Abstract
Aim: Considering that the effects of sex and oral contraceptives (OCs) on blood metabolites have been scarcely studied and the fact that protocol designs for clinical trials emphasise the use of contraception for women of childbearing potential, we examined if OCs and sex affect the serum levels of the physiologically relevant amino acids, carnitine and acylcarnitines, using metabolomics approaches. Methods: Healthy adult men and women were enrolled. They were drug free with the exception of women taking cyclic format OCs (ethinylestradiol + different progestins). OCs-free women were analysed during the follicular phase, and amino acids, free carnitine and acylcarnitines were measured using HPLC or LC-MS/MS, respectively. Results: Men had significantly higher leucine, isoleucine, methionine, asparagine, proline, valine, tyrosine, glutamine+glutamate, glutamate, histidine and citrulline than OCs-free women, while tryptophan was significantly lower in men. OCs use significantly decreased the levels of glycine, proline, histidine, lysine, hydroxyproline and ornithine and increased isoleucine levels when compared with non-user women. OCs use reduced, although not significantly, carnitine levels in women. Total esterified carnitines were higher in untreated women than in OCs users. Globally, the effect of OCs and sex was specific for the individual esterified carnitine. The observed metabolic changes were not attributable to renal or hepatic functions or to differences in body weight. Conclusions: The assessed parameters were specifically influenced by sex, highlighting the need to have reference values for women and men. The major novelty of this study is the demonstration that OCs specifically change the profiles of serum amino acids and carnitine, which suggests that OCs users and non-users should be represented in clinical trials.
Keywords: Amino acids, carnitine, acylcarnitines, metabolomics, oral contraceptives, sex
Introduction
Appreciation for the relevance of sex in medicine is expanding [1,2]; however, the majority of completed studies have not considered the influence of sex and gender on serum and plasma metabolites that have been linked to metabolomic processes involved in complex diseases. A recent study [3] found significant differences between men and women in 102 of the 131 metabolites that were tested.
Free carnitine participates in lipid metabolism and transfers long-chain fatty acids as acylcarnitine esters across the inner mitochondrial membrane for β-oxidation, which produces acetyl-CoA and chain-shortened acyl products to preserve cellular CoA homeostasis [4,5]. Free carnitine is found at higher levels in adult men than in adult women [6,7]; however, the influence of sex on acylcarnitine levels has not been studied in detail, although some differences have been described [3]. Free carnitine is largely derived from the diet and, to a lesser extent, from endogenous synthesis that requires lysine and methionine [8] and this process mainly occurs in the liver and kidney through the two iron-containing enzymes ε-N-trimethyllysine hydroxylase and γ-butyrobetaine [9,10]. Notably, serum carnitine levels can be regulated by sex steroid hormones because non-obese women with polycystic ovary syndrome have lower carnitine levels than healthy women [11]. Moreover, pregnant women have lower plasma concentrations of carnitine than non-pregnant women [12]. The decrease in plasma carnitine levels during pregnancy may be due to low iron status, which impairs the function of the required enzymes [13]. However, contrasting results have been reported [14].
In the past, laboratories have largely reported the amino acid levels in the plasma of men, but a few studies have shown significant differences between the plasma concentrations of several amino acids in young adult men and women [15,16].
There is a paucity of studies that have investigated in detail the effect of oral contraceptives (OCs) on plasma or serum levels of amino acids and acylcarnitines. It has been shown that tyrosine, cysteine and arginine are decreased in OCs users [17-19], while taurine, alanine, glycine are unchanged [19,20]. However, detailed investigations of the OCs-induced effects on plasma metabolites could be useful considering that a) OCs have been used by millions of women since the approval of the first contraceptive pill in 1960, b) OCs are able to change the endogenous milieu by varying the activity of the pituitary-ovarian and hypothalamus-pituitary-adrenal axes [21] and carbohydrate and lipid metabolism [19,21], and c) clinical trials for the approval of the drugs emphasise the need for contraception for women of childbearing potential [22].
The rationale of this study was to use metabolomic investigations, which address the large-scale detection and quantification of metabolites in biological media, to assess sex differences (men and premenopausal women) and the effect of OCs (women on and off OCs treatment) measuring the serum levels of as many as feasible amino acids and of free carnitine and acylcarnitines involved in lipid metabolism.
Methods
Ethics statement
This study was approved by the local ethical committee of Diabetologia Aziendale ASL 2 Olbia, Hospital San Giovanni di Dio. Verbal informed consent was obtained from each study participant (blood donors) prior to the collection of a separate blood aliquot during a voluntary blood donation.
Population
Sixty-seven healthy, non-smoking, adult women (26 OCs users and 41 non-users) with regular menstrual cycles (27-29 days) and 35 healthy, adult, non-smoking men were enrolled. All subjects were aged between 20-47 years, and they had no kidney, liver, heart, endocrine or infectious diseases and had not used chronic pharmaceutical treatments for at least two months prior to the start of the study. Women who did not use OCs were analysed within the first 10 days of their menstrual cycle and were free from OCs for at least 3 months to ensure an appropriate wash-out period. In contrast, OCs users had used OCs for at least 3 months. All of the included OCs were taken in a cyclic format and belonged to the third generation of combined oral contraceptives (ethinyl estradiol + different progestin).
Analysis of biochemical and blood tests
Fasting blood samples (between 8.00 and 10 AM) were obtained from the antecubital vein and collected using the appropriate anticoagulant. Next, the serum was aliquoted and used within 1 month after storage at -80°C.
Fasting glucose, total cholesterol, low-density lipoproteins, high-density lipoproteins, triglycerides, creatinine, uric acid, urea, total bilirubin, aspartate aminotransferase, alanine aminotransferase, gamma-glutamyl transpeptidase alkaline phosphatase, sideremia, total iron binding capacity (TIBC), % saturation, ferritin, red blood cells (RBC), haemoglobin (Hb), haematocrit and the mean corpuscular volume (MCV) were measured using standard laboratory procedures. The eGFR was calculated according to the modified MDRD formula (http://mdrd.com).
