Abstract
Background
Strains on the capacities of intensive care units (ICUs) may influence the quality of ICU-to-floor transitions.
Objective
To determine how 3 metrics of ICU capacity strain (ICU census, new admissions, and average acuity) measured on days of patient discharges influence ICU length of stay (LOS) and post–ICU discharge outcomes.
Design
Retrospective cohort study from 2001 to 2008.
Setting
155 ICUs in the United States.
Patients
200 730 adults discharged from ICUs to hospital floors.
Measurements
Associations between ICU capacity strain metrics and discharged patient ICU LOS, 72-hour ICU readmissions, subsequent in-hospital death, post–ICU discharge LOS, and hospital discharge destination.
Results
Increases in the 3 strain variables on the days of ICU discharge were associated with shorter preceding ICU LOS (all P < 0.001) and increased odds of ICU readmissions (all P< 0.050). Going from the 5th to 95th percentiles of strain was associated with a 6.3-hour reduction in ICU LOS (95% CI, 5.3 to 7.3 hours) and a 1.0% increase in the odds of ICU readmission (CI, 0.6% to 1.5%). No strain variable was associated with increased odds of subsequent death, reduced odds of being discharged home from the hospital, or longer total hospital LOS.
Limitation
Long-term outcomes could not be measured.
Conclusion
When ICUs are strained, triage decisions seem to be affected such that patients are discharged from the ICU more quickly and, perhaps consequentially, have slightly greater odds of being readmitted to the ICU. However, short-term patient outcomes are unaffected. These results suggest that bed availability pressures may encourage physicians to discharge patients from the ICU more efficiently and that ICU readmissions are unlikely to be causally related to patient outcomes.
Primary Funding Source
Agency for Healthcare Research and Quality; National Heart, Lung, and Blood Institute; and Society of Critical Care Medicine.
The high costs of critical care and projected shortages in providers of this care in the United States (1–6) will impede the ability to augment critical care supply in response to surges in demand engendered by an aging population (7, 8). Therefore, intensive care units (ICUs) will operate under conditions of increasing strain, providing care for greater numbers of more seriously ill patients (9).
Strain on ICU capacity may have particularly strong influences on decisions to discharge patients from ICUs. Providers may discharge patients sooner than desired to open ICU beds, and time constraints may influence the quality of communication during patient handoffs. Previous single-center studies of the effects of isolated elements of ICU capacity strain have yielded mixed results. Some studies have suggested that more new admissions and increased bed occupancy were associated with worse outcomes among discharged patients (10, 11), whereas others found that these strains on ICU capacity led to earlier patient discharges without changes in outcomes (12–14). Distinguishing between these competing findings has important policy implications because if patient outcomes are affected, this would suggest that potentially beneficial ICU time is being rationed under conditions of strain. By contrast, if strain causes reductions in ICU length of stay (LOS) without harming patients, this would suggest that strain causes clinicians to limit low-value extensions of ICU stays (15). Therefore, we explored the consequences of several metrics of ICU capacity strain (9) on patients who survived their initial ICU stays during hospitalization in a large, nationally representative sample of ICUs in the United States. Unlike previous studies, this approach yielded generalizable results and substantial power to detect changes in patient outcomes that we hypothesized would be influenced by the daily census, proportion of new admissions, and acuity of other patients in the ICU at the time when patients were discharged from the ICU.
Methods
Study Design
We conducted a cohort study of ICU patients who were discharged from an initial ICU admission to a hospital floor or step-down unit. The primary exposure variables we examined were 3 measures of ICU capacity strain, each calculated at the time of discharge from the ICU. Among these discharged patients, outcomes included initial ICU LOS and several post–ICU discharge outcomes.
Data Source
The cohort comprised patients admitted to ICUs in the United States included in the Project IMPACT database (Cerner Corporation, Kansas City, Missouri), which prospectively collects information on patients in 194 ICUs at 131 hospitals. Project IMPACT is a voluntary, fee-based, clinical information system comprising a large, diverse sample of ICUs that is commonly used for critical care outcomes research (16 –19). Data collectors who are certified by Project IMPACT capture detailed clinical information at each site from the time of ICU admission until hospital discharge. Because we were granted permission to use a specially prepared version of Project IMPACT that retained real date and time stamps for all patients' ICU admissions and discharges, we had a unique opportunity to determine how patients coinhabiting ICUs influenced each other's outcomes.
Patient Selection
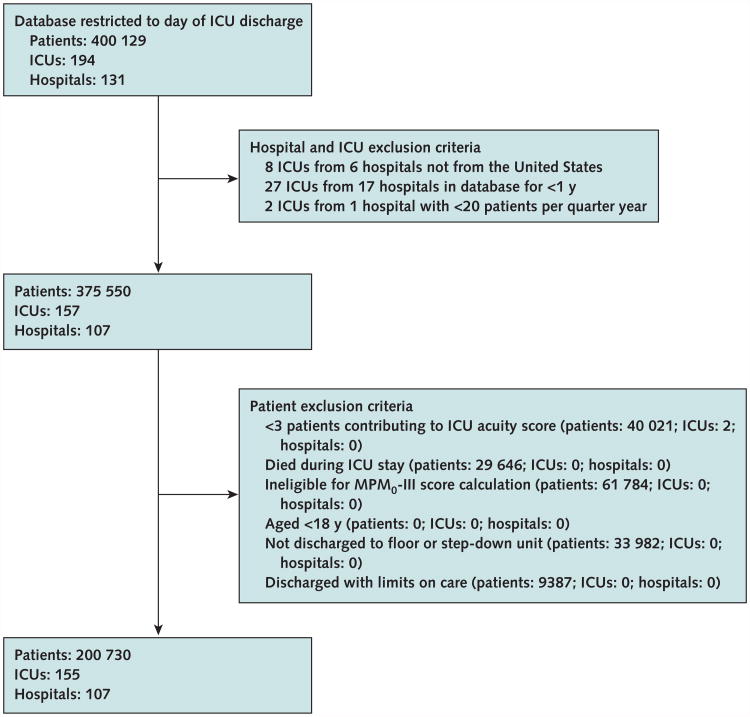
We included ICUs in the United States that provided at least 12 months of consecutive data during the study (Figure 1). We included all eligible patients except for those admitted to ICUs during periods of relative inactivity (admission of fewer than 20 patients per 3-month period) to account for ICUs that were either beginning or ending their participation in Project IMPACT. We then excluded patients who were younger than 18 years, were classified as moribund or had limits on their care beyond do-not-resuscitate orders at the time of ICU discharge (because subsequent death is an expected outcome in these patients), and were ineligible for Mortality Probability Model III (MPM0-III) score calculation (pediatric, burn, and coronary care patients). The MPM0-III is a commonly used ICU severity-of-illness adjustment system yielding condition-based scores that are calculated from physiologic and laboratory data obtained within 1 hour of ICU admission (20).
Figure 1. Study flow diagram.
ICU = intensive care unit; MPM0-III = Mortality Probability Model III.
To promote data independence, we analyzed only initial ICU discharges within a hospitalization. However, because Project IMPACT does not track patients across hospitalizations, those who were discharged from the hospital and then readmitted may have contributed more than 1 ICU admission.
Exposure Variables
We used 3 metrics of ICU capacity strain as primary exposures, each measured on the day of a patient's initial ICU discharge: census, admissions, and acuity (Table 1). The census included all patients spending at least 2 hours in the ICU each day, standardized to that ICU's mean, to account for differences in ICU size. Admissions represented the proportion of the daily census composed of new admissions that day; this variable accounted for possible differences in resource intensiveness between newly admitted and previously admitted ICU patients. Finally, acuity was calculated as the average predicted probability of death of other patients in the ICU, based on individual MPM0-III scores calculated on the day of admission. These measures stem from a conceptual model of ICU capacity strain (9), and each has been shown to be independently associated with ICU physicians' and nurses' ratings of daily workload, supporting their construct validity (21).
