatment for clinical application of this cell augmentation strategy. Low oxygen groups (5% and 10% O2) did not affect either cell survival or VEGF secretion. Moreover, cultivation after 24 h in 2% O2 was the only condition that induced significant changes in both parameters, while cultivation of hPBMNCs after 12 h showed no difference at any oxygen level. These observations suggest that 12 h was insufficient to induce augmentation of cellular functions in hypoxic conditions. In addition, we previously found that longer culture in hypoxic conditions decreased VEGF gene expression in rodent bone marrow-derived cells [12]. Taken together, 24 h is an optimum culture period for hypoxic preconditioning. With this assumption, the question remains whether the oxygen level established in our protocol is also practical for clinical applications. To clarify this question, we exposed hPBMNCs to 20% oxygen or low oxygen conditions (5 and 10%) and compared functional changes in these cells to those exposed to 2% oxygen. Among these four conditions, 2% oxygen was the only condition that was effective in enhancing the function of hPBMNCs. However, in the present study, we have not examined stricter oxygen levels of lower than 2%. For example, in bone marrow-derived cells, oxygen levels lower than 2% were also used in hypoxic preconditioning, and these hypoxic conditions also enhanced cellular functions such as proliferation, migration, and differentiation capacity [14,22,23]. Therefore, we need to test whether oxygen levels lower than 2% O2 are more effective in reinforcing cellular functions of hPBMNCs in future investigations.
In this study, we demonstrated that hypoxic preconditioning in 2% O2 for 24 h could be applied to hPBMNCs to improve blood flow in hindlimb ischemia mouse models (Figure 4C). Evidently, highly effective and minimally invasive therapeutic strategies are convenient for patients with peripheral artery diseases in practical medicine. PBMNCs can easily be isolated from peripheral blood of patients, and the therapeutic effects of PBMNC transplantation are more pronounced than those of bone marrow-derived cells [7,24]. Based on previous and present studies, we believe that therapeutic strategies involving PBMNCs are more favorable for patients with hindlimb ischemia and can provide relief from mental and physical exhaustion caused by treatment. Thus, this simple but powerful therapeutic strategy will be helpful to cure patients with severe hindlimb ischemia.
Acknowledgements
We thank to Mako Ohshima, Yukari Hironaka, Naomi Kojima, Yumi Yamamoto, and Tao-Sheng Li for their technical assistance and helpful comments. This work was supported by the JSPS-KAKENHI Grant-in-Aid for Scientific Research (C) (no. 25462162 to S.K.) and partly supported by the Pfizer Academic Contribution Grant (to K.H.).
References
- 1.Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease. Part I: angiogenic cytokines. Circulation. 2004;109:2487–2491. doi: 10.1161/01.CIR.0000128595.79378.FA. [DOI] [PubMed] [Google Scholar]
- 2.Isner JM, Feldman LJ. Gene therapy for arterial disease. Lancet. 1994;344:1653–1654. doi: 10.1016/s0140-6736(94)90454-5. [DOI] [PubMed] [Google Scholar]
- 3.Henry F, Paquet P, Pierard-Franchimont C, Pierard GE. [Leg heaviness and stasis microangiopathy. Preventive and therapeutic approaches] . Rev Med Liege. 2003;58:435–438. [PubMed] [Google Scholar]
- 4.Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, Amano K, Kishimoto Y, Yoshimoto K, Akashi H, Shimada K, Iwasaka T, Imaizumi T, Therapeutic Angiogenesis using Cell Transplantation (TACT) Study Investigators Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360:427–435. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- 5.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 6.Hojo Y, Ikeda U, Zhu Y, Okada M, Ueno S, Arakawa H, Fujikawa H, Katsuki T, Shimada K. Expression of vascular endothelial growth factor in patients with acute myocardial infarction. J Am Coll Cardiol. 2000;35:968–973. doi: 10.1016/s0735-1097(99)00632-4. [DOI] [PubMed] [Google Scholar]
- 7.Minamino T, Toko H, Tateno K, Nagai T, Komuro I. Peripheral-blood or bone-marrow mononuclear cells for therapeutic angiogenesis? Lancet. 2002:2083–2084. doi: 10.1016/s0140-6736(02)11977-5. author reply 2084. [DOI] [PubMed] [Google Scholar]
- 8.Moriya J, Minamino T, Tateno K, Shimizu N, Kuwabara Y, Sato Y, Saito Y, Komuro I. Long-term outcome of therapeutic neovascularization using peripheral blood mononuclear cells for limb ischemia. Circ Cardiovasc Interv. 2009;2:245–254. doi: 10.1161/CIRCINTERVENTIONS.108.799361. [DOI] [PubMed] [Google Scholar]
