Abstract
B-cell antigen receptor (BCR) signalling and its regulation through negative and positive regulators are critical for balancing B-cell response and function. Human Fc receptor like-2 (FCRL2), a member of the newly identified FCRL family, could influence B-cell signalling due to possession of both immunoreceptor tyrosine-based activation and inhibitory motifs (ITAM and ITIM). Since the natural ligand of FCRL2 has not been identified, we generated FCRL2-specific monoclonal antibodies (mAbs) and employed them to investigate the influence of FCRL2 stimulation on BCR signalling in an FCRL2-expressing B-cell line. Two anti-FCRL2 mAb-producing hybridoma clones (5A7-E7 and 3D8-G8) were selected. None of the mAbs displayed any cross-reactivity with the other members of the FCRL family including recombinant FCRL1, -3, -4 and -5, as tested by FACS and ELISA techniques. Engagement of the FCRL2 by these mAbs resulted in significant inhibition of BCR signalling mediators such as calcium mobilization and phosphorylation of the mitogen-activated protein kinases Erk, p38 and Jnk. These findings indicate that the FCRL2 ITIM motifs are functional and the anti-FCRL2 mAbs may mimic the natural ligand of FCRL2 by induction of inhibitory signals in B cells.
Keywords: B-cell receptor signalling, Fc receptor like-2, monoclonal antibody
Introduction
The signals transmitted through the B-cell antigen receptor (BCR) are central to B-cell development and function.1,2 Defective BCR signalling can result not only in impaired B-cell development and immunodeficiencies but also in a predisposition to autoimmunity.1 The BCR consists of the four chains of the membrane-bound immunoglobulin in association with a single chain of each of the accessory molecules Igα and Igβ. Both accessory molecules contain immunoreceptor tyrosine-based activation motifs (ITAMs) that are essential to transmembrane signalling pathways.3 When a B-cell encounters antigen, BCR-associated tyrosine kinases such as Lyn, Fyn and Lck phosphorylate tyrosine residues in the ITAMs. Phosphorylated ITAMs then interact with protein tyrosine kinases, such as Syk and Btk, which further phosphorylate the ITAMs, as well as facilitate the initiation of several different signalling pathways such as the phospholipase Cγ2 and phosphoinositide 3-kinase pathways, which are the two main BCR-mediated signal cascades.3,4 The outcome of the signalling cascade triggered by the BCR is regulation of transcription factor activation and gene expression.2 The regulation of a BCR-mediated response is achieved through balancing the activating and inhibitory signals. Therefore, agents that alter the strength of the BCR signalling could potentially alter the B-cell repertoire which may lead to autoimmunity.3 Beside classical BCR-negative (FcγRIIb, CD22, CD5 and CD72) and positive (CD19, CD21, CD45, CD38, CD81 and CD86) regulators,3,5–7 Fc receptor-like (FCRL) molecules have also been proposed as new potential important regulators of BCR signalling.
In humans, the FCRL family comprises eight members, which include transmembrane glycoproteins (FCRL1–6) and cytoplasmic proteins (FCRLA and FCRLB) whose genes are located in a region within chromosome 1q21 in the midst of Fc receptor genes.8 Based on the presence of ITAMs and/or immunoreceptor tyrosine-based inhibitory motifs (ITIMs) in their cytoplasmic tails, FCRLs have been initially proposed as immunomodulators,9–12 and indeed both activating and inhibitory effects on the BCR signalling have been reported for FCRL1 and FCRL2–5, respectively.5–7,13–15 FCRL2 (CD307b), the target of the current study, has four extracellular immunoglobulin domains, and one ITAM-like and two ITIMs in the cytoplasmic portion.10 Despite possessing the D2 and D3 immunoglobulin domains, which are used for Fc binding by FcγRI, FCRL2 has not been formally shown to have the ability to bind immunoglobulins.8,16 Its expression profile is unique to memory B cells of normal peripheral