Abstract
Purpose: Most of the patients with stage IIIA pN2 non-small cell lung cancer (NSCLC) develop recurrence after surgery. It is not clear whether post neoadjuvant chemotherapy tumor-associated macrophages is associated with recurrence. Patients and Methods: Stage IIIA pN2 NSCLC patients underwent cisplatin/docetaxel neoadjuvant chemotherapy and surgery were retrospectively enrolled. Immunohistochemical staining of CD68 was used to identify macrophages in surgical resected stored tissues. Results: The objective response rate of cisplatin/docetaxel was 68%, overall median disease-free survival (DFS) was 13.1 months and median overall survival (OS) 36.8. months. Multiple Cox regression analysis showed low total macrophage numbers and mediastinal lymph nodes downstaging were independent factors for longer DFS, whereas high islet/stromal macrophages ratio was an independent facto for OS. In patients downstaged to pN0, low total macrophage numbers was also associated with longer DFS. Conclusions: Low total macrophage number is an independent factor for better DFS in pN2 stage IIIA NSCLC patients receiving neoadjuvant chemotherapy and surgical resection, which association was kept in those downstaged to pN0. Further studies are warrant to confirm the predictive role of TAMs and their potential causative role in tumor recurrence.
Keywords: Lung cancer biology, immunochemistry, immunology
Introduction
Lung cancer, over 80% of which is non-small cell lung cancer (NSCLC), is the leading cause of cancer death worldwide [1]. Although NSCLC can be treated surgically with curative intent in the early stages, 30-70% of the patients develop recurrences [2,3]. Treatment with combined modalities in addition to surgery alone is thus evolving, particularly neoadjuvant chemotherapy for N2 stage IIIA disease. The 5-year survival rate for those who underwent surgical resection alone was unsatisfactory, from 8% to 23% in different reports [3,4]. Neoadjuvant chemotherapy theoretically allows early eradication of micrometastases, which are present in nearly 80% of patients with stage IIIA NSCLC [5], and inducing tumor downstaging to enable better local control in surgery. Two randomized control trials clearly demonstrated survival advantage associated with neoadjuvant chemotherapy followed by surgery compared with surgery alone in patients with stage IIIA-N2 NSCLC [2,6]. Although the benefit was not seen in two later trials [7,8], they were either enrolling more heterogeneous populations (stage I-IIIA) [8] or underpowered [7]. Nevertheless, even after neoadjuvant chemotherapy and adequate surgical resection, patients still had up to 60% locoregional relapse and 65% distant metastasis after a follow-up of 5 years [2,9]. Identification of prognostic factors would potentially help to select patients need more aggressive consolidation treatment. Indeed, complete resection and mediastinal downstaging after neoadjuvant chemotherapy are two strong favorable prognostic factors, and resection is thus recommended only for patients with mediastinal downstaging after chemotherapy [9,10].
Macrophages are the major component of immune cells infiltrating in tumor microenvironment [11]. Tumor-associate macrophages (TAMs), usually M2 skewed or alternatively activated, have been implicated in tumor invasion, immune suppression and tumor metastasis [12,13]. They also have long been known to be a prognostic factor in lung cancer. Earlier repor-ts demonstrated a negative correlation of the tumor infiltrating macrophage density with survival of patients with stage I-IIIA NSCLC after surgical resection [14,15]. We have recently reported in advanced NSCLC, the majority of TAM located in tumor stroma and expressed M2 markers, also predicted for poor treatment response to epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) and overall survival [16]. A prognostic role of TAMs in NSCLC patients receiving neoadjuvant chemotherapy and surgery has yet been tested.
In this retrospective study, based on the hypothesis that TAMs are associated with poor prognosis, we numbered TAMs in resected tumors of patients with pN2 stage IIIA NSCLC receiving neoadjuvant chemotherapy and surgery, and analyzed the predictive value of TAMs and other clinical factors for disease free survival (PFS) and overall survival (OS) after surgical resection.
Patients and methods
Ethics statement
This is a retrospective study approved by Institutional Review Board of Chang Gung Medical Foundation, Linkou branch. (IRB 101-4181B) and informed consent was deemed not required. The patient records was anonymized and de-identified prior to analysis.
Study population
Patients with pN2 stage IIIA NSCLC treated with docetaxel and cisplatin as neoadjuvant chemotherapy followed by complete surgical resection between 2005 and 2008 were enrolled. The inclusion criteria were histologically diagnosed N2 stage IIIA NSCLC patients, confirmed pathologically by video-assisted thoracoscopy (VATS) or mediastinoscopy, without any prior treatment before neoadjuvant chemotherapy, fit for chemotherapy and received subsequent surgery with curative intent. All patients were staged according to the TNM classification of the American Joint Committee on Cancer, 6th edition, by using bronchoscopy, computer tomography (CT), and/or positron emission tomography. The patients with concomitant malignancies or participating in a clinical trial as induction chemotherapy were excluded.