LC-MS/MS analysis for carnitines
Twenty microlitres of serum was spotted onto 903® Whatman filter paper (Dessel, Germany) and left to dry overnight. Amino acids and acylcarnitines quantisation was performed as reported elsewhere [23,24] using an API 4000 Triple-Quadrupole Mass Spectrometer (Applied Biosystems/MDS Sciex, Toronto, Canada) that was equipped with a TurboIonSpray source and coupled with a high performance liquid chromatograph Agilent 1200 series (Agilent Technologies, Waldbronn, Germany). The data were quantitatively analysed using the ChemoView v1.2 software by comparing the signal intensities of the analyte and its corresponding internal standard or the standard to the spectrum.
Quality control samples for LC-MS/MS analysis were provided by the Department for Screening Neonates, Centre for Disease Control and Prevention (Atlanta, GA, USA) (www.cdc.gov/labstandars) [25].
HPLC analysis for amino acids
Serum samples were analysed as previously described [26,27]. Amino acid analysis was performed using an Agilent Technologies 1200 Series LC System with an Agilent Zorbax Eclipse XDB-C18 analytical column (5 μm, 4.6 x 150 mm) and Agilent Eclipse XDB-C18 analytical Guard column (5 μm, 4.6 x 12.5 mm).
Amino acid concentrations were determined using an external calibration curve. The amino acids were identified and quantified by their retention time and absorption ratio by comparison with the ratio of authentic compounds in the calibration solution [28]. For the determination of cysteine a reduction step was carried out.
Quality controls were provided by ENRDIM (European Research Network for evaluation and improvement of screening, Diagnosis and treatment of Inherited disorders of Metabolism, Manchester, UK) (www.erndimqa.nl) [29].
Statistical analysis
The two class unpaired significance analysis of microarrays (SAM) with a false discovery rate (FDR < 0.1) approach was performed to identify parameters that were differentially-expressed in samples, using the SAM software (version 4.01 from Stanford University). The SAM was performed separately to asses sex differences (men versus premenopausal women) and the effect of OCs (women on and off OCs treatment). The strength of association between body weight and single amino acids was analysed using the Pearson Product Moment correlation coefficient when the data were normally distributed. The Spearman Product Moment correlation coefficient was used if the data displayed a non-Gaussian distribution.
Multiple linear regression analysis was performed to predict the association of free carnitine (dependent variable) with sideremia, TIBC, % saturation, ferritin, RBC, Hb and haematocrit, eGFR, esterified carnitines or the amino acids lysine, methionine, valine, leucine and isoleucine (independent variables) in the 3 studied populations using the SigmaStat software.
Results
Populations
72 parameters were measured in 102 subjects of both sexes. Of the 102 subjects who were enrolled in the study, 35 were men, and 67 were women. Of the 67 women, 26 were OCs users. Healthy men and women were age-matched but had significantly different body heights and body mass indices (BMI) (Table 1). These parameters were not different between OCs users and non-users women.
Table 1.
Study participant characteristics stratified by sex and OCs use
| Men (n=35) | Women (n=41) | Women OCs (n=26) | |
|---|---|---|---|
| Age (years) | 29 (22-42) | 28 (20-45) | 28 (21-47) |
| Height (m) | 1.8 (1.6-1.9)a | 1.6 (1.5-1.7) | 1.6 (1.5-1.7) |
| Weight (Kg) | 77 (58-95) | 53 (38-70) | 54 (43-73) |
| BMI (Kg/m2) | 25 (20-30)a | 21 (17-28) | 21 (18-26) |
Data are expressed as the median and range. n=number of subjects. Superscript letters represent statistical significance:
Men vs. Women.
Lipid profile, glycaemia and renal and hepatic functions
The general biochemical results from the laboratory tests were within the reported reference ranges (Tables 2, 3 and 4). Men had lower HDL-cholesterol and higher glycaemia and triglyceride levels than non-user of OCs (Table 2). In addition, OCs users had a significantly higher concentration of total cholesterol, HDL-cholesterol, and triglycerides versus non-users (Table 2).
Table 2.
Biomarkers of lipid and glucose metabolism stratified by sex and OCs use
| Men (n=35) | Women (n=41) | Women OCs (n=26) | |
|---|---|---|---|
| Glycaemia (mg/dl) | 83.6±12.9a | 77.3±10.5 | 75.6±10.0 |
| Total Cholesterol (mg/dl) | 173.5±29.5 | 184.8±30.4 | 222.8±41.5b |
| LDL-Cholesterol (mg/dl) | 103.2±28.3 | 108.9±22.8 | 125.8±31.3 |
| HDL-Cholesterol (mg/dl) | 47.0±9.5a | 60.8±11.0 | 73.6±13.7b |
| HDL/LDL | 0.4 (0.3-1.0) | 0.6 (0.4-0.9) | 0.6 (0.3-0.9) |
| Triglycerides (mg/dl) | 68.0 (30.0-155.0)a | 60.0 (32.0-160.0) | 87.0 (48.0-526.0)b |
Values are expressed as the mean±standard deviation or as the median and range. n=number of subjects. Superscript letters represent statistical significance:
Men vs. Women;
Women vs. Women OCs.
Table 3.
Biomarkers of renal and hepatic function stratified by sex and OCs use
| Men (n=35) | Women (n=41) | Women OCs (n=26) | |
|---|---|---|---|
| Creatinine (mg/ml) | 0.9 (0.7-1.2) | 0.7 (0.5-0.8) | 0.7 (0.6-0.9) |
| eGFR (ml/min/1.73 m2) | 102.5 (67.8-132.0) | 97.2 (74.3-123.0) | 97.2 (73.9-115.5) |
| Uric acid (mg/dl) | 5.4±1.0a | 3.4±0.7 | 3.3±0.8 |
| Urea (mg/dl) | 36.8±8.8a | 30.4±9.9 | 28.9±6.1 |
| Total bilirubin (mg/dl) | 0.9 (0.3-1.8) | 0.5 (0.3-1.8) | 0.5 (0.2-0.1) |
| Aspartate aminotransferase (U/l) | 20.0 (13.0-103.0)a | 16.0 (11.0-29.0) | 17.0 (7.0-22.0) |
| Alanine aminotransferase (U/l) | 23.0 (10.0-106.0)a | 14.0 (6.0-43.0) | 13.0 (7.0-22.0) |
| γ-Glutamyl transpeptidase (U/l) | 21.0 (9.0-67.0)a | 14.0 (7.0-24.0) | 11.0 (7.0-22.0) |
| Alkaline phosphate (U/l) | 70.1±14.1 | 57.8±10.9 | 52.9±11.8 |
Data are reported as the mean±standard deviation or as the median and range. n=number of subjects. Superscript letters represent statistical significance:
Men vs. Women.