blood.6,17,18 Moreover, leukaemic B cells of chronic lymphocytic leukaemia have been shown to over-express the FCRL2 protein, which has been introduced as a robust prognostic marker in these patients.17–20 Although the role of FCRL2 in chronic lymphocytic leukaemia is not clear, its over-expression might result in down-regulation of activation and proliferation of leukaemic cells, leading to immunodeficiency and altered immune regulation, which are frequently observed in chronic lymphocytic leukaemia.20 These reports strongly support the importance of FCRL2 in normal and malignant B-cell biology. In the only eligible study on the potential influence of FCRL2 on BCR signalling, Jackson et al.6 recently provided evidence that the FCRL2 has profound inhibitory effects on BCR signalling through recruitment and activation of the Src homology region 2 domain-containing phosphatase-1 (SHP-1). They used a chimeric FCRL2/FcγRIIB protein to determine the functional role of the ITAM-like and ITIM sequences of FCRL2.6 They showed that co-engagement of the chimeric FCRL2 receptor and BCR effectively inhibited calcium mobilization and both p38 and Erk mitogen-activated protein kinase (MAPK) activation.6 The applied model of co-ligating chimeric FcγRIIB/FCRL2 and the BCR does not resemble the physiological conditions, because FCRL2 molecules may not be recruited to the immune synapse and co-ligate with the BCR. Considering that the FCRL2 ligand is still unknown and that the potential influence of FCRL2 engagement on the BCR signalling both in normal and pathological conditions is only partially addressed, we generated anti-FCRL2 monoclonal antibodies (mAbs) to test the hypothesis that FCRL2 signalling can be triggered through binding of specific mAbs to the FCRL2 protein, which could mimic ligand–receptor binding. The influence of FCRL2 stimulation on the BCR signalling was assessed in the CA46 Burkitt's lymphoma (BL) cell line using these mAbs.
Materials and methods
Antibodies
Phosphospecific antibodies recognizing the active forms of Erk1/2 (Thr 202/Tyr204), p38 (Thr180/Tyr182), SAPK/Jnk (Thr183/Tyr185), IKKa (Ser180)/IKKb (Ser181), Ikβα (Ser32), Akt (Thr308) and Syk (Tyr525/526) were obtained from Cell Signaling Technology (Beverly, MA). Antibodies specific to phosphotyrosine (clone 4G10) and phospho-Vav1 (Tyr160) were purchased from Millipore (Billerica, MA) and BioSource (Nivelles, Belgium), respectively. Mouse anti-actin mAb (clone C4) was purchased from Millipore. Goat anti-human IgM Fcμ fragment (cat. no. 109-001-043) and donkey anti-goat immunoglobulin (H+L) antibodies (cat. no. 705-005-003) were obtained from Jackson ImmunoResearch (West Grove, PA) and goat IgG fraction to mouse immunoglobulin (cat. no. 55467) was purchased from MP Biomedicals (Solon, OH) and mouse anti-FcγRIIB mAb was obtained from Abcam (Cambridge, MA). Commercial mAb to FCRL2 (clone 7G7, mouse IgG1) was a generous gift from Prof. Polson (Genentech, South San Francisco, CA).
In flow cytometry experiments with unconjugated mAbs, the FITC-conjugated sheep anti-mouse immunoglobulin (Avicenna Research Institute, Tehran, Iran) and for biotinylated antibodies, streptavidin-phycoerythrin (DAKO, Glostrup, Denmark) were used as detectors. Horseradish peroxidase-conjugated donkey anti-rabbit IgG (GE Healthcare, Buckinghamshire, UK) and goat anti-mouse immunoglobulin (Sigma-Aldrich, St Louis, MO) were used for immunoblots.
Cell lines
CA46 and DG75 BL cell lines and the Chinese hamster ovary (CHO) cell line (National Cell Bank of Iran, Tehran, Iran) were cultured in RPMI-1640 (Gibco, Big Cabin, OK) culture medium supplemented with 10% fetal bovine serum (CM10) (Biochrom AG, Berlin, Germany), penicillin (100 U/ml) (ICN Biomedicals, Aurora, OH) and streptomycin (100 μg/ml) (Sigma). CA46 and DG75 cell lines express IgM on their surface but do not express the FcγRIIB.21,22 All cell lines were cultured at 37° in a humidified incubator supplied with 5% CO2 atmosphere.