Table 1. ICU Capacity Strain Variables and Definitions.
| Variable* | Operational Definition | Method of Calculation |
|---|---|---|
| Census† | Standardized ICU census | The difference between the number of patients spending at least 2 h in the ICU that day (12:00 a.m.–11:59 p.m.) and the mean census for that year, divided by the yearly SD of that ICU's census. |
| Admissions | Proportion of new admissions | The number of new admissions on that day divided by the raw (nonstandardized) ICU census that day. |
| Acuity‡ | Average predicted probability of death of the other patients in the ICU | The sum of the probability of death of other ICU patients divided by the number of other patients in the ICU on that day, excluding the index patient. |
ICU = intensive care unit.
All candidate capacity strain variables are measured on the index patient's day of ICU discharge. Calculations included all patients (including those who died in the ICU) who were present on the day of an index patient's discharge.
A 2-h cutoff was chosen to account for possible coding errors in bed assignment that were quickly corrected.
Probabilities of death derived from Mortality Probability Model III scores.
Risk Adjustment
We assessed and adjusted for confounding by severity of illness using the MPM0-III scores of the discharged patients (20), plus variables indicating whether these patients were mechanically ventilated or received vasoactive infusions (vasopressors or inotropes) during their initial ICU stays. We further adjusted for discharged patient demographic and admission characteristics (Table 2).
Table 2. Discharged Patient Characteristics*.
| Characteristic | Patients (n = 200 730) |
|---|---|
| Men | 108 308 (54.0) |
| Age | |
| ≤64 y | 111 587 (55.6) |
| 65–74 y | 39 676 (19.8) |
| 74–84 y | 36 744 (18.3) |
| ≥85 y | 12 723 (6.3) |
| Race | |
| White | 153 818 (76.6) |
| Black | 29 349 (14.6) |
| Other | 17 563 (8.7) |
| Insurance status | |
| Private | 60 168 (30.0) |
| Medicare | 98 368 (49.0) |
| Medicaid | 17 116 (8.5) |
| Self-pay | 17 179 (8.6) |
| Government/other | 7899 (3.9) |
| Functional status | |
| Independent | 157 642 (78.5) |
| Partially dependent | 30 897 (15.4) |
| Fully dependent | 12 191 (6.1) |
| Source of ICU admission | |
| Emergency department | 79 788 (39.7) |
| Another hospital | 11 846 (5.9) |
| General care | 23 900 (11.9) |
| Step-down unit | 5234 (2.6) |
| Procedure | 72 105 (35.9) |
| SNF/acute rehabilitation | 1043 (0.5) |
| Another ICU | 3059 (1.5) |
| Other | 3755 (1.9) |
| Type of ICU admission | |
| Surgical (scheduled) | 49 244 (24.5) |
| Surgical (unscheduled) | 26 721 (13.3) |
| Medical | 124 765 (62.2) |
| Mean MPM0 -III–predicted probability of death (SD) | 10.9 (1.2) |
| Mechanical ventilation (any) | 66 142 (33.0) |
| Vasoactive infusions (any) | 31 02 (15.5) |
| Median ICU LOS (IQR), h | 48 (26–91) |
| Median post–ICU discharge LOS (IQR), h | 96 (47–188) |
| 72-h ICU readmissions | 6473 (3.2) |
| Subsequent in-hospital death Hospital discharge destination | 7947 (4.0) |
| Hospital discharge destination | |
| Home | 127 110 (63.3) |
| SNF | 54 975 (27.4) |
| Another hospital | 5181 (2.6) |
| Other | 5525 (2.8) |
ICU = intensive care unit; IQR = interquartile range; LOS = length of stay; MPM0-III = Mortality Probability Model III; SNF = skilled nursing facility
Values are numbers (percentages) unless otherwise indicated.
Outcomes
We focused on ICU discharges to hospital floors or step-down units to ensure that all patients were eligible for all outcomes. We first evaluated LOS (in hours) during the initial ICU admission. Length of stay was calculated as the number of hours from ICU admission to ICU discharge. Second, we assessed readmissions to the ICU within 72 hours. We chose 72 hours as the cutoff in base analyses because it represents the median interval between ICU discharge and readmission for U.S. patients (16, 22). Third, we calculated in-hospital mortality rates for all patients from the time of initial ICU discharge through hospital discharge, including patients who died during an ICU readmission. Fourth, we evaluated post–ICU discharge hospital LOS. The sum of this variable plus initial ICU LOS equals total hospital LOS. Finally, we evaluated patients' ultimate destination at hospital discharge and specifically whether patients were discharged to home.
Statistical Analysis
Bivariate analyses evaluating each measure of ICU capacity strain's association with each outcome were done using linear and logistic regression for continuous and categorical variables, respectively. We used locally weighted scatterplot smoothing curves to determine whether continuous variables could be modeled linearly or required transformation (23). Log transformation was done for discharged patients' MPM0-III scores and for the outcomes of ICU LOS and post–ICU discharge hospital LOS. In multi-variable regression models, we included ICU year (each ICU identified separately during each calendar year) as a fixed effect to mitigate potential confounding by differences among ICUs or in ICU practices over time (24). We included terms for each continuous strain variable and tested for 2-way interactions between strain variables for all regression models.
Several secondary analyses assessed the robustness of our findings. First, we examined the relationship between strain and ICU LOS using median regression to account for a positively skewed ICU LOS distribution. Second, we evaluated ICU readmissions at 48 hours instead of 72 hours because the former have been endorsed as a quality metric (16, 25). Third, we tested additional elements of ICU acuity by adding variables representing the percentages of mechanically ventilated patients and of patients requiring vasoactive infusions in the ICU each day. Fourth, we repeated our analyses in a data set restricted to the top quartiles of either median ICU acuity or median percentage of occupancy to focus on the highest-acuity ICUs and those operating closest to physical capacity, respectively. Fifth, we evaluated the influence of strain on a group of patients who may be particularly susceptible to it, specifically those receiving mechanical ventilation or having a diagnosis of acute respiratory failure on admission.
Sixth, to explore the possibility that our results were influenced by a healthy survivor effect (that is, on high-strain days some patients who were marginally eligible for discharge died in the ICU such that those who are discharged are healthier), we evaluated the influence of ICU capacity strain on the odds of a patient dying on the day of discharge. Seventh, we restricted our analyses to exclude ICUs with more than 30% elective surgical patients because these patients had an increased likelihood of having an expected or scheduled discharge. Eighth, we reexamined ICU LOS for discharged patients after including patients admitted to ICUs with fewer than 20 patients per quarter year and those with fewer than 4 patients contributing MPM0-III scores. Lastly, we evaluated the potential effects of transfers by including patients having intrahospital and interhospital ICU transfers in the analyses. For intrahospital transfers, observed hospital outcomes were used. For interhospital transfers, we estimated outcomes on the basis of the observed mortality rate (24%) among patients admitted to Project IMPACT ICUs via interhospital transfer. Specifically, we randomly assigned 24% of interhospital transfer patients as deceased and used bootstrap resampling with 100 iterations to generate 95% CIs for the results of this secondary analysis. All analyses were done using SAS, version 9.3 (SAS Institute, Cary, North Carolina), and Stata, version 12 (StataCorp, College Station, Texas). This study was considered to be exempt by the Institutional Review Board of the University of Pennsylvania (Philadelphia, Pennsylvania).
Role of the Funding Source
This work was supported by the Agency for Healthcare Research and Quality; National Heart, Lung, and Blood Institute; and Society of Critical Care Medicine. The funding sources were not involved in the design of the study or in the decision to submit the manuscript for publication.
Results
A total of 200 730 eligible patients survived their initial ICU admission and were discharged to a hospital floor or step-down unit (Figure 1). Among such patients, 3.2% had an ICU readmission within 72 hours, 4.0% died in the hospital after their initial ICU discharge, and 63.3% were ultimately discharged directly home from the hospital (Table 2). Many of the foregoing measures varied considerably across the included ICUs (data not shown), consistent with the diversity of the ICUs in Project IMPACT (Appendix Table 1, available at www.annals.org).