Table 4.
Biomarkers of iron metabolism stratified by sex and OCs use
| Men (n=35) | Women (n=41) | Women OCs (n=26) | |
|---|---|---|---|
| RBC (1012/l) | 5.4 (4.3-7.1)a | 4.8 (4.0-6.3) | 4.7 (3.8-6.2) |
| Hb (g/dl) | 14.4±1.3a | 12.8±1.0 | 12.8±1.1 |
| Haematocrit (%) | 43.0±2.9a | 39.5±2.7 | 38.9±2.3 |
| MCV (fl) | 82.2 (17.7-92.5) | 83.8 (61.7-90.8) | 82.9 (62.1-92.9) |
| Sideremia (µg/l) | 89.0 (42.0-200.0) | 82.0 (16.0-192.0) | 83.0 (28.0-218.0) |
| TIBC (µg/dl) | 390.0 (269.0-642.0) | 389.0 (264.0-492.0) | 455.0 (328.0-680.0)b |
| % saturation | 26.1±9.1 | 24.0±10.3 | 20.2±10.8 |
| Ferritin (ng/ml) | 117.5 (24.6-307.4)a | 44.5 (9.0-214.0) | 40.4 (4.3-106.0) |
Values are expressed as the mean±standard deviation or as the median and range. n=number of subjects. Superscript letters represent statistical significance:
Men vs. Women;
Women vs. Women OCs.
As expected, uric acid and urea were higher in men compared with women (Table 3); creatinine was higher in men although it did not reach the statistical significance. In addition, aspartate aminotransferase, alanine aminotransferase, γ-glutamyl transpeptidase were higher in men than in women. Moreover, alanine aminotransferase, γ-gluta-myl transpeptidase, alkaline phosphatase and total bilirubin were lower in OCs users compared with non-users, although the differences did not reach statistical significance. The levels of aspartate aminotransferase did not differ between OCs users and non-users, and no differences were detected in eGFR and creatinine.
Evaluation of iron metabolism
Men showed higher levels of RBC, Hb, haematocrit and ferritin, compared with women. Additionally, sideremia, TIBC, and the MCV did not statistically differ between the groups and OCs use did not significantly influence these parameters, with the exception of TIBC that was significantly higher in OCs user than in non-users (Table 4).
Serum amino acid levels
Nonpolar and polar amino acids
The analysis of amino acids revealed that the serum levels of alanine, serine, phenylalanine and cysteine were not significantly different among the studied groups (Table 5), although cysteine tend to be lower in OCs users. Leucine, isoleucine, methionine, asparagine, proline, valine and tyrosine were significantly higher in men than in women who did not use OCs, whereas tryptophan was significantly lower in men (Table 5). OCs users showed significantly lower levels of glycine and proline and significantly higher levels of isoleucine than non-users.
Table 5.
Nonpolar and polar amino acid levels stratified by sex and OCs use
| Men (n=41) | Women (n=43) | Women OCs (n=26) | |
|---|---|---|---|
| Alanine (μM) | 255.8±70.0 | 263.5±62.5 | 235.8±69.9 |
| Serine (μM) | 202.0 (123.0-503.0) | 213.3 (123.4-387.0) | 211.4 (57.7-1387.0) |
| Leucine (μM) | 220.0 (118.0-481.0)a | 150.2 (49.5-287.3) | 138.7 (44.6-281.0) |
| Isoleucine (μM) | 113.0 (57.0-255.0)a | 73.9 (46.1-136.0) | 78.0 (20.0-523.0)b |
| Methionine (μM) | 17.6 (14.8-40.6)a | 15.9 (11.3-23.2) | 13.8 (9.6-27.9) |
| Phenylalanine (μM) | 66.3 (49.7-109.3) | 54.7 (39.3-146.1) | 52.3 (41.1-116.5) |
| Asparagine (μM) | 159 (41.0-259.0)a | 82 (46.2-180.7) | 84.5 (18.1-268.0) |
| Tryptophan (μM) | 9.0 (2.0-58.0)a | 53.2 (5.0-87.6) | 52.0 (1.0-67.5) |
| Glycine (μM) | 204.8 (159.6-351.8) | 237.3 (137.9-384.4) | 176.7 (112.3-359.9)b |
| Proline (μM) | 269.0 (99.0-900.0)a | 216.1 (3.1-566.0) | 109.5 (2.9-222.0)b |
| Valine (μM) | 251.6 (175.5-467.9)a | 205.0 (171.6-320.7) | 208.5 (145.8-313.5) |
| Tyrosine (μM) | 79.5±20.5a | 71.9±14.3 | 63.5±18.9 |
| Cysteine (μM) | 5.0 (3.0-15.0) | 11.2 (2.0-399.0) | 10.0 (2.0-17.6) |
Values are expressed as the mean±standard deviation or as the median (range). n=number of subjects. Superscript letters represent statistical significance:
Men vs. Women;
Women vs. Women OCs.
Charged amino acids
The analysis of amino acids revealed that the serum levels of aspartic acid, arginine and taurine were not significantly different among the studied groups (Table 6). Glutamine+glutamate, glutamate, histidine and citrulline were significantly higher in men than in women who did not use OCs (Table 6). OCs users showed significantly higher levels of histidine and lower levels of lysine, hydroxyproline and ornithine when compared with women who did not use OCs (Table 6).
Table 6.