FCRL plasmid construct and transfection
The FCRL1-pCMV6-XL5 (SC123097, NM_052938, 2657 bp), FCRL2-pCMV6-XL5 (TC305281, NM_030764, 1600 bp) and FCRL4-pCMV6-XL5 (SC305326, NM_031282, 1600 bp) DNA ready for transfection were obtained from OriGene (Rockville, MD). Full-length FCRL1, -2 and -4 cDNAs were subcloned into pCMV6-Neo mammalian expression vector (OriGene) using NotI restriction enzyme (Invitrogen, Carlsbad, CA). The new constructs were designated as FCRL-pCMV6-Neo. The CHO cell line was transfected with FCRL-pCMV6-Neo or pCMV6-Neo mock vectors using JetPEI transfection kit (Polyplus, Illkirch, France) as described elsewhere23 and cultured in CM10 medium containing 600 μg/ml of neomycin (Roche, Mannheim, Germany). The FCRL-expressing clones were screened by flow cytometry using commercial polyclonal goat anti-human FCRL proteins (R&D Systems, Minneapolis, MN) and the clones with the highest expression of recombinant FCRL proteins were used in the experiments.
Cloning and expression of FCRL2 protein in a prokaryotic system
The extracellular region of FCRL2 protein was produced in Escherichia coli, as described in detail elsewhere.24 Briefly, the FCRL2-pCMV6-Neo construct was applied as a template for amplification of the extracellular region of FCRL2 by PCR using FCRL2-specific primers (NcoI-FCRL2 sense: CATGGATATGCTGCTGTGGTCATTGCTGGTCAT; NotI-FCRL2 antisense: GCGGCCGCTCCAGCTGTCATGAGGTCTCTTCTA). The PCR product was purified and cloned into the pET28b(+) expression vector (Novagen, Madison, WI) and introduced into BL21-DE3 E. coli strain (Novagen). Expression of the recombinant protein was induced by 1 mm isopropyl-1-thio-β-d-galactoside (Sigma). Cultured bacteria were centrifuged and the pellet was lysed completely by sonication. The lysate was then centrifuged and the inclusion bodies in the pellet were dissolved in buffer A (NaH2Po4 100 mm, Tris-base 10 mm, urea 8 m, NaCl 100 mm, pH8) containing 30 mm imidazole and centrifuged at 18 500 g for 10 min. The filtrate was subsequently applied to buffer A equilibrated Ni–NTA agarose resin (Qiagen, Hilden, Germany). After coupling the FCRL2-His-tag recombinant protein to Ni–NTA resin, the unbound fraction was washed with buffer A supplemented with 30 mm imidazole. Bound proteins were first renatured by a continuous descending (8–0·01 m) gradient of urea and finally eluted by gradual ascending (80, 300 and 1000 mm) concentrations of imidazole in buffer A. The eluted recombinant proteins were immediately dialysed against PBS. Protein purity was determined by SDS–PAGE and immunoblotting techniques.
Production of anti-FCRL2 mAbs
BALB/c mice were intraperitoneally immunized with recombinant FCRL2 protein (5 μg mixed with complete Freund's adjuvant) (R&D Systems). Four weeks after the first immunization, booster injections were performed four times with an interval of 2 weeks (3 μg mixed with incomplete Freund's adjuvant, intraperitoneally). Two days before the cell fusion, 6 μg of recombinant FCRL2 protein (without any adjuvant) was injected intravenously. Spleen cells of hyperimmunized mice were fused with SP2/0, a mouse myeloma cell line, using polyethylene glycol.25 Antibody-producing hybridoma cells were screened with the immunizing protein by ELISA and subsequently cloned by limiting dilution. Positive clones were further screened, using the FCRL2 protein expressing CHO stable transfectant, by flow cytometry. The reactivity of mAbs with FCRL2 protein was also checked by immunoblotting. Furthermore, the cross-reactivities of the produced mAbs with the other members of the FCRL family were checked using either stable CHO cell lines expressing FCRL proteins or recombinant FCRL proteins by flow cytometry and ELISA. The recombinant FCRL proteins employed in ELISA were either expressed in eukaryotic (FCRL1, -3 and -5, R&D Systems) or prokaryotic (FCRL2, produced in our laboratory) systems. Reactivities of FCRL1, -3 and -5 proteins were checked by ELISA using either polyclonal FCRL-specific antibodies produced in our laboratory or prepared commercially (R&D Systems). No recombinant FCRL4 protein was available for this study. The isotypes of established mAbs were determined using a mouse mAb isotyping kit (Zymed, San Francisco, CA) and the results were confirmed by an indirect capture ELISA using isotype-specific mAbs to IgG1 and IgG2a heavy chains (Sigma).