Treatment and response evaluation
Patients received neoadjuvant docetaxel 60 mg/m2 as 1-hour intravenous infusion on day 1 and cisplatin 75 mg/m2 as a 3 hours intravenous infusion on day 1 every 3 weeks. Cycle numbers given were based on clinicians’ discretion according to clinical and radiologic response.
Clinical response was defined by using the Response Evaluation Criteria in Solid Tumors (RECIST) criteria [17]. A resection was considered complete (R0) when there was no residual tumor detected at the bronchial or vascular margins and no residual disease in the mediastinal area. Mediastinal downstaging was defined as no gross and microscopic disease in the resected mediastinal nodes. Pathologic complete response (pCR) was defined as the absence of viable tumor detected in the resected surgical specimen on pathologic examination [18]. Drug related toxicities were evaluated by the National Cancer Institute Common Toxicity Criteria version 3.0.
The patients were restaged by chest CT and/or positron emission tomography after the neoadjuvant chemotherapy. Every patient received an anatomic lung resection and mediastinal lymph node dissection in an attempt to eradicate the whole tumor. Lobectomy or bilobectomy was performed according to the lesion locations on chest CT before surgery. Postoperative treatment was left to clinicians’ discretion. Adjuvant chemotherapy was usually given to patients with residual lymph nodes metastasis. Those without residual tumor were followed up every 3 months until recurrence. The survival data were censored at December 31, 2012.
Immunohistochemical staining
Totally 28 stored paraffin-embedded, formalin-fixed, surgically resected specimens were collected for immunohistochemical (IHC) studies. Tissue sections were cut, placed onto glass slides, de-waxed in xylene and alcohols and then washed with phosphate-buffered saline twice as our previous study [16]. Macrophages were stained with an mouse anti-human CD68 monoclonal antibody (Clone PG-M1 from Dako, Glostrup, Denmark.).
Analysis and validation of immunohistochemical staining
Analysis of IHC was performed blindly with respect to the clinical outcome. The five most representative high-power fields (200X magnified) per slide were manually selected using an Olympus BX50 microscope (Olympus, Southall, United Kingdom). Nucleated CD68-positive cells with macrophage morphology (Figure 1) were counted manually independently by two researchers, including a pathologist, and expressed as islet, stromal or total macrophages as previous described [19]. The two sets of data were compared to assess the reproducibility and, hence, the validity of the results.
Figure 1.
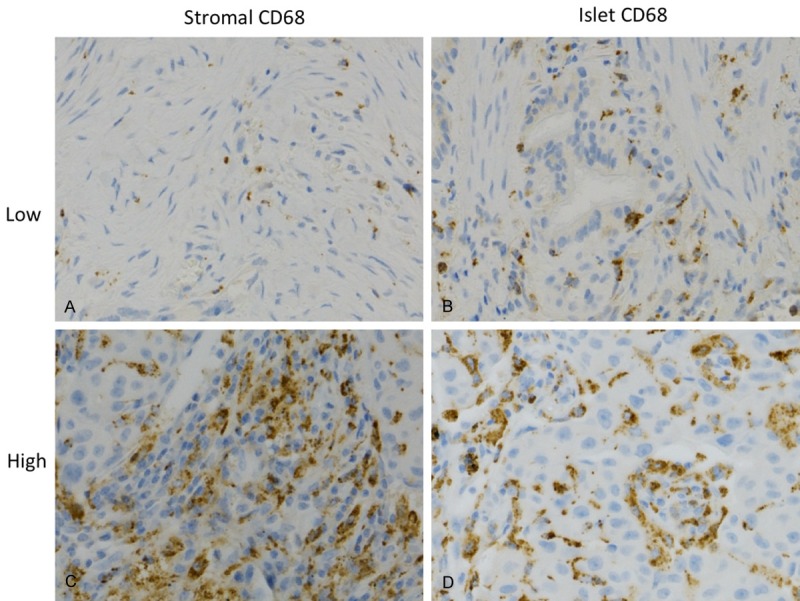
Immunohistochemical staining demonstrated low and high CD68+ macrophages (brown) in tumor stroma (A and C), and tumor islet (B and D).
The mediums of islet (113), stromal (113) and total macrophages (222) numbers per high power field (200X), and that of islet/stromal macrophage ratio (1.33) were chosen as cut-off levels for determination between high and low groups. Representative pictures of low and high CD68+ macrophages in islet and stromal area are shown in Figure 1.
Statistical analysis
The categorized variables were compared by Fisher exact test and continuous variables by the Mann-Whitney U test. The median follow-up was computed by the reverse Kaplan-Meier method [20]. Disease-free survival (DFS) was calculated from initiation of treatment to the date of last follow-up, the first clinical or radiologic evidence of tumor relapses, or progression, whereas Overall survival (OS) from initiation of treatment to the date of last follow-up or death. The Kaplan-Meier estimation was used to plot survival curves, and log-rank tests to compare the difference between groups. Univariate analysis was performed to investigate the association between an outcome (DFS and OS) and each individual prognostic factor. Time to event was analyzed by fitting multiple Cox regression model. All analyses were performed by using SPSS version 20 (SPSS Inc, Chicago, IL) and Prism 5 (version 5.01, Graphpad Software, San Diego, CA). Statistical significance was considered when p<0.05 by two sided.