Charged amino acid levels stratified by sex and OCs use
| Men (n=41) | Women (n=43) | Women OCs (n=26) | |
|---|---|---|---|
| Aspartic acid (μM) | 29.2 (21.8-50.1) | 26.4 (17.3-74.9) | 29.2 (19.8-68.2) |
| Arginine (μM) | 41.4 (24.3-107.3) | 45.1 (27.1-172.5) | 44.2 (23.8-184.1) |
| Taurine (μM) | 230.0 (95.0-534.0) | 204.1 (109.0-401.6) | 188.0 (60.9-793.0) |
| Glutamate + Glutamine (μM) | 585.0 (281.0-1342.0)a | 412.1 (174.4-614.0) | 340.5 (50.0-2060.0) |
| Glutamate (μM) | 159.9 (118.4-260.0)a | 137.7 (75.4-301.2) | 123.1 (71.8-219.8) |
| Histidine (μM) | 129.0 (63.0-244.0)a | 99.9 (69.6-200.0) | 117.7 (33.5-117.1)b |
| Lysine (μM) | 252.0 (103.0-1135.0) | 234.0 (127.0-429.0) | 163.5 (83.0-251.0)b |
| Hydroxyproline (μM) | 15.5 (5.0-102.0) | 13.1 (3.3-136.1) | 4.2 (0.0-25.5)b |
| Ornitine (μM) | 64.4 ± 19.3 | 58.8 ± 15.1 | 45.4 ± 14.9b |
| Citrulline (μM) | 24.3 (11.6-47.3)a | 22.0 (13.6-34.0) | 18.4 (11.0-36.7) |
Values are expressed as the mean±standard deviation or as the median (range). n=number of subjects. Superscript letters represent statistical significance:
Men vs. Women;
Women vs. Women OCs.
Correlations
In untreated women, tryptophan was negatively correlated with body weight (r=-0.435, P=0.009), while lysine presented a positive correlation with body weight (r=0.51, P < 0.001) (Figure 1A). In OCs users, body weight was positively associated with valine (r=0.449, P=0.02) and negatively correlated with cysteine (r=-0.499, P=0.015) (Figure 1B). In men, none of the tested amino acids were associated with body weight.
Figure 1.
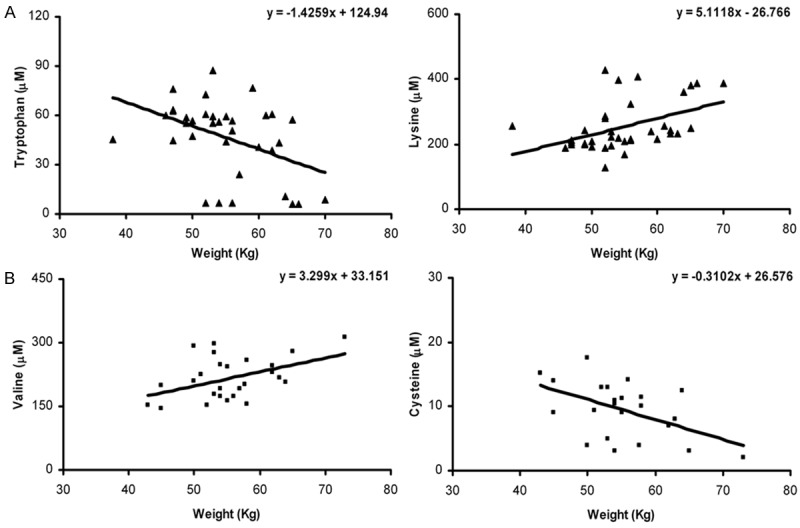
Panel A. Body weight correlation with tryptophan and lysine levels in women (▲). Panel B. Body weight correlation with tryptophan and lysine in OCs users (■).
Serum carnitine and acylcarnitine levels
Our results confirm previous findings that showed that the serum levels of free carnitine are higher in men than in women [6,7]. Notably, OCs use reduced the levels of free carnitine of about 18%, which suggests that they are regulated by sex steroid hormones (Figure 2A). Total esterified carnitines were higher in untreated women compared with OCs users (Figure 2A), while no differences were detected in the esterified/free ratio (Figure 2B). Analysis of the esterified carnitines revealed that OCs use reduced acetyl carnitine (C2) levels compared with those of non-users. No significant differences were detected in the other analysed esterified carnitines (Tables 7, 8).
Figure 2.
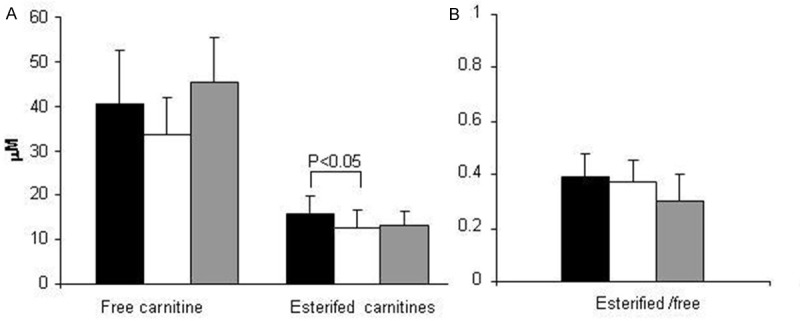
Panel A. Free carnitine concentration and total esterified carnitines were measured in women (n=43, black bars), OCs users (n=26, white bars) and men (n=41, grey bars). Panel B. The esterified/free carnitine concentration was calculated in women (n=43, black bars), OCs users (n=26, white bars) and men (n=41, grey bars). Values are expressed as the mean±standard deviation. The connectors represent significant differences.
Table 7.
Saturated Acylcarnitine levels stratified by sex and OCs use
| Men (n=41) | Women (n=43) | Women OCs (n=26) | |
|---|---|---|---|
| C2 (μM) | 9.8±2.9 | 12.6±3.6 | 9.5±3.6b |
| C3 (μM) | 0.5±0.2 | 0.5±0.2 | 0.4±0.1 |
| C4 (μM) | 0.2 (0.1-1.0) | 0.2 (0.1-0.8) | 0.3 (0.1-0.8) |
| C5 (μM) | 0.1 (0.07-0.3) | 0.09 (0.05-0.3) | 0.09 (0.06-0.2) |
| C6 (μM) | 0.06±0.02 | 0.06±0.02 | 0.05±0.01 |
| C8 (μM) | 0.2 (0.1-0.6) | 0.3 (0.07-0.7) | 0.2 (0.1-0.4) |
| C10 (μM) | 0.3 (0.09-1.1) | 0.3 (0.03-1.2) | 0.3 (0.1-0.5) |
| C12 (μM) | 0.09 (0.04-0.2) | 0.1 (0.02-0.3) | 0.07 (0.03-0.2) |
| C14 (μM) | 0.06 (0.03-0.1) | 0.05 (0.03-0.1) | 0.05 (0.03-0.2) |
| C16 (μM) | 0.2 (0.08-0.3) | 0.1 (0.06-0.2) | 0.1 (0.06-0.2) |
| C18 (μM) | 0.08(0.04-0.1) | 0.07 (0.03-0.1) | 0.06 (0.03-0.1) |
Values are expressed as the mean±standard deviation or as the median (range). n=number of subjects. Superscript letters represent statistical significance:
Women vs. Women OCs.