- 9.Akita T, Murohara T, Ikeda H, Sasaki K, Shimada T, Egami K, Imaizumi T. Hypoxic preconditioning augments efficacy of human endothelial progenitor cells for therapeutic neovascularization. Lab Invest. 2003;83:65–73. doi: 10.1097/01.lab.0000050761.67879.e4. [DOI] [PubMed] [Google Scholar]
- 10.Kubo M, Li TS, Suzuki R, Shirasawa B, Morikage N, Ohshima M, Qin SL, Hamano K. Hypoxic preconditioning increases survival and angiogenic potency of peripheral blood mononuclear cells via oxidative stress resistance. Am J Physiol Heart Circ Physiol. 2008;294:H590–595. doi: 10.1152/ajpheart.00856.2007. [DOI] [PubMed] [Google Scholar]
- 11.Kubo M, Li TS, Kamota T, Ohshima M, Qin SL, Hamano K. Increased expression of CXCR4 and integrin alphaM in hypoxia-preconditioned cells contributes to improved cell retention and angiogenic potency. J Cell Physiol. 2009;220:508–514. doi: 10.1002/jcp.21803. [DOI] [PubMed] [Google Scholar]
- 12.Li TS, Hamano K, Suzuki K, Ito H, Zempo N, Matsuzaki M. Improved angiogenic potency by implantation of ex vivo hypoxia prestimulated bone marrow cells in rats. Am J Physiol Heart Circ Physiol. 2002;283:H468–473. doi: 10.1152/ajpheart.00261.2002. [DOI] [PubMed] [Google Scholar]
- 13.Efimenko A, Starostina E, Kalinina N, Stolzing A. Angiogenic properties of aged adipose derived mesenchymal stem cells after hypoxic conditioning. J Transl Med. 2011;9:10. doi: 10.1186/1479-5876-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leroux L, Descamps B, Tojais NF, Seguy B, Oses P, Moreau C, Daret D, Ivanovic Z, Boiron JM, Lamaziere JM, Dufourcq P, Couffinhal T, Duplaa C. Hypoxia preconditioned mesenchymal stem cells improve vascular and skeletal muscle fiber regeneration after ischemia through a Wnt4-dependent pathway. Mol Ther. 2010;18:1545–1552. doi: 10.1038/mt.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kudo T, Hosoyama T, Samura M, Katsura S, Nishimoto A, Kugimiya N, Fujii Y, Li TS, Hamano K. Hypoxic preconditioning reinforces cellular functions of autologous peripheral blood-derived cells in rabbit hindlimb ischemia model. Biochem Biophys Res Commun. 2014;444:370–375. doi: 10.1016/j.bbrc.2014.01.054. [DOI] [PubMed] [Google Scholar]
- 16.Kubo M, Li TS, Kurazumi H, Takemoto Y, Ohshima M, Murata T, Katsura S, Morikage N, Furutani A, Hamano K. Hypoxic preconditioning enhances angiogenic potential of bone marrow cells with aging-related functional impairment. Circ J. 2012;76:986–994. doi: 10.1253/circj.cj-11-0605. [DOI] [PubMed] [Google Scholar]
- 17.Kalra VK, Shen Y, Sultana C, Rattan V. Hypoxia induces PECAM-1 phosphorylation and transendothelial migration of monocytes. Am J Physiol. 1996;271:H2025–2034. doi: 10.1152/ajpheart.1996.271.5.H2025. [DOI] [PubMed] [Google Scholar]
- 18.Winning S, Splettstoesser F, Fandrey J, Frede S. Acute hypoxia induces HIF-independent monocyte adhesion to endothelial cells through increased intercellular adhesion molecule-1 expression: the role of hypoxic inhibition of prolyl hydroxylase activity for the induction of NF-kappa B. J Immunol. 2010;185:1786–1793. doi: 10.4049/jimmunol.0903244. [DOI] [PubMed] [Google Scholar]
- 19.Zund G, Uezono S, Stahl GL, Dzus AL, McGowan FX, Hickey PR, Colgan SP. Hypoxia enhances induction of endothelial ICAM-1: role for metabolic acidosis and proteasomes. Am J Physiol. 1997;273:C1571–1580. doi: 10.1152/ajpcell.1997.273.5.C1571. [DOI] [PubMed] [Google Scholar]
- 20.Kim MH, Jin E, Zhang HZ, Kim SW. Robust angiogenic properties of cultured human peripheral blood-derived CD31(+) cells. Int J Cardiol. 2013;166:709–715. doi: 10.1016/j.ijcard.2011.11.097. [DOI] [PubMed] [Google Scholar]
- 21.Kim SW, Kim H, Cho HJ, Lee JU, Levit R, Yoon YS. Human peripheral blood-derived CD31+ cells have robust angiogenic and vasculogenic properties and are effective for treating ischemic vascular disease. J Am Coll Cardiol. 2010;56:593–607. doi: 10.1016/j.jacc.2010.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malladi P, Xu Y, Chiou M, Giaccia AJ, Longaker MT. Effect of reduced oxygen tension on chondrogenesis and osteogenesis in adipose-derived mesenchymal cells. Am J Physiol Cell Physiol. 2006;290:C1139–1146. doi: 10.1152/ajpcell.00415.2005. [DOI] [PubMed] [Google Scholar]
- 23.Merceron C, Vinatier C, Portron S, Masson M, Amiaud J, Guigand L, Cherel Y, Weiss P, Guicheux J. Differential effects of hypoxia on osteochondrogenic potential of human adipose-derived stem cells. Am J Physiol Cell Physiol. 2010;298:C355–364. doi: 10.1152/ajpcell.00398.2009. [DOI] [PubMed] [Google Scholar]
- 24.Huang PP, Yang XF, Li SZ, Wen JC, Zhang Y, Han ZC. Randomised comparison of G-CSF-mobilized peripheral blood mononuclear cells versus bone marrow-mononuclear cells for the treatment of patients with lower limb arteriosclerosis obliterans. Thromb Haemost. 2007;98:1335–1342. doi: 10.1160/th07-02-0137. [DOI] [PubMed] [Google Scholar]