Results
Patient characteristics
Between January 2005 and December 2008, totally 39 newly diagnosed potentially operable N2 stage IIIA patients were screened. Eleven patients were excluded without pathological confirmation of N2 diseases. Finally, twenty-eight (71%) patients (15 men, mean age 59 years, range 41-78 years) were enrolled for analysis. Most patients were non-smokers (17, [60%]) and adenocarcinoma (20, [71%]) was the predominant histology. The baseline characteristics and treatment response were summarized at Table 1.
Table 1.
Baseline Clinical Characteristics and Treatment Response
| Characteristics | All |
|---|---|
| Gender, No. (%) | |
| Male | 15 (53.5) |
| Female | 13 (46.5) |
| Mean Age, (range) | 59±9 (41-78) |
| Age, y, no (%) | |
| <60 y | 13 (46.5) |
| ≥60 y | 15 (53.5) |
| ECOGa Performance status, No. (%) | |
| 0 | 3 (10.7) |
| 1 | 25 (89.3) |
| Smoking, No. (%) | |
| Never smoker | 17 (60.7) |
| Smoker | 11 (39.3) |
| Histology, No. (%) | |
| Adenocarcinoma | 20 (71.4) |
| Squamous cell carcinoma | 6 (21.4) |
| Poorly differentiated carcinoma | 2 (7.1) |
| Clinical Response, No. (%) | |
| PRb | 19 (67.8) |
| SDc | 9 (32.2) |
| Post operative T, No. (%) | |
| T1 | 19 (67.8) |
| T2 | 8 (28.6) |
| T3 | 1 (3.6) |
| T4 | 0 (0.0) |
| Post operative N, No. (%) | |
| N0 | 19 (67.8) |
| N1 or N2 | 9 (32.2) |
| Infiltrating macrophages, medium, (IQRd) | |
| Stromal macrophage | 121 (59-164) |
| Islet macrophage | 105 (63-144) |
| Islet/Stromal macrophage | 1.21 (0.43-1.83) |
| Total macrophage | 228 (154-321) |
| Disease Free Survival, Median, (95% CI) | 13.1 (5.0-21.3) |
| Overall Survival, Median, (95% CI) | 36.8 (28.2-45.7) |
ECOG: Eastern Cooperative Oncology Group;
PR: partial response;
SD: stable disease;
IQR: interquartile range.
Neoadjuvant treatment response and toxicity
Nine (32%) patients received 2 cycles, 9 (32%) received 3 cycles, and 10 (36%) received 4 cycles of cisplatin/docetaxel. The objective response rate (ORR) was 67.8% (19 patients), all were partial response (PR). There were 9 patients (32%) with stable disease (SD) and no (0%) progressive disease.
Only 20 of 28 patients were evaluable for drug related toxicity. Ten (50%) patients developed leukopenia and 9 (45%) anemia during neoadjuvant chemotherapy. Frequent non-hematologic toxicities were nausea/vomiting (10 of 20, [50%]), fatigue (13 of 20, [65%]), and alopecia (6 of 16, [37.5%]). Most of the side effects were grade 1 or 2, and only one (5%) grade 3 leukopenia was recorded.
Surgical resection, pathologic response, nodal downstaging and postoperative treatment
All patients had complete resection (R0). Most patients received lobectomy (27 of 28, [96%]) and only one bilobectomy. Systemic lymph node dissection was performed in every patient. Most patients (19, 67.8%) had tumor shrinkage to T1. Residual pN2 was presented in 4 (14%) patients, whereas mediastinal downstaging in 18 (81%) patients, including 5 (18%) pN1 and 19 (68%) pN0 (Table 1). No pathologic complete response was achieved in these patients.
All 4 patients with residual pN2 disease underwent cisplatin/docetaxel adjuvant chemotherapy, whilst one out of two patients with pN1 residual disease received the same treatment. None of the pN0 disease received adjuvant therapy.
Relapse and survival
The median follow up time was 63 months (95% CI, 60.8-65.7 months). By December 31, 2012, 21 (75%) patients experienced relapse and 16 (57%) had died, all of which died of relapse. The median DFS was 13.1 months (95% CI, 5.0-21.3 months), and the median OS 36.8 months (95% CI, 24.0-72.1 months) (Table 1). There were 5 locoregional relapses and 16 distant metastases, the latter being 8 in the lungs and 8 in the brains as the first metastatic site. All patients with tumor relapse received chemotherapy and/or epidermal growth factor receptor tyrosine kinase inhibitors, and all those with brain metastasis received palliative radiotherapy.