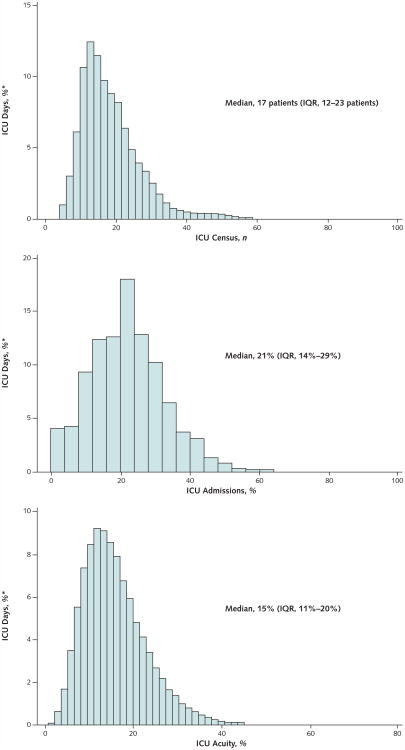
Figure 2 displays the distributions of the 3 capacity strain variables when measured on the days of ICU discharge. In bivariate analyses, these capacity strain variables each displayed mixed associations with the 5 outcomes (Appendix Table 2, available at www.annals.org). The variables were not highly correlated with each other (ICU census and admissions [r = 0.23], ICU census and acuity [r = − 0.03], and ICU admissions and acuity [r = − 0.095]), enabling their joint inclusion in multivariable models. After additional adjustment for patient characteristics, each variable was inversely associated with ICU LOS (all P < 0.001) (Appendix Table 3, available at www.annals.org). There was a significant interaction between ICU admissions and acuity (P = 0.015) such that the effect of admissions on the decrease in ICU LOS was greater during times of higher acuity (Appendix Figure, available at www.annals.org). Hospital LOS after ICU discharge was longer among patients discharged on days with higher census (P = 0.002), shorter among patients discharged on days with increased admissions (P < 0.001), and not significantly associated with ICU acuity (P = 0.076) (Appendix Table 3).
Figure 2. Capacity strain variables when measured on the day of ICU discharge.
ICU = intensive care unit; IQR = interquartile range.
*The unit of analysis was each ICU day, and the y-axis plots the proportion of these ICU days during which the corresponding value of strain was experienced.
Elevations in the strain variables were associated with greater odds of 72-hour ICU readmission in the fully adjusted model (Table 3). For every 1-unit change in census, the odds of 72-hour ICU readmission increased by 3% (P = 0.030). Similarly, 10% increases in ICU admissions and acuity corresponded to 3% (P = 0.053) and 5% (P = 0.019) increased odds of ICU readmission, respectively.
Table 3. Discharge Day Capacity Strain and the Odds of ICU Readmission, Subsequent In-Hospital Death, and Being Discharged Home.
| Predictor* | 72-h ICU Readmission | Subsequent In-Hospital Death | Home as Discharge Destination | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| ICU capacity strain† | ||||||
| Census | 1.03 (1.00–1.06) | 0.030 | 1.02 (1.00–1.05) | 0.10 | 0.99 (0.98–1.00) | 0.22 |
| Admissions | 1.03 (1.00–1.06) | 0.053 | 0.97 (0.94–0.99) | 0.011 | 1.01 (1.00–1.03) | 0.015 |
| Acuity | 1.05 (1.01–1.10) | 0.019 | 1.00 (0.96–1.04) | 0.87 | 0.98 (0.97–1.00) | 0.76 |
| Discharged patient factors | ||||||
| Age | ||||||
| ≤64 y | Reference | Reference | Reference | |||
| 65–74 y | 1.11 (1.02–1.20) | 0.014 | 1.36 (1.25–1.47) | <0.001 | 0.71 (0.68–0.73) | <0.001 |
| 74–84 y | 1.08 (0.99–1.18) | 0.078 | 1.83 (1.69–1.98) | <0.001 | 0.42 (0.40–0.43) | <0.001 |
| ≥85 y | 0.96 (0.84–1.08) | 0.47 | 2.25 (2.05–2.48) | <0.001 | 0.24 (0.23–0.25) | <0.001 |
| Race | ||||||
| White | Reference | Reference | Reference | |||
| Black | 0.84 (0.77–0.91) | <0.001 | 0.98 (0.91–1.05) | 0.61 | 1.03 (1.00–1.07) | 0.051 |
| Other | 0.89 (0.79–1.00) | 0.047 | 0.89 (0.79–1.00) | 0.055 | 1.42 (1.35–1.49) | <0.001 |
| Sex | ||||||
| Male | Reference | Reference | Reference | |||
| Female | 0.90 (0.85–0.95) | <0.001 | 0.92 (0.88–0.97) | 0.001 | 0.89 (0.87–0.91) | <0.001 |
| Insurance | ||||||
| Private | Reference | Reference | Reference | |||
| Medicare | 1.18 (1.09–1.28) | <0.001 | 1.32 (1.22–1.43) | <0.001 | 0.67 (0.65–0.69) | <0.001 |
| Medicaid | 1.17 (1.06–1.30) | 0.002 | 1.25 (1.12–1.39) | <0.001 | 0.73 (0.70–0.76) | <0.001 |
| Self-pay | 0.75 (0.67–0.85) | <0.001 | 0.72 (0.63–0.83) | <0.001 | 1.07 (1.02–1.12) | 0.004 |
| Government/other | 0.88 (0.74–1.04) | 0.150 | 0.93 (0.77–1.13) | 0.47 | 0.85 (0.80–0.91) | <0.001 |
| Source of ICU admission | ||||||
| Emergency department | Reference | Reference | Reference | |||
| Another hospital | 1.24 (1.11–1.38) | <0.001 | 1.15 (1.03–1.28) | 0.010 | 1.05 (1.00–1.11) | 0.027 |
| General care | 1.46 (1.35–1.58) | <0.001 | 2.19 (2.05–2.34) | <0.001 | 0.85 (0.82–0.88) | <0.001 |
| Step-down unit | 1.32 (1.14–1.53) | <0.001 | 2.07 (1.84–2.32) | <0.001 | 0.88 (0.82–0.94) | <0.001 |
| Procedure | 1.07 (0.97–1.20) | 0.171 | 1.20 (1.08–1.33) | 0.001 | 1.52 (1.45–1.59) | <0.001 |
| SNF/rehabilitation facility | 1.59 (1.18–2.15) | 0.002 | 2.38 (1.92–2.95) | <0.001 | 0.09 (0.08–0.11) | <0.001 |
| Another ICU | 1.86 (1.58–2.19) | <0.001 | 1.63 (1.40–1.91) | <0.001 | 0.71 (0.65–0.77) | <0.001 |
| Other | 0.96 (0.77–1.05) | 0.68 | 1.20 (0.98–1.46) | 0.071 | 1.26 (1.15–1.37) | <0.001 |
| Type of ICU admission | ||||||
| Surgical (scheduled) | Reference | Reference | Reference | |||
| Surgical (unscheduled) | 1.31 (1.20–1.43) | <0.001 | 1.09 (0.99–1.20) | 0.068 | 0.61 (0.58–0.63) | <0.001 |
| Medical | 1.22 (1.09–1.36) | <0.001 | 1.33 (1.20–1.48) | <0.001 | 0.96 (0.92–1.00) | 0.101 |
| Mechanical ventilation (any) | 1.26 (1.18–1.34) | <0.001 | 1.14 (1.08–1.21) | <0.001 | 0.56 (0.54–0.57) | <0.001 |
| Vasopressor use (any) | 1.25 (1.16–1.33) | <0.001 | 1.48 (1.40–1.57) | <0.001 | 0.76 (0.74–0.79) | <0.001 |
ICU = intensive care unit; OR = odds ratio; SNF = skilled nursing facility.