Table 8.
Unsaturated acylcarnitine levels stratified by sex and OCs use
| Men (n=41) | Women (n=43) | Women OCs (n=26) | |
|---|---|---|---|
| C5:1 (μM) | 0.04 (0.03-0.07) | 0.03 (0.01-0.06) | 0.03 (0.02-0.08) |
| C6:1 (μM) | 0.2 (0.09-0.4) | 0.1 (0.07-0.4) | 0.2 (0.1-0.5) |
| C8:1 (μM) | 0.14 (0.07-0.5) | 0.11 (0.05-0.3) | 0.15 (0.09-0.4) |
| C10:1 (μM) | 0.2 (0.1-0.5) | 0.2 (0.04-0.5) | 0.2 (0.1-0.3) |
| C10:2 (μM) | 0.07 (0.03-0.2) | 0.05 (0.02-0.1) | 0.09 (0.03-0.2) |
| C12:1 (μM) | 0.09 (0.04-0.2) | 0.09 (0.03-0.2) | 0.09 (0.05-0.2) |
| C14:1 (μM) | 0.10 (0.03-0.2) | 0.09 (0.02-0.3) | 0.07 (0.01-0.2) |
| C14:2 (μM) | 0.09 (0.05-0.2) | 0.07 (0.03-0.1) | 0.08 (0.05-0.2) |
| C16:1 (μM) | 0.05±0.01 | 0.04±0.01 | 0.05±0.02 |
| C18:1 (μM) | 0.16±0.06 | 0.15±0.05 | 0.14±0.05 |
| C18:2 (μM) | 0.08±0.03 | 0.06±0.02 | 0.06±0.02 |
Values are expressed as the mean±standard deviation or as the median (range). n=number of subjects.
Correlations
Multiple linear regression analysis revealed that none of the chosen independent variables (sideremia, TIBC, % saturation, ferritin, RBC, Hb, hematocrit, eGFR, lysine, methionine, valine, leucine, isoleucine and the esterified carnitines) predicted the values of free carnitine in the two female populations (data not shown). However, in men, the independent variables C2, propionylcarnitine (C3), octenoyl carnitine (C8:1) and oleylcarnitine (C18:1) predicted the value of free carnitine (P=0.04, P=0.007, P=0.038 and P=0.043, respectively).
Discussion
Sex differences
This study showed that the levels of several amino acids, such as leucine, isoleucine, methionine, asparagine, proline, valine, tyrosine, histidine, glutamate and citrulline are significantly higher in men than in women. However, no differences in the levels of alanine, serine, phenylalanine, cysteine, aspartate, arginine, or taurine were observed between men and women. Globally, these results are in line with the limited previous reports that suggest that plasma and serum amino acid levels are higher in men than in women [15,16,30-33]. However, some studies [15,32,34] do not take into account that amino acid levels are age dependent [34] and are influenced by hormonal fluctuations in women [35]. Tryptophan is lower in men compared with women and it has been reported that men exhibit higher brain synthesis of serotonin [30], which may explain the lower levels of that molecule in men than in women.
In men, no correlations exist between body weight and serum amino acid levels. Notably, no association has been found between body weight and amino acids that play a key role in muscle synthesis, such as leucine [36]. Conversely, lysine and tryptophan are positively and negatively associated with body weight, respectively, in women who did not use OCs. Overall, these results suggest that body weight does not accurately reflect serum amino acid levels, with some exceptions in women. Furthermore, these differences between men and women depend on single amino acid, which suggests that more specific mechanisms are involved, as in the case of leucine [36,37] and tryptophan [38].
In line with previous results [7,39-41], serum carnitine concentrations are lower in young, healthy women when compared with those of age-matched men. Although the two enzymes involved in carnitine synthesis contain iron with a catalytic function [9,10], no correlations have been found between levels of carnitine and biomarkers of iron metabolism. Notably, the lower level of methionine found in women could contribute to the difference in carnitine synthesis between women and men. The renal handling of carnitine does not seem to be influenced by sex because no correlations have been detected between eGFR and carnitine.
Effects of OCs in women
OCs induce specific and significant reductions in glycine, proline, hydroxyproline, lysine and ornithine, and elevations in isoleucine and histidine. Limited studies have reported that OCs users have similar or reduced glycine levels [17,42] and decreased tyrosine, ornithine and proline levels [17,18,42]. Consistent with prior reports, taurine and alanine are not different [19,20]. A discrepancy exists between current and previous work regarding serum cysteine and arginine levels [19] that could be attributed to the different analysed population. Here, we only included non-smoking subjects, while the previous population was not stratified for smoking habits. Other groups showed that regular smoking elevates arginine levels in women [43], which suggests the importance of the influence of regular smoking on biochemical parameters. Notably, valine and cysteine are positively and negatively associated with body weight, respectively, in OCs users, whereas in women who do not use OCs, lysine and tryptophan are positively and negatively associated with body weight, which indicates that the hormonal milieu could have a strong influence on the relationships between amino acids and body weight.
The major novel finding of this study is the demonstration that OCs use induces specific alterations in the set of serum carnitines. In particular, OCs use decreases free carnitine and increases unsaturated acylcarnitines although the difference is not statistically significant, which indicates the importance of sex hormones in carnitine handling, as previously suggested [11,12,44]. The decrease in carnitine may be due to a decrease in lysine, which is the precursor of carnitine, but a direct correlation between carnitine and lysine has not been found, which is not surprising because the majority of carnitine is obtained from exogenous sources [8]. The variations that are induced by OCs do not depend upon body dimension or renal function because the two female populations had similar height, weight and renal function. Importantly, the decrease in serum-free carnitine is associated with systemic carnitine deficiency [45].
Biological effects of low carnitine levels may not be clinically significant until they reach less than 10-20% of normal [46,47]. Here we highlighted a decrease of about 18% in OCs users, levels that could therefore be of some clinical significance if OCs are co-administered with drug that induce carnitine deficiency, e.g. cefditoren, pivoxil and valproic acid [48]. Indeed, our data also suggested that OCs should be carefully used in clinical situations that produce carnitine deficiency such as coeliac disease [49].