Prognostic factors for survival outcomes
Univariate analysis of prognostic factors for DFS and OS were summarized in Table 2. Whilst mediastinal downstaging, low total numbers of macrophage and high islet/stromal ratio were significantly associated with longer DFS, only high islet/stromal macrophage ratio was significantly associated with longer OS. Median DFS was 16.3 months (95% CI: 7.1-25.5 months) vs. 5.3 months (95% CI: 4.2-6.8 months, p=0.001) and 5.5 months (95% CI: 4.2-6.7 months) vs. 33.4 months (95% CI: 7.5-59.3 months, p=0.001) for pN0 vs. pN1-2 and high total macrophage numbers vs. low total macrophage numbers, respectively (Figure 2A and 2B). Median DFS and OS were 20.7 (95% CI: 11.4-30.0 months) vs 5.5 months (95% CI: 4.4-6.6 months, p=0.04) and unreached vs. 34.8 months (95% CI: 21.8-47.9 months, p<0.01) for high islet/stromal macrophage ratio vs. low ratio, respectively (Figure 2C and 2D).
Table 2.
Univariate factor analysis of disease free survival and overall survival
| Characteristics | No | Disease Free Survival | Overall Survival | ||
|---|---|---|---|---|---|
|
| |||||
| Median (95% CI) | P | Median (95% CI) | P | ||
| Gender | |||||
| Man | 15 | 13.4 (5.0-21.8) | 0.76 | 36.1 (22.1-50.1) | 0.60 |
| Woman | 13 | 9.3 (6.2-20.7) | 40.5 (22.5-58.5) | ||
| Age | |||||
| <60 y | 13 | 13.2 (3.8-22.5) | 0.21 | 48.0 (20.9-75.2) | 0.17 |
| ≥60 y | 15 | 13.4 (16.7-46.1) | 36.0 (36.1-48.7) | ||
| Smoking | |||||
| Never smoker | 17 | 9.3 (8.4-22.5) | 0.74 | 40.6 (32.6-48.4) | 0.88 |
| Smoker | 11 | 13.4 (6.3-20.4) | 27.4 (0-70.1) | ||
| Histology | |||||
| Adenocarcinoma | 21 | 13.4 (4.6-22.2) | 0.42 | 48.0 (25.0-71.1) | 0.14 |
| Non-adenocarcinoma | 7 | 7.6 (3.3-11.9) | 27.4 (15.3-39.4) | ||
| Clinical Response | |||||
| PRa | 18 | 15.4 (10.3-20.5) | 0.68 | 36.1 (3.9-68.3) | 0.63 |
| SDb | 10 | 7.4 (3.6- 11.3) | 40.5 (31.0-50.0) | ||
| Mediastinal downstaging | |||||
| N0 | 19 | 16.3 (7.1-25.5) | 0.001 | 52.8 (14.0-91.4) | 0.187 |
| N1 or N2 | 9 | 5.3 (4.2-6.5) | 36.8 (27.6-46.0) | ||
| Islet/stromal macrophage ratio | |||||
| < median | 14 | 5.5 (4.4-6.6) | 0.04 | 34.8 (21.8-47.9) | <0.01 |
| ≥ median | 14 | 20.7 (11.4-30.0) | unreached | ||
| Total macrophages | |||||
| < median | 14 | 33.4 (7.5-59.3) | 0.001 | 36.1 (0-75.4.2) | 0.72 |
| ≥ median | 14 | 5.5 (4.2-6.7) | 36.8 (27.1-46.4) | ||
PR: partial response;
SD: stable disease.
Figure 2.
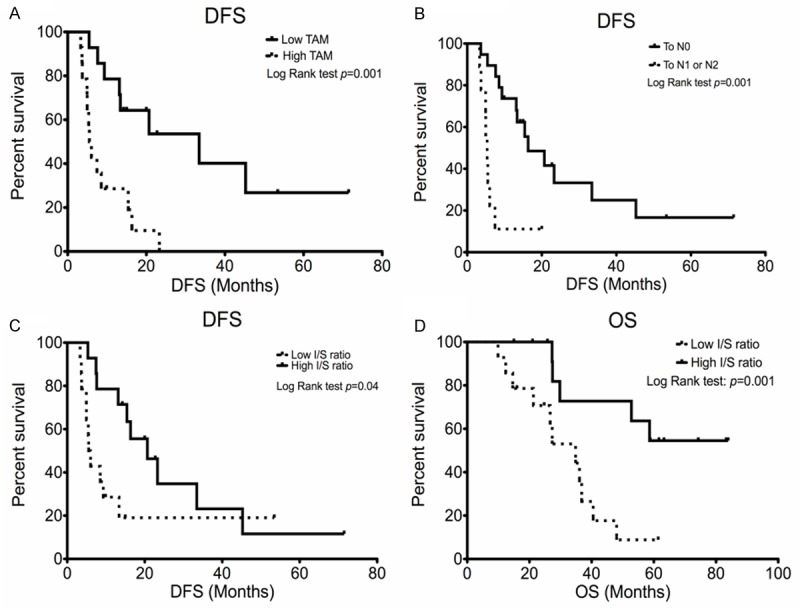
Kaplan-Meier disease free survival curves of (A) total macrophages, (B) mediastinal downstaging, (C) islet/stromal macrophage ratio and (D) overall survival of islet/stromal macrophage ratio. Data were dichotomized at the median value for each parameter.