Entered in fully adjusted logistic regression with ICU year modeled as a fixed effect.
The OR for ICU census corresponds to a 1-unit change in standardized census, whereas the ORs for ICU admissions and acuity correspond to a 10% change in the proportion of new admissions and the predicted probability of death of the other patients in the ICU at the time of ICU discharge, respectively.
No capacity strain variable was associated with increased odds of subsequent in-hospital death or decreased odds of being discharged directly home from the hospital (Table 3). Discharge on days with increased admissions was associated with lower odds of subsequent in-hospital death (odds ratio [OR], 0.97 [95% CI, 0.94 to 0.99]) and higher odds of being discharged directly home from the hospital (OR, 1.01 [CI, 1.00 to 1.03]). No significant 2-way interactions occurred between any 2 strain variables when dichotomous outcomes were assessed.
To illustrate the magnitude of the regression findings, increases in all strain variables from the 5th to the 95th percentiles of their respective distributions resulted in a 6.3-hour (CI, 5.3 to 7.3 hours) reduction in expected ICU LOS for patients surviving their initial ICU stay and a 2.0-hour (CI, 1.0 to 3.0 hours) decrease in expected post–ICU discharge hospital LOS. Thus, corresponding increases in the 3 capacity strain variables resulted in an overall reduction of 8.3 hours in expected total hospital LOS (Table 4). In addition to this decrease in total hospital LOS, raising all 3 variables from the 5th to the 95th percentiles resulted in a 1.0% (CI, 0.6% to 1.5%) increase in the probability of being readmitted to the ICU within 72 hours of ICU discharge and no significant change in mortality rates or hospital discharge destination (Table 4).
Table 4. Expected Outcomes Based on Percentiles of ICU Capacity Strain*.
| Capacity Strain | ICU LOS, h | Post–ICU Discharge Hospital LOS, h | Probability of 72-h ICU Readmission, % | Probability of Subsequent Death, % | Probability of Being Discharged Home, % |
|---|---|---|---|---|---|
| 5% | 55.1 | 100.3 | 2.9 | 4.0 | 66.0 |
| 10% | 54.4 | 100.1 | 3.0 | 4.1 | 65.7 |
| 25% | 53.4 | 99.5 | 3.2 | 4.1 | 66.0 |
| 50% | 52.0 | 99.4 | 3.3 | 4.1 | 65.6 |
| 75% | 50.7 | 98.8 | 3.5 | 4.0 | 65.6 |
| 90% | 49.4 | 98.5 | 3.8 | 4.0 | 65.6 |
| 95% | 48.8 | 98.3 | 3.9 | 4.0 | 65.5 |
ICU = intensive care unit; LOS = length of stay.
All expected values and probabilities were derived from the fully adjusted model by entering values for capacity strain at given percentiles of their respective distributions.
Similar to our primary analyses, analyses accounting for ICU transfer practices yielded null associations between the 3 strain variables and death: census (OR, 1.02 [CI, 0.95 to 1.05]), admissions (OR, 0.98 [CI, 0.95 to 1.00]), and acuity (OR, 1.00 [CI, 0.96 to 1.04]). Similar results were also produced in the other 8 secondary analyses (Appendix Table 4, available at www.annals.org). We found no consistent evidence that the effects of strain on ICU LOS varied significantly by an ICU's physician staffing model, academic affiliation, or service as a critical care fellowship training site (Appendix Table 5, available at www.annals.org).
Discussion
This study shows that the circumstances under which patients are discharged from ICUs to hospital floors vary considerably from day to day within each ICU and that these variations affect patient flow without changing short-term outcomes. Specifically, ICU census, admissions, and acuity were associated with critical care use such that when these measures were elevated, initial ICU LOS was shorter than it would be otherwise. Of note, the observation that patients discharged on days with high-capacity strain had slightly higher odds of ICU readmission suggests that the reduction in ICU LOS induced by variations in capacity strain are sufficiently large to influence potentially consequential triage decisions. Nonetheless, these effects on triage did not influence in-hospital death or a patient's odds of being discharged home. Together, these results suggest that rather than causing the rationing of beneficial care (12, 13, 26–28), strain spurs providers to reduce their provision of what seems to be low-value care by critically re-examining needs for ICU-level care and transferring patients who could be equally well-managed outside the ICU setting.
Distinguishing reductions in low-value care provision from withholding clearly beneficial care has important clinical and ethical implications (15). Avoiding waste serves the interests of individual patients and society simultaneously, whereas rationing beneficial care entails tradeoffs between these interests (26, 27). To our knowledge, our study provides the most robust evidence to date that provision of critical care is not based solely on a clinician's assessment of individual patient needs. Instead, external factors, including the needs of other patients, influence decision making. Of note, under current conditions in ICUs in the United States, these influences do not seem to harm patients, at least in the short term. Indeed, our data support the hypothesis that systematic reductions in critical care use could be obtained without adversely affecting patients (29–31).
Although reductions in ICU LOS may influence outcomes under conditions of higher strain in the future, our study suggests that this general conclusion has been true for some time. Our results are consistent with a single-center study from 3 decades ago suggesting that when ICU bed occupancy was high, ICU patients were more rapidly discharged without having adverse outcomes (12). Whereas that study had limited generalizability and power to detect changes in patient outcomes, our study of more than 200 000 patients discharged from 155 ICUs in the United States could have detected very small effects had they been present.
Similar questions about the value of certain types of ICU care were raised by a recent study demonstrating that New York hospitals that manage most patients with diabetic ketoacidosis in non-ICU settings achieve similar risk-adjusted hospital LOS and mortality rates as hospitals that initially admit most of these patients to the ICU (32). This suggests that many ICUs admit patients who would benefit equally from a lower level of care.
Although the reductions in ICU LOS we noted were modest, decreasing ICU LOS by even a few hours for all patients admitted to the nearly 100 000 ICU beds in the United States each year (33) could reduce the overall use of critical care. Thus, we may accommodate part of the surging demand for critical care by increasing ICU efficiency rather than using the higher-cost approach of building more ICU beds (33–38).
Our study is also consistent with previous investigations showing that singular metrics of strain are associated with more frequent ICU readmissions (10, 11, 39). However, our observation that variations in ICU capacity strain influence readmission rates without ultimately affecting patient outcomes suggests that ICU readmissions may not be causally related to the quality of ICU care (40). This suggests the need for caution in adopting ICU readmission rates as a measure of ICU quality (25, 41). Indeed, our results suggest that such a policy may yield the unintended consequence of causing physician reluctance to discharge patients in an efficient manner.
Our study has limitations. First, the ICUs participating were not randomly selected. Nonetheless, Project IMPACT ICUs represent the diversity of U.S. critical care with respect to ICU size, location, academic affiliation, and the characteristics and illnesses of patients (16). Further, our hierarchical design allowed assessments of the effects of strain within ICUs, with those effects then averaged across ICUs, promoting the internal validity of the comparisons (24). Second, we have not experimentally quantified the value of several extra hours of critical care. Because randomly assigning similar patients to longer versus shorter ICU stays would be ethically challenging, we believe that our design provides reasonable alternative evidence that low-value extensions of ICU stays are commonly provided in the United States. Third, we could not directly measure the outcomes of patients transferred to other hospitals during times of high or low strain. However, these transfers comprised only 2.3% of Project IMPACT ICU discharges, and a secondary analysis that used bootstrap resampling to estimate these patients' deaths provided results nearly identical to our primary analyses. Fourth, because Project IMPACT does not collect post–hospital discharge data, we could not measure the effects of strain on long-term outcomes or account for possible hospital readmissions. However, any adverse effects of capacity strain on the day of ICU discharge would most likely manifest close to the time of discharge. Fifth, our study does not measure the influence of strain on patients discharged directly home or to skilled nursing facilities or other post–acute care settings (Appendix Table 6, available at www.annals.org). Sixth, we did not have data on other exogenous influences of capacity strain, such as the availability of hospital beds. Because reduced availability of hospital beds would diminish the ability to discharge patients from the ICU, strain may have had greater effects on ICU LOS if hospital beds were plentiful. Finally, our study was not designed to help clinicians identify when specific patients are ready for ICU discharge.