The reduction of free carnitine is important because it is involved in energy metabolism, and recent papers highlight the role of the carnitine pathway in cardiovascular diseases [50]. Short- and medium-chain acylcarnitine levels are significantly increased [50] in patients with acute myocardial infarction, and the unsaturated acylcarnitine accumulation that is observed in myocardial ischemia contributes to membrane dysfunction in ischemic zones [51]. Indeed, previous studies have shown heart dysfunction due to carnitine deficiency [52,53].
Finally, the simultaneous increase in unsaturated acylcarnitine and reduction in short-chain acylcarnitine in women who use OCs highlight the need for the evaluation of the carnitine serum profile in OCs users.
We conclude that sex specifically influences serum metabolomic profile. Considering that men have a larger total blood volume (approximately 6-8%) than women [54], the metabolite concentration in blood is diluted in men compared with women, resulting in larger differences than those presented in our current study.
Notably, OCs use induces specific alterations in the serum metabolic status, which implies that OCs users and non-users should be represented in clinical trials. Additionally, these findings imply that in a clinical setting that alters carnitine profiles, OCs should be used with caution.
We are confident that these types of studies will improve drug therapy for women who currently experience nearly twice the number of adverse events as men [55]. Indeed, the knowledge of the normal ranges for serum amino acids, carnitine and acylcarnitine is important for clinical and diagnostic purposes and for interpreting results from dietary and metabolic experiments, considering that current studies are mostly focused on men.
Acknowledgements
This research was funded by a grant from Regione Sardegna “Progetti di Farmacovigilanza attiva, finanziabili attraverso i fondi fv 2008/09” and from Istituto Superiore di Sanità “Piattaforma italiana per lo Studio della Polimorbidilità: scenario epidemiologico, aspetti clinici e farmacologici, prospettiva di genere e contesto farmaco-economico”. We express deep gratitude to Novartis Pharma and the Italian Pharmacological Society for providing the “Gender Innovation” Award to IC. This work was also supported by POR Campania FSE 2007-2013 project CRÈME. We are grateful to the Major of Osilo and all of Municipal Hall for the laboratory space that they have dedicated to National Sex -Gender Laboratory.
Disclosure of conflict of interest
The authors report no conflicts of interest that could be perceived as prejudicing the impartiality of this research.
References
- 1.Legato MJ. Principles of Gender-Specific. Amsterdam, Boston: Elsevier Academic Press; 2009. [Google Scholar]
- 2.Franconi F, Carru C, Spoletini I, Malorni W, Vella S, Mercuro G, Deidda M, Rosano G. A GENS-based approach to cardiovascular pharmacology: impact on metabolism, pharmacokinetics and pharmacodynamics. Ther Deliv. 2011;2:1437–1453. doi: 10.4155/tde.11.117. [DOI] [PubMed] [Google Scholar]
- 3.Mittelstrass K, Ried JS, Yu Z, Krumsiek J, Gieger C, Prehn C, Roemisch-Margl W, Polonikov A, Peters A, Theis FJ, Meitinger T, Kronenberg F, Weidinger S, Wichmann HE, Suhre K, Wang-Sattler R, Adamski J, Illig T. Discovery of sexual dimorphisms in metabolic and genetic biomarkers. PLoS Genet. 2011;7:e1002215. doi: 10.1371/journal.pgen.1002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sewell AC, Bohles HJ. Acylcarnitines in intermediary metabolism. Eur J Pediatr. 1995;154:871–877. doi: 10.1007/BF01957495. [DOI] [PubMed] [Google Scholar]
- 5.Steiber A, Kerner J, Hoppel CL. Carnitine: a nutritional, biosynthetic, and functional perspective. Mol Aspects Med. 2004;25:455–473. doi: 10.1016/j.mam.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Slupsky CM, Rankin KN, Wagner J, Fu H, Chang D, Weljie AM, Saude EJ, Lix B, Adamko DJ, Shah S, Greiner R, Sykes BD, Marrie TJ. Investigations of the effects of gender, diurnal variation, and age in human urinary metabolomic profiles. Anal Chem. 2007;79:6995–7004. doi: 10.1021/ac0708588. [DOI] [PubMed] [Google Scholar]
- 7.Reuter SE, Evans AM, Chace DH, Fornasini G. Determination of the reference range of endogenous plasma carnitines in healthy adults. Ann Clin Biochem. 2008;45:585–592. doi: 10.1258/acb.2008.008045. [DOI] [PubMed] [Google Scholar]
- 8.Vaz FM, Wanders RJ. Carnitine biosynthesis in mammals. Biochem J. 2002;361:417–429. doi: 10.1042/0264-6021:3610417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaz FM, Ofman R, Westinga K, Back JW, Wanders RJ. Molecular and biochemical characterization of rat epsilon -N-trimethyllysine hydroxylase, the first enzyme of carnitine biosynthesis. J Biol Chem. 2001;276:33512–33517. doi: 10.1074/jbc.M105929200. [DOI] [PubMed] [Google Scholar]
- 10.Lindstedt G, Lindstedt S. Cofactor requirements of gamma-butyrobetaine hydroxylase from rat liver. J Biol Chem. 1970;245:4178–4186. [PubMed] [Google Scholar]
- 11.Fenkci SM, Fenkci V, Oztekin O, Rota S, Karagenc N. Serum total L-carnitine levels in non-obese women with polycystic ovary syndrome. Hum Reprod. 2008;23:1602–1606. doi: 10.1093/humrep/den109. [DOI] [PubMed] [Google Scholar]