To determine the independent factors for PFS and OS, multivariate analysis was performed and included significant factors and those with p<0.05 for PFS in univariate analysis (Table 3). Mediastinal downstaging and low total macrophages were both independent factors for longer PFS (HR: 0.22; 95% CI: 0.07-0.74, p=0.02 and HR: 4.21; 95% CI: 1.83-15.3, p=0.01, respectively). High islet/stromal macrophage ratio is the only independent factor for longer OS (HR: 0.25; 95% CI: 0.07-0.82, p=0.02).
Table 3.
Multivariate analysis for disease free survival and overall survival
| Disease Free Survival | Overall Survival | |||
|---|---|---|---|---|
|
|
||||
| Characteristics | HR (95% CI) | P value | HR (95% CI) | P value |
| Mediastinal downstaging | 0.22 (0.07-0.74) | 0.02 | 0.73 (0.13-3.94) | 0.71 |
| High Islet/stromal macrophage ratio | 0.39 (0.15-1.05) | 0.06 | 0.25 (0.07-0.82) | 0.02 |
| High Total macrophages | 4.21 (1.83-15.3) | 0.01 | 0.86 (0.17-4.15) | 0.85 |
Relapses occurred in 72.7-100% in each subgroup, and distant failure was the major type of the relapse. There was no significant difference in types of relapse in each subgroup.
Correlation of TAMs with survival in the subgroup downstaged to pN0
To avoid the confounding with mediastinal status and to clarify the prognosis role of TAMs, we further studied the patients with nodal downstaged to N0 (Table 4). The median number of total macrophages (202) in these patients was used as the cut-off value. Patients with high total macrophage numbers had significantly shorter median DFS (13.4 months, 95% CI: 8.2-18.6 months) compared with those with low numbers (33.4 months, 95% CI: 6.3-60 months, Log-Rank test p=0.01) (Figure 3A). OS was similar in both groups (median 52.8 months, 95% CI: 10-95.6 months vs. unreached; Log-Rank test p=0.88) (Figure 3B). With regard to types of relapse, 2 (22.2%) and 6 (66.7%) patients with high total macrophage numbers experienced locoregional and distant metastasis, respectively. And the recurrence pattern was similar with 1 (10%) locoregional and 4 (40%) distant metastasis in those with low total macrophage numbers (p=0.26).
Table 4.
Clinical characteristics and outcomes in patients downstaged to pN0
| Low total macrophage (n=10) | High total macrophage (n=9) | p value | |
|---|---|---|---|
| Gender, No. (%) | |||
| Male | 6 (60) | 6 (66.7) | 1.00 |
| Female | 4 (40) | 3 (33.3) | |
| Mean Age, year (range) | 64 (56-69) | 57 (44-71) | 0.09 |
| Age, y, no (%) | |||
| <60 y | 2 (20) | 5 (55.6) | 0.17 |
| ≥60 y | 8 (80) | 4 (44.4) | |
| ECOGa Performance status, No. (%) | |||
| 0 | 0 (0) | 1 (11.1) | 0.47 |
| 1 | 10 (100) | 8 (88.9) | |
| Smoking, No. (%) | |||
| Never smoker | 6 (60) | 3 (33.3) | 0.37 |
| Smoker | 4 (40) | 6 (66.7) | |
| Histology, No. (%) | |||
| Adenocarcinoma | 8 (87.5) | 5 (55.6) | 0.35 |
| Non-adenocarcinoma | 2 (12.5) | 4 (44.4) | |
| Clinical Response, No. (%) | |||
| PRb | 8 (80) | 7 (77.8) | 1.000 |
| SDc | 2 (20) | 2 (22.2) | |
| Post Neoadjuvant pT, No. (%) | |||
| T1 | 8 (80) | 6 (44.5) | 0.51 |
| T2 | 2 (20) | 3 (44.4) | |
| T3 | 0 (0) | 0 (0) | |
| T4 | 0 (0) | 0 (0) | |
| Relapse patterns, No. (%) | |||
| No relapse | 5 (50) | 1 (11.1) | 0.26 |
| Locoregional | 1 (10) | 2 (22.2) | |
| Distant | 4 (40) | 6 (66.7) | |
| Disease Free Survival, | |||
| Median, (95% CI) | 33.4 (6.3-60) | 13.4 (8.2-18.6) | 0.01 |
| Overall Survival, | |||
| Median, (95% CI) | 52.8 (10.0-95.6) | 54.5 (33.4-75.5) | 0.88 |
ECOG: Eastern Cooperative Oncology Group;
PR: partial response;
SD: stable disease.
Figure 3.