ELISA
Cross-reactivities of the established mAbs were determined by ELISA. MaxiSorp 96-well plates (Nunc, Wiesbaden, Germany) were coated with 0·1 μg/well of eukaryotic recombinant FCRL1, -3 and -5 proteins (R&D Systems) and 1 μg/well of prokaryotic recombinant FCRL2 (produced in our laboratory) for 1·5 hr at 37° in 100 μl PBS. The wells were then blocked with 200 μl PBS-Tween (PBST) supplemented with 1% BSA (v/v) as blocking buffer. After washing with PBST, 100 μl of anti-FCRL2 mAbs (10 μg/ml) were added to the wells and incubated for 1 hr at 37°. The wells were then washed three times with PBST followed by adding 100 μl of horseradish peroxidase-conjugated sheep anti-mouse immunoglobulin (Avicenna Research Institute) and incubation for 45 min at 37°. In parallel, commercial and home-made biotin-conjugated anti-FCRL polyclonal antibodies (pAbs) were included as positive controls as follows. Reactivities of recombinant FCRL1 and -5 proteins were confirmed by commercial biotinylated goat anti-human FCRL pAbs (R&D Systems) and reactivity of recombinant FCRL3 was approved by biotinylated rabbit anti-FCRL pAb produced in our laboratory (data not shown). For detection of these pAbs, horseradish peroxidase-streptavidin (DAKO) was applied diluted 1 : 5000 in PBST. After washing the wells with PBST, 3,3',5,5'-Tetramethylbenzidine substrate (Invitrogen) was added and the reaction was stopped with 20% H2SO4. The colorimetric analysis of the reactions was performed at 450 nm in a microplate reader (Bio-Rad, Berkeley, CA).
Flow cytometry
Cell lines were washed with PBS containing 1% BSA and then incubated with primary antibodies for 45 min in the dark at 4°. In these experiments purified mAbs to FCRL2 and commercial mAb to FCRL2 were used at 10 μg/ml and biotinylated goat anti-human FCRL pAbs were applied at 2·5 μg/ml. Mouse anti-FcγRIIB mAb was employed at 20 μg/ml for indirect staining. Unconjugated and biotin-conjugated antibodies were detected with FITC-conjugated sheep anti-mouse immunoglobulin (Avicenna Research Institute) and Streptavidin-phycoerythrin (DAKO), respectively as secondary conjugates according to the manufacturer's instructions. The anti-ENV11 mAb (IgG1/κ) specific to HIV envelope protein (Avicenna Research Institute) and biotinylated sheep immunoglobulin were used to eliminate non-specific binding of the primary antibodies. Stained cells were subsequently analysed by flow cytometry (FACScalibur, Becton- Dickinson, San Jose, CA). Forward scatter and side scatter signals were recorded in the linear mode and fluorescence signals in the logarithmic mode. Dead cells and debris were gated out using scatter properties of the cells. Data were analysed using CellQuest Pro (BD Biosciences) and FlowJo (Tree Star Inc., Ashland, OR) analysis software programs.
Calcium mobilization
Calcium influx was measured using Fluo-4FF (Molecular Probes Europe BV, Leiden, the Netherlands) as described previously.26 Briefly, cells (200 × 103/sample) were suspended in 200 μl RPMI-1640 without phenol red (Invitrogen) supplemented with 25 mm HEPES (pH 7·4), 5 μm Fluo-4FF and 500 μm sulfinpyrazone (Sigma-Aldrich). Cells were incubated for 45 min at 37°, pelleted by centrifugation, and resuspended in 500 μl of the same medium without Fluo-4FF. Baseline fluorescence was recorded for 30 seconds at 37°. Cells were subsequently incubated with a cocktail of 2·5–10 μg/ml of anti-IgM antibody (goat anti-human IgM Fcμ fragment pAb) and/or 15 μg/ml mAbs to FCRL2 as well as secondary antibodies [donkey anti-goat Ig antibody (5·0 μg/ml) and goat IgG fraction to mouse immunoglobulin (10 μg/ml)]. Anti-Env11 mAb was applied as isotype-matched negative control. Fluorescent light emission was recorded continuously for 180–320 seconds at 37° by flow cytometry.