In summary, the census, proportion of new admissions, and acuity of patients in an ICU all influence bed allocation decisions without impacting important outcomes for patients having ICU-to-floor transitions. Rather than confirming fears of critical care rationing, these results suggest that many patients in ICUs in the United States are too well to benefit from ongoing critical care and that bed pressures prompt physicians to allocate ICU resources more efficiently.
Context.
At times, intensive care units (ICUs) have too many acutely ill patients for their staffing. One response is to discharge patients from the ICU who probably would have otherwise remained.
Contribution
When ICUs were strained, patients stayed for a shorter time and were somewhat more likely to be readmitted to the ICU. However, there were no increases in patient mortality rates, no greater overall length of hospital stay, and no reductions in the odds of being discharged from the hospital to home.
Caution
Long-term outcomes were not studied.
Implication
When ICUs are strained, discharge decisions seem to be efficient without being harmful.
Acknowledgments
The authors thank the data collectors for Project IMPACT and the Cerner Corporation (which is the sole proprietor of Project IMPACT), particularly Andrew Kramer, PhD, and Maureen Stark, for the use of Project IMPACT data for research purposes. They also thank Michael O. Harhay, MPH, for his assistance with the statistical analyses.
Grant Support: By the Agency for Healthcare Research and Quality (K08HS018406); the National Heart, Lung, and Blood Institute (T32HL098054); and the Society of Critical Care Medicine (Vision Grant).
Appendix
Appendix Table 1. Project IMPACT ICU Organizational Characteristics.
| ICU Characteristic | ICU Years, n (%)* | Patients, n (%)† |
|---|---|---|
| Location | ||
| Urban | 389 (58.6) | 118 804 (59.3) |
| Suburban | 190 (28.6) | 51 009 (25.5) |
| Rural | 85 (12.8) | 30 391 (15.2) |
| Number of beds | ||
| 5–12 | 281 (42.8) | 57 595 (29.0) |
| 13–16 | 135 (20.6) | 36 777 (18.5) |
| 17–21 | 127 (19.3) | 54 008 (27.2) |
| 22–66 | 114 (17.3) | 50 197 (25.3) |
| Type | ||
| Academic | 154 (23.1) | 49 852 (24.8) |
| City/county/state | 30 (4.5) | 7873 (3.9) |
| Community | 482 (72.4) | 143 005 (71.2) |
| Model | ||
| Closed | 47 (7.1) | 14 300 (7.1) |
| Open with mandatory critical care | 154 (23.3) | 57 713 (28.8) |
| Open without mandatory critical care | 461 (69.6) | 128 717 (64.1) |
| Affiliation with medical school | 567 (85.1) | 171 507 (85.4) |
| Critical care fellowship program | 255 (38.3) | 78 153 (38.9) |
| Night coverage | ||
| Critical care physician | 172 (25.8) | 62 048 (30.9) |
| Attending/other physician | 169 (25.4) | 53 831 (26.8) |
| Fellow | 46 (6.9) | 17 222 (8.6) |
| Resident | 205 (30.8) | 48 684 (24.3) |
| Other | 74 (11.1) | 18 945 (9.4) |
ICU = intensive care unit.
Data presented by ICU year, which reflects the combination of each ICU for each year that it contributed data to the sample. The overall sample included 155 ICUs from 2001 to 2008, but because not all ICUs contributed data for this entire period, the total number of ICU years was 658.
Percentages in the ICU characteristics of location and number of beds were calculated with adjusted denominators of 200 204 and 198 577 patients, respectively, due to missing data. All other percentages were calculated with denominators of 200 730 patients.
Appendix Table 2. Bivariate Analysis*.
| Capacity Strain Variable | Value | |
|---|---|---|
| LOS | β (95% CI) | P Value |
| ICU | ||
| Census | −0.027 (−0.032 to −0.023) | <0.001 |
| Admissions | −0.84 (−0.87 to −0.80) | <0.001 |
| Acuity | 0.37 (0.31 to 0.42) | <0.001 |
| Postdischarge | ||
| Census | −0.01 (−0.013 to −0.003) | 0.001 |
| Admissions | −0.71 (−0.75 to −0.66) | <0.001 |
| Acuity | 0.036 (0.030 to 0.042) | <0.001 |
| 72-h ICU readmissions | OR (95% CI) | P Value |
| Census | 1.01 (0.98 to 1.04) | 0.43 |
| Admissions | 0.99 (0.96 to 1.01) | 0.27 |
| Acuity | 1.05 (1.01 to 1.08) | 0.008 |
| In-hospital death | OR (95% CI) | P Value |
| Census | 1.01 (0.98 to 1.03) | 0.66 |
| Admissions | 0.93 (0.92 to 0.95) | <0.001 |
| Acuity | 1.18 (1.15 to 1.22) | <0.001 |
| Hospital discharge destination | OR (95% CI) | P Value |
| Census | 1.01 (1.00 to 1.03) | 0.005 |
| Admissions | 1.05 (1.04 to 1.06) | <0.001 |
| Acuity | 0.89 (0.88 to 0.91) | <0.001 |
ICU = intensive care unit; LOS = length of stay; OR = odds ratio.
Linear and logistic regression used to evaluate the relationship between each strain variable and either continuous or dichotomous outcomes, respectively.
Appendix Table 3. Influence of ICU Capacity Strain on ICU LOS and Post–ICU Discharge Hospital LOS*.
| Capacity Strain Variable | β (95% CI) | P Value |
|---|---|---|
| ICU LOS | ||
| Census | −0.007 (−0.011 to −0.003) | <0.001 |
| Admissions | −0.226 (−0.260 to −0.192) | <0.001 |
| Acuity | −0.128 (−0.183 to −0.074) | <0.001 |
| Post–ICU discharge hospital LOS | ||
| Census | 0.008 (0.003 to 0.013) | 0.002 |
| Admissions | −0.172 (−0.217 to −0.127) | <0.001 |
| Acuity | 0.065 (−0.007 to 0.137) | 0.076 |
ICU = intensive care unit; LOS = length of stay.
Linear coefficients reported for the fully adjusted model.
Appendix Figure. Interaction between ICU admissions and acuity on predicted ICU LOS.
Each line represents the relationship between ICU admissions and predicted ICU LOS stratified by deciles of ICU acuity ranging from 6% (lowest decile, depicted by lightest gray line) to 29% (highest decile, depicted by black line) average predicted probability of death of other patients in the ICU. Results stem from fully adjusted models. ICU = intensive care unit; LOS = length of stay.