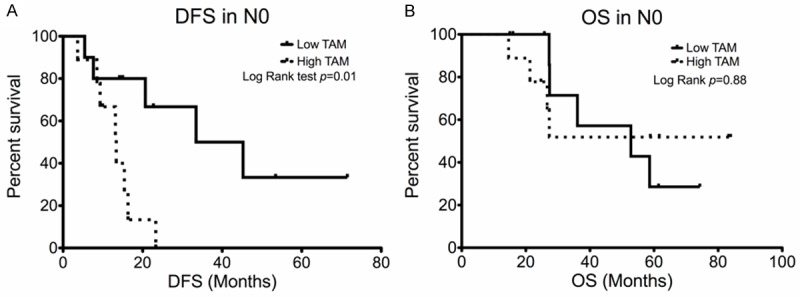
Kaplan-Meier survival curve of patients down-staged to N0 with different (A) total macrophages in patients downstaged to N0. (A) Disease-free survival and (B) Overall survival.
Discussion
This study confirms the prognostic role of mediastinal downstaging for DFS and demonstrates that numbers of total macrophages also have the same prognostic role. In addition, we showed that islet/stromal macrophage ratio is correlated with for OS. Furthermore, in patients downstaged to pN0, high numbers of total macrophage were associated with a shorter DFS. The present study also confirms a high objective response rate of docetaxel-cisplatin neoadjuvant chemotherapy, which were well tolerated.
Several prognostic factors had been reported in stage III pN2 patients receiving neoadjuvant chemotherapy and subsequent surgery, with complete surgical resection and mediastinal downstaging being the most important [9,10,21,22]. In the present study, all patients had complete resection. An independent role of mediastinal downstaging for predicting DFS is confirmed. However, this factor was not significant for OS in multivariate analysis. Although out result is conflicted with that reported by Betticher, et. al. [9,10], it is in agreement with most others ones by Garrido, et. al.[21] and Elias, et. al.[22] and a retrospective report by Liao et. al.[23].
The prognostic roles of TAM have been tested for NSCLC in different settings. Chen et al first demonstrated that overall TAM was a poor prognostic factor for OS in surgically resected NSCLC [14,15]. Further analysis by their micro-anatomic localization showed that stromal TAMs [19,24] were frequently negatively associated with patient survival whilst islet TAMs or islet/stromal macrophage ratio were always positively correlated [19,25,26]. More recent data suggested that islet macrophages expressed anti-tumor M1 phenotype [25] whilst more stromal ones were pro-tumor M2 skewed [27]. Generally, more TAMs were M2 macrophages compared to M1 macrophages in NSCLC [27]. In line with this concept, the present study showed islet/stromal macrophage ratio an independent factor for OS, which further extends to a more homogenous population, e.g. stage IIIA pN2 patients post neoadjuvant chemotherapy and surgery.
Interestingly, total macrophage, instead of islet/stromal macrophage ratio, is an independent predictive factor for DFS. This role was further confirmed in the pN0 group, in which any confounding with mediastinal downstaging can be ignored. Thus total macrophage may be used to identify patients who need further treatment after surgery, even in patients with mediastinal clearance. As distant metastasis is the major site of first relapse, adjuvant chemotherapy and/or radiotherapy might be necessary and need to be studied in further trials.
It is not clear why total macrophages but not islet/stromal macrophage ratio are predictive of DFS. Nevertheless, it is in agreement with our recent report showing that total macrophages are predictive of poor efficacy, including progression-free survival (PFS), in response to EGFR-TKI [16]. Interestingly, high total macrophage numbers were associated with low islet/stromal macrophage ratio (median, 1.21; IQR: 0.43-1.83 vs. 1.83; IQR: 0.89-2.50) and therefore supporting their M2-skewed tumor supporting phenotype as in advanced NSCLC [16]. Of note, recent gene profile experiments indicate several distinct populations of TAMs that often share features of both types [28]. In vitro studies also showed that, coculture with normal macrophages induced higher invasive potentials and activation of genes associated with angiogenesis and metastasis in lung cancer cell lines [15]. These studies support a potentially causative role of TAM, irrespective of their binary phenotypes.
There are limitations in the present study. First, the retrospective nature and small sample size might overlook confounding factors and was underpowered. Second, although CD68 is widely used as a marker of macrophages, it is also expressed on other cell types, such as immature CD1a-restrictive dendritic cells and fibroblasts. Nevertheless, this bias might have been minimized by the fact that only very low ratio of CD68+ cells were fibroblast or dendritic cells [16] and that macrophages were also determined by morphology.
Conclusion
Lower total macrophages, in addition to mediastinal downstaging, could independently predict better DFS in pN2 stage IIIA NSCLC patients underwent neoadjuvant chemotherapy and surgical resection. This role of TAMs is also kept in patients downstaged to pN0. The present study thus identified a novel marker that could be used to select patients who need more aggressive post-surgical treatment. A prospective study is warranted to confirm the finding herein.