- 12.Cederblad G, Fahraeus L, Lindgren K. Plasma carnitine and renal-carnitine clearance during pregnancy. Am J Clin Nutr. 1986;44:379–383. doi: 10.1093/ajcn/44.3.379. [DOI] [PubMed] [Google Scholar]
- 13.Keller U, van der Wal C, Seliger G, Scheler C, Ropke F, Eder K. Carnitine status of pregnant women: effect of carnitine supplementation and correlation between iron status and plasma carnitine concentration. Eur J Clin Nutr. 2009;63:1098–1105. doi: 10.1038/ejcn.2009.36. [DOI] [PubMed] [Google Scholar]
- 14.Ringseis R, Hanisch N, Seliger G, Eder K. Low availability of carnitine precursors as a possible reason for the diminished plasma carnitine concentrations in pregnant women. BMC Pregnancy Childbirth. 2010;10:17. doi: 10.1186/1471-2393-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armstrong MD, Stave U. A study of plasma free amino acid levels. II. Normal values for children and adults. Metabolism. 1973;22:561–569. doi: 10.1016/0026-0495(73)90069-3. [DOI] [PubMed] [Google Scholar]
- 16.Caballero B, Gleason RE, Wurtman RJ. Plasma amino acid concentrations in healthy elderly men and women. Am J Clin Nutr. 1991;53:1249–1252. doi: 10.1093/ajcn/53.5.1249. [DOI] [PubMed] [Google Scholar]
- 17.Moller SE. Effect of oral contraceptives on tryptophan and tyrosine availability: evidence for a possible contribution to mental depression. Neuropsychobiology. 1981;7:192–200. doi: 10.1159/000117851. [DOI] [PubMed] [Google Scholar]
- 18.Moller SE, Maach-Moller B, Olesen M, Madsen B, Madsen P, Fjalland B. Tyrosine metabolism in users of oral contraceptives. Life Sci. 1995;56:687–695. doi: 10.1016/0024-3205(94)00502-j. [DOI] [PubMed] [Google Scholar]
- 19.Campesi I, Sanna M, Zinellu A, Carru C, Rubattu L, Bulzomi P, Seghieri G, Tonolo G, Palermo M, Rosano G, Marino M, Franconi F. Oral contraceptives modify DNA methylation and monocyte-derived macrophage function. Biol Sex Differ. 2012;3:4. doi: 10.1186/2042-6410-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rose DP, Leklem JE, Brown RR, Potera C. Effect of oral contraceptives and vitamin B6 supplements on alanine and glycine metabolism. Am J Clin Nutr. 1976;29:956–960. doi: 10.1093/ajcn/29.9.956. [DOI] [PubMed] [Google Scholar]
- 21.Brunton L, Chabner B, Knollmann B, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. Estrogens and Progestins; p. 12. manca l’anno. [Google Scholar]
- 22.Cain J, Lowell J, Thorndyke L, Localio AR. Contraceptive requirements for clinical research. Obstet Gynecol. 2000;95:861–866. doi: 10.1016/s0029-7844(00)00824-3. [DOI] [PubMed] [Google Scholar]
- 23.Millington DS, Kodo N, Norwood DL, Roe CR. Tandem mass spectrometry: a new method for acylcarnitine profiling with potential for neonatal screening for inborn errors of metabolism. J Inherit Metab Dis. 1990;13:321–324. doi: 10.1007/BF01799385. [DOI] [PubMed] [Google Scholar]
- 24.Millington D, Kodo N, Terada N, Roe D, Chase D. The analysis of diagnostic markers of genetic disorders to human blood and urine using tandem mass spectrometry with liquid secondary ion mass spectrometry. Int J Mass Spectrom Ion Proc. 1991;111:211–228. [Google Scholar]
- 25.Whitley R, Hannon W, Dietzen D, Rinaldo P. Pre-analytical, analytical, and post-analytical issues related to follow-up testing of positive newborn screens. In: Bennett M, editor. Laboratory Medicine Practice Guidelines. Follow-up Testing for Metabolic Diseases Identified by Expanded Newborn Screening Using Tandem Mass Spectrometry. Washington, DC: 2009. pp. 9–29. [DOI] [PubMed] [Google Scholar]
- 26.Turnell DC, Cooper JD. Rapid assay for amino acids in serum or urine by pre-column derivatization and reversed-phase liquid chromatography. Clin Chem. 1982;28:527–531. [PubMed] [Google Scholar]
- 27.Schuster R. Determination of amino acids in biological, pharmaceutical, plant and food samples by automated precolumn derivatization and high-performance liquid chromatography. J Chromatogr. 1988;431:271–284. doi: 10.1016/s0378-4347(00)83096-0. [DOI] [PubMed] [Google Scholar]
- 28.Parvy PR, Bardet JI, Rabier DM, Saudubray JM, Kamoun PP. Ion-exchange chromatography and clinical criteria in the screening of the aminoacidopathies. Clin Chim Acta. 1988;176:269–277. doi: 10.1016/0009-8981(88)90186-6. [DOI] [PubMed] [Google Scholar]
- 29.Fowler B, Burlina A, Kozich V, Vianey-Saban C. Quality of analytical performance in inherited metabolic disorders: the role of ERNDIM. J Inherit Metab Dis. 2008;31:680–689. doi: 10.1007/s10545-008-1025-4. [DOI] [PubMed] [Google Scholar]
- 30.Nishijima S, Sugaya K, Fukuda T, Miyazato M, Ashimine S, Ogawa Y. Serum amino acids as indicators of cerebrospinal neuronal activity in patients with micturition disorders. Int J Urol. 2006;13:1479–1483. doi: 10.1111/j.1442-2042.2006.01653.x. [DOI] [PubMed] [Google Scholar]
- 31.Zlotnik A, Gruenbaum BF, Mohar B, Kuts R, Gruenbaum SE, Ohayon S, Boyko M, Klin Y, Sheiner E, Shaked G, Shapira Y, Teichberg VI. The effects of estrogen and progesterone on blood glutamate levels: evidence from changes of blood glutamate levels during the menstrual cycle in women. Biol Reprod. 2011;84:581–586. doi: 10.1095/biolreprod.110.088120. [DOI] [PubMed] [Google Scholar]
- 32.Pitkanen HT, Oja SS, Kemppainen K, Seppa JM, Mero AA. Serum amino acid concentrations in aging men and women. Amino Acids. 2003;24:413–421. doi: 10.1007/s00726-002-0338-0. [DOI] [PubMed] [Google Scholar]