Appendix Table 4. Secondary Analysis*.
| Variable | Value | |
|---|---|---|
| Median regression of ICU capacity strain on predicted ICU LOS | β (95% CI) | P Value |
| Census | −0.01 (−0.02 to −0.01) | <0.001 |
| Admissions | −0.14 (−0.18 to −0.10) | <0.001 |
| Acuity | −0.09 (−0.15 to −0.03) | <0.001 |
| Influence of ICU capacity strain on 48-h ICU readmissions | OR (95% CI) | P Value |
| Census | 1.04 (1.01 to 1.07) | 0.014 |
| Admissions | 1.04 (1.01 to 1.07) | 0.013 |
| Acuity | 1.06 (1.01 to 1.11) | 0.021 |
| Influence of severity of illness predictors on ICU acuity | OR (95% CI) | P Value |
| Acuity | 1.05 (1.01 to 1.10) | 0.023 |
| Acuity + % mechanically ventilated | 1.05 (1.00 to 1.10) | 0.028 |
| Acuity + % vasoactive infusions | 1.06 (1.07 to 1.11) | 0.030 |
| Acuity + % mechanically ventilated + vasoactive infusions | 1.06 (1.00 to 1.11) | 0.041 |
| Influence of ICU capacity strain on ICU LOS in high-acuity ICUs† | β (95% CI) | P Value |
| Census | −0.015 (−0.02 to −0.01) | <0.001 |
| Admissions | −0.24 (−0.31 to −0.17) | <0.001 |
| Acuity | −0.19 (−0.29 to −0.10) | <0.001 |
| Influence of ICU capacity strain on 72-h ICU readmissions in high-acuity ICUs† | OR (95% CI) | P Value |
| Census | 1.07 (1.01 to 1.13) | 0.022 |
| Admissions | 1.07 (1.02 to 1.13) | 0.008 |
| Acuity | 1.00 (0.93 to 1.07) | 0.98 |
| Influence of ICU capacity strain on in-hospital death in high-acuity ICUs† | OR (95% CI) | P Value |
| Census | 1.02 (0.98 to 1.08) | 0.33 |
| Admissions | 0.95 (0.91 to 0.99) | 0.018 |
| Acuity | 0.97 (0.92 to 1.03) | 0.36 |
| Influence of ICU capacity strain on hospital discharge destination in high-acuity ICUs† | OR (95% CI) | P Value |
| Census | 0.99 (0.96 to 1.01) | 0.30 |
| Admissions | 1.02 (1.00 to 1.05) | 0.038 |
| Acuity | 0.97 (0.94 to 0.99) | 0.019 |
| Influence of ICU capacity strain on post–ICU discharge hospital LOS in high-acuity ICUs† | β (95% CI) | P Value |
| Census | 0.015 (0.005 to 0.025) | 0.003 |
| Admissions | −0.145 (−0.237 to −0.053) | 0.002 |
| Acuity | 0.066 (−0.051 to 0.184) | 0.27 |
| Influence of ICU capacity strain on ICU LOS in high-occupancy ICUs† | β (95% CI) | P Value |
| Census | −0.004 (−0.012 to 0.004) | 0.34 |
| Admissions | −0.206 (−0.291 to −0.121) | −0.001 |
| Acuity | −0.082 (−0.215 to 0.052) | 0.23 |
| Influence of ICU capacity strain on ICU LOS in patients with acute respiratory failure or receiving mechanical ventilation | β (95% CI) | P Value |
| Census | −0.005 (−0.014 to 0.003) | 0.22 |
| Admissions | −0.23 (−0.30 to −0.15) | <0.001 |
| Acuity | −0.12 (−0.24 to −0.004) | 0.041 |
| Influence of ICU capacity strain on 72-h ICU readmissions in patients with acute respiratory failure or receiving mechanical ventilation | OR (95% CI) | P Value |
| Census | 1.07 (1.01 to 1.14) | 0.023 |
| Admissions | 1.03 (0.97 to 1.09) | 0.39 |
| Acuity | 0.99 (0.91 to 1.08) | 0.88 |
| Influence of ICU capacity strain on in-hospital death in patients with acute respiratory failure or receiving mechanical ventilation | OR (95% CI) | P Value |
| Census | 1.02 (0.97 to 1.07) | 0.50 |
| Admissions | 0.96 (0.92 to 1.01) | 0.102 |
| Acuity | 0.98 (0.91 to 1.05) | 0.54 |
| Influence of ICU capacity strain on hospital discharge destination in patients with acute respiratory failure or receiving mechanical ventilation | OR (95% CI) | P Value |
| Census | 1.01 (0.98 to 1.04) | 0.60 |
| Admissions | 1.00 (0.98 to 1.03) | 0.95 |
| Acuity | 1.00 (0.96 to 1.04) | 0.92 |
| Influence of ICU capacity strain on post–ICU discharge hospital LOS in patients with acute respiratory failure or receiving mechanical ventilation | OR (95% CI) | P Value |
| Census | 0.004 (−0.007 to 0.014) | 0.51 |
| Admissions | −0.11 (−0.20 to −0.01) | 0.032 |
| Acuity | 0.03 (-0.12 to 0.19) | 0.65 |
| Influence of ICU capacity strain on death on the day of ICU discharge | OR (95% CI) | P Value |
| Census | 0.92 (0.90 to 0.94) | <0.001 |
| Admissions | 1.02 (1.00 to 1.04) | 0.053 |
| Acuity | 0.99 (0.97 to 1.02) | 0.63 |
| Influence of ICU capacity strain on ICU LOS in ICUs with <30% scheduled surgical admissions‡ | β (95% CI) | P Value |
| Census | −0.005 (−0.009 to −0.0004) | 0.032 |
| Admissions | −0.246 (−0.288 to −0.205) | <0.001 |
| Acuity | −0.142 (−0.205 to −0.079) | <0.001 |
| Influence of ICU capacity strain on 72-h ICU readmissions in ICUs with <30% scheduled surgical admissions‡ | OR (95% CI) | P Value |
| Census | 1.05 (1.01 to 1.08) | 0.008 |
| Admissions | 1.04 (1.01 to 1.07) | 0.020 |
| Acuity | 1.04 (0.99 to 1.09) | 0.096 |
| Influence of ICU capacity strain on subsequent in-hospital death in ICUs with <30% scheduled surgical admissions‡ | OR (95% CI) | P Value |
| Census | 1.02 (0.99 to 1.05) | 0.13 |
| Admissions | 0.97 (0.94 to 0.99) | 0.035 |
| Acuity | 0.99 (0.95 to 1.04) | 0.76 |
| Influence of ICU capacity strain on hospital discharge destination in ICUs with <30% scheduled surgical admissions‡ | OR (95% CI) | P Value |
| Census | 0.99 (0.98 to 1.01) | 0.41 |
| Admissions | 1.01 (0.99 to 1.03) | 0.081 |
| Acuity | 0.99 (0.97 to 1.04) | 0.56 |
| Influence of ICU capacity strain on post–ICU discharge hospital LOS in ICUs with <30% scheduled surgical admissions‡ | β (95% CI) | P Value |
| Census | 2.11 (0.73 to 3.48) | 0.003 |
| Admissions | −25.9 (−38.7 to −13.0) | <0.001 |
| Acuity | 2.58 (−17.0 to 22.1) | 0.80 |
| Influence of ICU capacity strain on discharged patients' ICU LOS, including those admitted to ICUs with <20 patients per quarter year and to ICUs with <4 patients contributing MPM0-III score | β (95% CI) | P Value |
| Census | −0.007 (−0.011 to −0.004) | <0.001 |
| Admissions | −0.241 (−0.274 to −0.209) | <0.001 |
| Acuity | −0.084 (−0.132 to −0.036) | 0.001 |
| Influence of ICU capacity strain on death among patients discharged to a floor bed, step-down unit, or another ICU in the same hospital | OR (95% CI) | P Value |
| Census | 1.02 (1.00 to 1.05) | 0.082 |
| Admissions | 0.98 (0.94 to 0.99) | 0.013 |
| Acuity | 1.00 (0.96 to 1.04) | 0.95 |
| Influence of capacity strain on death among patients discharged to a floor bed, step-down unit, or another ICU using a bootstrap resampling analysis§ | OR (95% CI) | |
| Census | 1.02 (0.95 to 1.05) | |
| Admissions | 0.98 (0.95 to 1.00) | |
| Acuity | 1.00 (0.96 to 1.04) | |
ICU = intensive care unit; LOS = length of stay; MPM0-III = Mortality Probability Model III; OR = odds ratio.
All analyses done with the fully adjusted model.
A high-acuity ICU is defined as the upper quartile for median yearly acuity (median predicted probability of death ≥17.5%). A high-occupancy ICU is defined as the upper quartile of median ICU census by the number of operational ICU beds.
ICUs with <30% scheduled surgical admissions account for 24% of ICUs.