Acknowledgements
Po-Hao, Feng, is also a PhD student at Graduate Institute of Clinical Medical Science, Chang Gung University. This work is submitted in partial fulfillment of the requirement for PhD. This study is supported by grants from Chang Gung Memorial Hospital (CMRPG300181 to K-Y. L.) and National Science Council (NSC 102-2314-B-182A-040 to P-H. F., K-Y. L. and C-T. Y.). The funder had no role in study design, data collection, data analysis, decision to publish or preparation of the manuscript.
Declaration of conflict of interst
None to declare.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Rosell R, Gomez-Codina J, Camps C, Maestre J, Padille J, Canto A, Mate JL, Li S, Roig J, Olazabal A, et al. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer. N Engl J Med. 1994;330:153–158. doi: 10.1056/NEJM199401203300301. [DOI] [PubMed] [Google Scholar]
- 3.Andre F, Grunenwald D, Pignon JP, Dujon A, Pujol JL, Brichon PY, Brouchet L, Quoix E, Westeel V, Le Chevalier T. Survival of patients with resected N2 non-small-cell lung cancer: evidence for a subclassification and implications. J. Clin. Oncol. 2000;18:2981–2989. doi: 10.1200/JCO.2000.18.16.2981. [DOI] [PubMed] [Google Scholar]
- 4.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111:1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 5.Bunn PA Jr. Future directions in clinical research for lung cancer. Chest. 1994;106:399S–407S. doi: 10.1378/chest.106.6_supplement.399s. [DOI] [PubMed] [Google Scholar]
- 6.Roth JA, Atkinson EN, Fossella F, Komaki R, Bernadette Ryan M, Putnam JB Jr, Lee JS, Dhingra H, De Caro L, Chasen M, Hong WK. Long-term follow-up of patients enrolled in a randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. Lung Cancer. 1998;21:1–6. doi: 10.1016/s0169-5002(98)00046-4. [DOI] [PubMed] [Google Scholar]
- 7.Nagai K, Tsuchiya R, Mori T, Tada H, Ichinose Y, Koike T, Kato H. A randomized trial comparing induction chemotherapy followed by surgery with surgery alone for patients with stage IIIA N2 non-small cell lung cancer (JCOG 9209) J Thorac Cardiovasc Surg. 2003;125:254–260. doi: 10.1067/mtc.2003.15. [DOI] [PubMed] [Google Scholar]
- 8.Depierre A, Milleron B, Moro-Sibilot D, Chevret S, Quoix E, Lebeau B, Braun D, Breton JL, Lemarie E, Gouva S, Paillot N, Brechot JM, Janicot H, Lebas FX, Terrioux P, Clavier J, Foucher P, Monchatre M, Coetmeur D, Level MC, Leclerc P, Blanchon F, Rodier JM, Thiberville L, Villeneuve A, Westeel V, Chastang C. Preoperative chemotherapy followed by surgery compared with primary surgery in resectable stage I (except T1N0), II, and IIIa non-small-cell lung cancer. J. Clin. Oncol. 2002;20:247–253. doi: 10.1200/JCO.2002.20.1.247. [DOI] [PubMed] [Google Scholar]
- 9.Betticher DC, Hsu Schmitz SF, Totsch M, Hansen E, Joss C, von Briel C, Schmid RA, Pless M, Habicht J, Roth AD, Spiliopoulos A, Stahel R, Weder W, Stupp R, Egli F, Furrer M, Honegger H, Wernli M, Cerny T, Ris HB. Prognostic factors affecting long-term outcomes in patients with resected stage IIIA pN2 non-small-cell lung cancer: 5-year follow-up of a phase II study. Br J Cancer. 2006;94:1099–1106. doi: 10.1038/sj.bjc.6603075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Betticher DC, Hsu Schmitz SF, Totsch M, Hansen E, Joss C, von Briel C, Schmid RA, Pless M, Habicht J, Roth AD, Spiliopoulos A, Stahel R, Weder W, Stupp R, Egli F, Furrer M, Honegger H, Wernli M, Cerny T, Ris HB. Mediastinal lymph node clearance after docetaxel-cisplatin neoadjuvant chemotherapy is prognostic of survival in patients with stage IIIA pN2 non-small-cell lung cancer: a multicenter phase II trial. J. Clin. Oncol. 2003;21:1752–1759. doi: 10.1200/JCO.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 11.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 12.Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33:119–126. doi: 10.1016/j.it.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Chen JJ, Yao PL, Yuan A, Hong TM, Shun CT, Kuo ML, Lee YC, Yang PC. Up-regulation of tumor interleukin-8 expression by infiltrating macrophages: its correlation with tumor angiogenesis and patient survival in non-small cell lung cancer. Clin Cancer Res. 2003;9:729–737. [PubMed] [Google Scholar]