- 33.Bancel E, Strubel D, Bellet H, Polge A, Peray P, Magnan de Bornier B. Effect of the age and the sex on plasma concentration of amino acids. Ann Biol Clin (Paris) 1994;52:667–670. [PubMed] [Google Scholar]
- 34.Proenza AM, Crespi C, Roca P, Palou A. Gender related differences in the effect of aging on blood amino acid compartmentation. J Nutr Biochem. 2001;12:431–440. doi: 10.1016/s0955-2863(01)00157-7. [DOI] [PubMed] [Google Scholar]
- 35.Moller SE, Moller BM, Olesen M, Fjalland B. Effects of oral contraceptives on plasma neutral amino acids and cholesterol during a menstrual cycle. Eur J Clin Pharmacol. 1996;50:179–184. doi: 10.1007/s002280050089. [DOI] [PubMed] [Google Scholar]
- 36.Tipton KD, Elliott TA, Ferrando AA, Aarsland AA, Wolfe RR. Stimulation of muscle anabolism by resistance exercise and ingestion of leucine plus protein. Appl Physiol Nutr Metab. 2009;34:151–161. doi: 10.1139/H09-006. [DOI] [PubMed] [Google Scholar]
- 37.Lamont LS, McCullough AJ, Kalhan SC. Gender differences in the regulation of amino acid metabolism. J Appl Physiol. 2003;95:1259–1265. doi: 10.1152/japplphysiol.01028.2002. [DOI] [PubMed] [Google Scholar]
- 38.Nishizawa S, Benkelfat C, Young SN, Leyton M, Mzengeza S, de Montigny C, Blier P, Diksic M. Differences between males and females in rates of serotonin synthesis in human brain. Proc Natl Acad Sci U S A. 1997;94:5308–5313. doi: 10.1073/pnas.94.10.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cederblad G. Plasma carnitine and body composition. Clin Chim Acta. 1976;67:207–212. doi: 10.1016/0009-8981(76)90261-8. [DOI] [PubMed] [Google Scholar]
- 40.Borum PR. Plasma carnitine compartment and red blood cell carnitine compartment of healthy adults. Am J Clin Nutr. 1987;46:437–441. doi: 10.1093/ajcn/46.3.437. [DOI] [PubMed] [Google Scholar]
- 41.Takiyama N, Matsumoto K. Age-and sex-related differences of serum carnitine in a Japanese population. J Am Coll Nutr. 1998;17:71–74. doi: 10.1080/07315724.1998.10720458. [DOI] [PubMed] [Google Scholar]
- 42.Amatayakul K, Laokuldilok T, Koottathep S, Dejsarai W, Prapamontol T, Srirak N, Tansuhaj A, Uttaravichai C. The effect of oral contraceptives on protein metabolism. J Med Assoc Thai. 1994;77:509–516. [PubMed] [Google Scholar]
- 43.Campesi I, Carru C, Zinellu A, Occhioni S, Sanna M, Palermo M, Tonolo G, Mercuro G, Franconi F. Regular cigarette smoking influences the transsulfuration pathway, endothelial function, and inflammation biomarkers in a sex-gender specific manner in healthy young humans. Am J Transl Res. 2013;5:497–509. [PMC free article] [PubMed] [Google Scholar]
- 44.Gacias M, Perez-Marti A, Pujol-Vidal M, Marrero PF, Haro D, Relat J. PGC-1beta regulates mouse carnitine-acylcarnitine translocase through estrogen-related receptor alpha. Biochem Biophys Res Commun. 2012;423:838–843. doi: 10.1016/j.bbrc.2012.06.051. [DOI] [PubMed] [Google Scholar]
- 45.Lango R, Smolenski RT, Narkiewicz M, Suchorzewska J, Lysiak-Szydlowska W. Influence of L-carnitine and its derivatives on myocardial metabolism and function in ischemic heart disease and during cardiopulmonary bypass. Cardiovasc Res. 2001;51:21–29. doi: 10.1016/s0008-6363(01)00313-3. [DOI] [PubMed] [Google Scholar]
- 46.Magoulas PL, El-Hattab AW. Systemic primary carnitine deficiency: an overview of clinical manifestations, diagnosis, and management. Orphanet J Rare Dis. 2012;7:68. doi: 10.1186/1750-1172-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flanagan JL, Simmons PA, Vehige J, Willcox MD, Garrett Q. Role of carnitine in disease. Nutr Metab (Lond) 2010;7:30. doi: 10.1186/1743-7075-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tamai I. Pharmacological and pathophysiological roles of carnitine/organic cation transporters (OCTNs: SLC22A4, SLC22A5 and Slc22a21) Biopharm Drug Dispos. 2013;34:29–44. doi: 10.1002/bdd.1816. [DOI] [PubMed] [Google Scholar]
- 49.Lerner A, Gruener N, Iancu TC. Serum carnitine concentrations in coeliac disease. Gut. 1993;34:933–935. doi: 10.1136/gut.34.7.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khan HA, Alhomida AS, Madani HA, Sobki SH. Carnitine and acylcarnitine profiles in dried blood spots of patients with acute myocardial infarction. Metabolomics. 2013;9:828–838. [Google Scholar]
- 51.Ford DA, Han X, Horner CC, Gross RW. Accumulation of unsaturated acylcarnitine molecular species during acute myocardial ischemia: metabolic compartmentalization of products of fatty acyl chain elongation in the acylcarnitine pool. Biochemistry. 1996;35:7903–7909. doi: 10.1021/bi960552n. [DOI] [PubMed] [Google Scholar]
- 52.Regitz V, Shug AL, Fleck E. Defective myocardial carnitine metabolism in congestive heart failure secondary to dilated cardiomyopathy and to coronary, hypertensive and valvular heart diseases. Am J Cardiol. 1990;65:755–760. doi: 10.1016/0002-9149(90)91383-h. [DOI] [PubMed] [Google Scholar]
- 53.Ino T, Sherwood WG, Benson LN, Wilson GJ, Freedom RM, Rowe RD. Cardiac manifestations in disorders of fat and carnitine metabolism in infancy. J Am Coll Cardiol. 1988;11:1301–1308. doi: 10.1016/0735-1097(88)90296-3. [DOI] [PubMed] [Google Scholar]
- 54.Guyton A. Textbook of Medical Physiology. Philadelphia: Saunders; 1986. [Google Scholar]
- 55.Rademaker M. Do women have more adverse drug reactions? Am J Clin Dermatol. 2001;2:349–351. doi: 10.2165/00128071-200102060-00001. [DOI] [PubMed] [Google Scholar]