Bootstrap resampling analyses were done on all patients who died after their index ICU discharge to a floor bed, step-down unit, or another ICU in the same hospital. To account for expected death among patients transferred to another ICU, we took a 24% random sample of patients discharged to another hospital's ICU and coded them as deceased. This percentage was chosen because it represented the mortality rate for patients transferred to Project IMPACT ICUs from other hospitals. We then evaluated the relationship of strain and death using our fully adjusted model. We repeated this process 100 times to derive a mean point estimate and bootstrap CI.
Appendix Table 5. ICU Characteristics as Effect Modifiers on the Relationship Between ICU Capacity Strain and ICU LOS*.
| ICU Strain Variable | Closed Staffing Model β (95% CI) | Open Staffing Model β (95% CI) | Interaction P Value |
|---|---|---|---|
| Model | |||
| Census | −0.004 (−0.019 to 0.010) | −0.007 (−0.011 to −0.004) | 0.56 |
| Admissions | −0.328 (−0.490 to −0.166) | −0.220 (−0.255 to −0.185) | 0.115 |
| Acuity | −0.256 (−0.469 to −0.043) | −0.114 (−0.171 to −0.058) | 0.189 |
| Type | Academic ICU β (95% CI) | Community ICU β (95% CI) | Interaction P Value |
| Census | −0.000 (−0.008 to 0.008) | −0.009 (−0.013 to −0.005) | 0.062 |
| Admissions | −0.228 (−0.308 to −0.148) | −0.224 (−0.262 to −0.186) | 0.88 |
| Acuity | −0.263 (−0.401 to −0.125) | −0.099 (−0.158 to −0.040) | 0.020 |
| Critical care fellowship program | Critical Care Fellowship β (95% CI) | No Critical Care Fellowship β (95% CI) | Interaction P Value |
| Census | −0.002 (−0.008 to 0.004) | −0.010 (−0.015 to −0.006) | 0.073 |
| Admissions | −0.247 (−0.310 to −0.185) | −0.212 (−0.253 to 0.171) | 0.78 |
| Acuity | −0.232 (−0.333 to −0.131) | −0.082 (−0.146 to −0.018) | 0.024 |
ICU = intensive care unit; LOS = length of stay.
Linear coefficients were derived from fully adjusted model.
Appendix Table 6. ICU Survivor Discharge Destinations.
| Location | Patients, n (%) |
|---|---|
| Hospital floor or step-down unit | 200 730 (86.2) |
| Home | 14 794 (6.4) |
| Another ICU | 5243 (2.3) |
| Another hospital (non-ICU) | 2731 (1.2) |
| SNF or rehabilitation facility | 2109 (1.0) |
| Hospice | 194 (0.1) |
| Psychiatric facility | 3569 (1.5) |
| Other | 3592 (1.5) |
ICU = intensive care unit; SNF = skilled nursing facility.
Footnotes
Potential Conflicts of Interest: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M13-0377.
Reproducible Research Statement: Study protocol and statistical code: Available from Dr. Wagner (email, jason.wagner@uphs.upenn.edu). Data set: Available with permission from Cerner Corporation (2800 Rockcreek Parkway, Kansas City, MO 64117).
Current author addresses and author contributions are available at www.annals.org.
Author Contributions: Conception and design: J. Wagner, S.E.S. Brown, B.L. Strom, S.D. Halpern.
Analysis and interpretation of data: J. Wagner, N.B. Gabler, S.E.S. Brown, S.D. Halpern.
Drafting of the article: J. Wagner, N.B. Gabler.
Critical revision of the article for important intellectual content: J. Wagner, S.E.S. Brown, B.L. Strom, S.D. Halpern.
Final approval of the article: J. Wagner, N.B. Gabler, S.J. Ratcliffe, S.E.S. Brown, B.L. Strom, S.D. Halpern.
Provision of study materials or patients: S.D. Halpern.
Statistical expertise: N.B. Gabler, S.J. Ratcliffe, S.E.S. Brown.
Obtaining of funding: S.D. Halpern
Administrative, technical, or logistic support: N.B. Gabler, S.E.S. Brown, S.D. Halpern.
Collection and assembly of data: N.B. Gabler.
References
- 1.Kelley MA. Critical care workforce crisis: time to look in the mirror. Crit Care Med. 2008;36:1385–6. doi: 10.1097/CCM.0b013e31816a0bd3. Editorial. [DOI] [PubMed] [Google Scholar]
- 2.Halpern NA, Pastores SM, Greenstein RJ. Critical care medicine in the United States 1985-2000: an analysis of bed numbers, use, and costs. Crit Care Med. 2004;32:1254–9. doi: 10.1097/01.ccm.0000128577.31689.4c. [DOI] [PubMed] [Google Scholar]
- 3.Pronovost PJ, Angus DC, Dorman T, Robinson KA, Dremsizov TT, Young TL. Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review. JAMA. 2002;288:2151–62. doi: 10.1001/jama.288.17.2151. [DOI] [PubMed] [Google Scholar]
- 4.Angus DC, Kelley MA, Schmitz RJ, White A, Popovich J, Jr Committee on Manpower for Pulmonary and Critical Care Societies (COMPACCS) Caring for the critically ill patient. Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: can we meet the requirements of an aging population? JAMA. 2000;284:2762–70. doi: 10.1001/jama.284.21.2762. [DOI] [PubMed] [Google Scholar]
- 5.Kelley MA, Angus D, Chalfin DB, Crandall ED, Ingbar D, Johanson W, et al. The critical care crisis in the United States: a report from the profession. Chest. 2004;125:1514–7. doi: 10.1378/chest.125.4.1514. [DOI] [PubMed] [Google Scholar]
- 6.Cooper RA. Weighing the evidence for expanding physician supply. Ann Intern Med. 2004;141:705–14. doi: 10.7326/0003-4819-141-9-200411020-00012. [DOI] [PubMed] [Google Scholar]
- 7.Angus DC, Shorr AF, White A, Dremsizov TT, Schmitz RJ, Kelley MA Committee on Manpower for Pulmonary and Critical Care Societies (COMPACCS) Critical care delivery in the United States: distribution of services and compliance with Leapfrog recommendations. Crit Care Med. 2006;34:1016–24. doi: 10.1097/01.CCM.0000206105.05626.15. [DOI] [PubMed] [Google Scholar]
- 8.Howell MD. Managing ICU throughput and understanding ICU census. Curr Opin Crit Care. 2011;17:626–33. doi: 10.1097/MCC.0b013e32834b3e6e. [DOI] [PubMed] [Google Scholar]
- 9.Halpern SD. ICU capacity strain and the quality and allocation of critical care. Curr Opin Crit Care. 2011;17:648–57. doi: 10.1097/MCC.0b013e32834c7a53. [DOI] [PubMed] [Google Scholar]
- 10.Baker DR, Pronovost PJ, Morlock LL, Geocadin RG, Holzmueller CG. Patient flow variability and unplanned readmissions to an intensive care unit. Crit Care Med. 2009;37:2882–7. doi: 10.1097/ccm.0b013e3181b01caf. [DOI] [PubMed] [Google Scholar]
- 11.Chrusch CA, Olafson KP, McMillan PM, Roberts DE, Gray PR. High occupancy increases the risk of early death or readmission after transfer from intensive care. Crit Care Med. 2009;37:2753–8. doi: 10.1097/CCM.0b013e3181a57b0c. [DOI] [PubMed] [Google Scholar]
- 12.Strauss MJ, LoGerfo JP, Yeltatzie JA, Temkin N, Hudson LD. Rationing of intensive care unit services. An everyday occurrence. JAMA. 1986;255:1143–6. [PubMed] [Google Scholar]
- 13.Singer DE, Carr PL, Mulley AG, Thibault GE. Rationing intensive care—physician responses to a resource shortage. N Engl J Med. 1983;309:1155–60. doi: 10.1056/NEJM198311103091905. [DOI] [PubMed] [Google Scholar]