- 15.Chen JJ, Lin YC, Yao PL, Yuan A, Chen HY, Shun CT, Tsai MF, Chen CH, Yang PC. Tumor-associated macrophages: the double-edged sword in cancer progression. J. Clin. Oncol. 2005;23:953–964. doi: 10.1200/JCO.2005.12.172. [DOI] [PubMed] [Google Scholar]
- 16.Chung FT, Lee KY, Wang CW, Heh CC, Chan YF, Chen HW, Kuo CH, Feng PH, Lin TY, Wang CH, Chou CL, Chen HC, Lin SM, Kuo HP. Tumor-associated macrophages correlate with response to epidermal growth factor receptor-tyrosine kinase inhibitors in advanced non-small cell lung cancer. Int J Cancer. 2012;131:E227–235. doi: 10.1002/ijc.27403. [DOI] [PubMed] [Google Scholar]
- 17.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 18.Mouillet G, Monnet E, Milleron B, Puyraveau M, Quoix E, David P, Ducolone A, Molinier O, Zalcman G, Depierre A, Westeel V. Pathologic complete response to preoperative chemotherapy predicts cure in early-stage non-small-cell lung cancer: combined analysis of two IFCT randomized trials. J Thorac Oncol. 2012;7:841–849. doi: 10.1097/JTO.0b013e31824c7d92. [DOI] [PubMed] [Google Scholar]
- 19.Welsh TJ, Green RH, Richardson D, Waller DA, O’Byrne KJ, Bradding P. Macrophage and mast-cell invasion of tumor cell islets confers a marked survival advantage in non-small-cell lung cancer. J. Clin. Oncol. 2005;23:8959–8967. doi: 10.1200/JCO.2005.01.4910. [DOI] [PubMed] [Google Scholar]
- 20.Clark TG, Bradburn MJ, Love SB, Altman DG. Survival analysis part I: basic concepts and first analyses. Br J Cancer. 2003;89:232–238. doi: 10.1038/sj.bjc.6601118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garrido P, Gonzalez-Larriba JL, Insa A, Provencio M, Torres A, Isla D, Sanchez JM, Cardenal F, Domine M, Barcelo JR, Tarrazona V, Varela A, Aguilo R, Astudillo J, Muguruza I, Artal A, Hernando-Trancho F, Massuti B, Sanchez-Ronco M, Rosell R. Long-term survival associated with complete resection after induction chemotherapy in stage IIIA (N2) and IIIB (T4N0-1) non small-cell lung cancer patients: the Spanish Lung Cancer Group Trial 9901. J. Clin. Oncol. 2007;25:4736–4742. doi: 10.1200/JCO.2007.12.0014. [DOI] [PubMed] [Google Scholar]
- 22.Elias AD, Skarin AT, Leong T, Mentzer S, Strauss G, Lynch T, Shulman L, Jacobs C, Abner A, Baldini EH, Frei E 3rd, Sugarbaker DJ. Neoadjuvant therapy for surgically staged IIIA N2 non-small cell lung cancer (NSCLC) Lung Cancer. 1997;17:147–161. doi: 10.1016/s0169-5002(97)00658-2. [DOI] [PubMed] [Google Scholar]
- 23.Liao WY, Chen JH, Wu M, Shih JY, Chen KY, Ho CC, Yang JC, Yu CJ. Neoadjuvant Chemotherapy With Docetaxel-Cisplatin in Patients With Stage III N2 Non-Small-Cell Lung Cancer. Clinical Lung Cancer. 2013;14:418–424. doi: 10.1016/j.cllc.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Dai F, Liu L, Che G, Yu N, Pu Q, Zhang S, Ma J, Ma L, You Z. The number and microlocalization of tumor-associated immune cells are associated with patient’s survival time in non-small cell lung cancer. BMC Cancer. 2010;10:220. doi: 10.1186/1471-2407-10-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohri CM, Shikotra A, Green RH, Waller DA, Bradding P. Macrophages within NSCLC tumour islets are predominantly of a cytotoxic M1 phenotype associated with extended survival. Eur Respir J. 2009;33:118–126. doi: 10.1183/09031936.00065708. [DOI] [PubMed] [Google Scholar]
- 26.Kim DW, Min HS, Lee KH, Kim YJ, Oh DY, Jeon YK, Lee SH, Im SA, Chung DH, Kim YT, Kim TY, Bang YJ, Sung SW, Kim JH, Heo DS. High tumour islet macrophage infiltration correlates with improved patient survival but not with EGFR mutations, gene copy number or protein expression in resected non-small cell lung cancer. Br J Cancer. 2008;98:1118–1124. doi: 10.1038/sj.bjc.6604256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma J, Liu L, Che G, Yu N, Dai F, You Z. The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer. 2010;10:112. doi: 10.1186/1471-2407-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ojalvo LS, King W, Cox D, Pollard JW. High-density gene expression analysis of tumor-associated macrophages from mouse mammary tumors. Am J Pathol. 2009;174:1048–1064. doi: 10.2353/ajpath.2009.080676. [DOI] [PMC free article] [PubMed] [Google Scholar]