- 14.Walther SM, Jonasson U. A prospective cohort study of 6-month mortality in a community hospital experiencing a gradual reduction in critical care services. Intensive Care Med. 2001;27:700–5. doi: 10.1007/s001340100899. [DOI] [PubMed] [Google Scholar]
- 15.Brody H. From an ethics of rationing to an ethics of waste avoidance. N Engl J Med. 2012;366:1949–51. doi: 10.1056/NEJMp1203365. [DOI] [PubMed] [Google Scholar]
- 16.Brown SE, Ratcliffe SJ, Kahn JM, Halpern SD. The epidemiology of intensive care unit readmissions in the United States. Am J Respir Crit Care Med. 2012;185:955–64. doi: 10.1164/rccm.201109-1720OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy MM, Rapoport J, Lemeshow S, Chalfin DB, Phillips G, Danis M. Association between critical care physician management and patient mortality in the intensive care unit. Ann Intern Med. 2008;148:801–9. doi: 10.7326/0003-4819-148-11-200806030-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chalfin DB, Trzeciak S, Likourezos A, Baumann BM, Dellinger RP DELAY-ED study group. Impact of delayed transfer of critically ill patients from the emergency department to the intensive care unit. Crit Care Med. 2007;35:1477–83. doi: 10.1097/01.CCM.0000266585.74905.5A. [DOI] [PubMed] [Google Scholar]
- 19.Glance LG, Li Y, Osler TM, Dick A, Mukamel DB. Impact of patient volume on the mortality rate of adult intensive care unit patients. Crit Care Med. 2006;34:1925–34. doi: 10.1097/01.CCM.0000226415.93237.84. [DOI] [PubMed] [Google Scholar]
- 20.Higgins TL, Teres D, Copes WS, Nathanson BH, Stark M, Kramer AA. Assessing contemporary intensive care unit outcome: an updated Mortality Probability Admission Model (MPM0-III) Crit Care Med. 2007;35:827–35. doi: 10.1097/01.CCM.0000257337.63529.9F. [DOI] [PubMed] [Google Scholar]
- 21.Vranas K, Ratcliffe SJ, Harhay MO, Cooney E, Halpern SD, Prasad M. A prospective study of the determinants of intensive care unit (ICU) capacity strain. Am J Respir Crit Care Med. 2012;185:A1608. [Google Scholar]
- 22.Kramer AA, Higgins TL, Zimmerman JE. Intensive care unit readmissions in U.S. hospitals: patient characteristics, risk factors, and outcomes. Crit Care Med. 2012;40:3–10. doi: 10.1097/CCM.0b013e31822d751e. [DOI] [PubMed] [Google Scholar]
- 23.Cleveland WS. Robust locally weighted regression and smoothing scatter-plots. J Am Stat Assoc. 1979;74:829–36. [Google Scholar]
- 24.Localio AR, Berlin JA, Ten Have TR, Kimmel SE. Adjustments for center in multicenter studies: an overview. Ann Intern Med. 2001;135:112–23. doi: 10.7326/0003-4819-135-2-200107170-00012. [DOI] [PubMed] [Google Scholar]
- 25.Rhodes A, Moreno RP, Azoulay E, Capuzzo M, Chiche JD, Eddleston J, et al. Task Force on Safety and Quality of European Society of Intensive Care Medicine (ESICM) Prospectively defined indicators to improve the safety and quality of care for critically ill patients: a report from the Task Force on Safety and Quality of the European Society of Intensive Care Medicine (ESICM) Intensive Care Med. 2012;38:598–605. doi: 10.1007/s00134-011-2462-3. [DOI] [PubMed] [Google Scholar]
- 26.Truog RD, Brock DW, Cook DJ, Danis M, Luce JM, Rubenfeld GD, et al. Task Force on Values, Ethics, and Rationing in Critical Care (VERICC) Rationing in the intensive care unit. Crit Care Med. 2006;34:958–63. doi: 10.1097/01.CCM.0000206116.10417.D9. [DOI] [PubMed] [Google Scholar]
- 27.Young MJ, Brown SE, Truog RD, Halpern SD. Rationing in the intensive care unit: to disclose or disguise? Crit Care Med. 2012;40:261–6. doi: 10.1097/CCM.0b013e31822d750d. [DOI] [PubMed] [Google Scholar]
- 28.Ward NS, Teno JM, Curtis JR, Rubenfeld GD, Levy MM. Perceptions of cost constraints, resource limitations, and rationing in United States intensive care units: results of a national survey. Crit Care Med. 2008;36:471–6. doi: 10.1097/CCM.0B013E3181629511. [DOI] [PubMed] [Google Scholar]
- 29.Wunsch H. Is there a Starling curve for intensive care? Chest. 2012;141:1393–9. doi: 10.1378/chest.11-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wunsch H, Linde-Zwirble WT, Harrison DA, Barnato AE, Rowan KM, Angus DC. Use of intensive care services during terminal hospitalizations in England and the United States. Am J Respir Crit Care Med. 2009;180:875–80. doi: 10.1164/rccm.200902-0201OC. [DOI] [PubMed] [Google Scholar]
- 31.Zimmerman JE, Kramer AA. A model for identifying patients who may not need intensive care unit admission. J Crit Care. 2010;25:205–13. doi: 10.1016/j.jcrc.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Gershengorn HB, Iwashyna TJ, Cooke CR, Scales DC, Kahn JM, Wunsch H. Variation in use of intensive care for adults with diabetic ketoacidosis*. Crit Care Med. 2012;40:2009–15. doi: 10.1097/CCM.0b013e31824e9eae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halpern NA, Pastores SM. Critical care medicine in the United States 2000-2005: an analysis of bed numbers, occupancy rates, payer mix, and costs. Crit Care Med. 2010;38:65–71. doi: 10.1097/CCM.0b013e3181b090d0. [DOI] [PubMed] [Google Scholar]
- 34.Seymour CW, Iwashyna TJ, Ehlenbach WJ, Wunsch H, Cooke CR. Hospital-level variation in the use of intensive care. Health Serv Res. 2012;47:2060–80. doi: 10.1111/j.1475-6773.2012.01402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts RR, Frutos PW, Ciavarella GG, Gussow LM, Mensah EK, Kampe LM, et al. Distribution of variable vs. fixed costs of hospital care. JAMA. 1999;281:644–9. doi: 10.1001/jama.281.7.644. [DOI] [PubMed] [Google Scholar]
- 36.Luce JM, Rubenfeld GD. Can health care costs be reduced by limiting intensive care at the end of life? Am J Respir Crit Care Med. 2002;165:750–4. doi: 10.1164/ajrccm.165.6.2109045. [DOI] [PubMed] [Google Scholar]
- 37.Kahn JM. Understanding economic outcomes in critical care. Curr Opin Crit Care. 2006;12:399–404. doi: 10.1097/01.ccx.0000244117.08753.38. [DOI] [PubMed] [Google Scholar]
- 38.Seymour CW, Kahn JM. Addressing the growth in intensive care: comment on “Intensive care unit admitting patterns in the Veterans Affairs health care system”. Arch Intern Med. 2012;172:1226. doi: 10.1001/archinternmed.2012.3773. [DOI] [PubMed] [Google Scholar]
- 39.Cooper GS, Sirio CA, Rotondi AJ, Shepardson LB, Rosenthal GE. Are readmissions to the intensive care unit a useful measure of hospital performance? Med Care. 1999;37:399–408. doi: 10.1097/00005650-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Kramer AA, Higgins TL, Zimmerman JE. The association between ICU readmission rate and patient outcomes. Crit Care Med. 2013;41:24–33. doi: 10.1097/CCM.0b013e3182657b8a. [DOI] [PubMed] [Google Scholar]
- 41.Angus DC. Grappling with intensive care unit quality—does the readmission rate tell us anything? Crit Care Med. 1998;26:1779–80. doi: 10.1097/00003246-199811000-00008. Editorial. [DOI] [PubMed] [Google Scholar]