Western blot
FCRL2 signalling was studied in CA46 (FCRL2-positive) and DG75 (negative for FCRL molecules) BL cell lines. The cells were stimulated under different conditions using different concentrations of anti-IgM antibody (goat anti-human IgM Fcμ fragment pAb) and mAbs to FCRL2. For detection of FCRL-mediated signalling on the BCR pathway, saturating amounts of anti-IgM antibody and mAbs to FCRL2 were added simultaneously or separately and then appropriate concentrations of secondary antibodies [(donkey anti-goat immunoglobulin (H+L) antibody (5·0 μg/ml) and goat IgG fraction to mouse immunoglobulin (10 μg/ml)] were immediately added to the mixture. Subsequently, cells (1 × 106 to 3 × 106/sample) were lysed in 1% (v/v) Triton X-100 in 20 mm Tris–HCl (pH 8), 150 mm NaCl (in the presence of 0·2 mg/ml sodium orthovanadate, 1 μg/ml pepstatin, leupeptin, aprotinin, and 10 mm PMSF). Protein concentration was calculated using the BCA protein assay kit (Pierce, Rockford, IL) and cell lysates were resolved by SDS–PAGE and transferred onto nitrocellulose membranes. Immunoblots were performed using peroxidase-labelled secondary antibodies (GE healthcare) and a chemiluminescence detection kit (Pierce).
Statistical analysis
Statistical analyses of the results were performed using Student's t-test using the SPSS statistical package version 13.0 (SPSS, Chicago, IL). P-values of < 0·05 were considered significant.
Results
Generation and characterization of monoclonal anti-FCRL2 antibodies
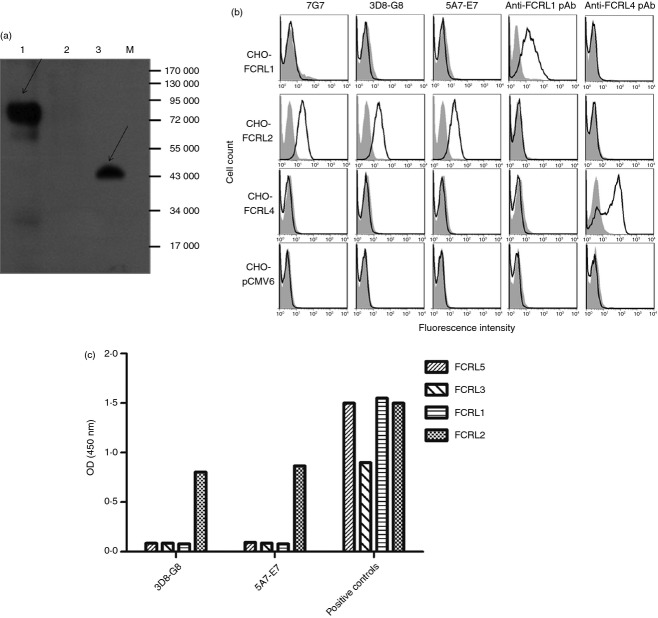
Two hybridoma clones (5A7-E7 and 3D8-G8) were selected from a number of FCRL2-specific hybridomas based on their isotype (IgG1), reactivity with native FCRL2 by flow cytometry and ELISA and lack of cross-reactivity with the other members of the FCRL family. WB analysis revealed that both mAbs detected a 75 000 molecular weight (MW) molecule corresponding to full-length FCRL2 expressed in the FCRL2-transfected CHO cell line and a 45 000 MW recombinant protein representing the extracellular region of FCRL2 expressed in a prokaryotic system (produced in our laboratory). Representative results obtained for mAb 3D8-G8 are illustrated in Fig.1(a). Cross-reactivity of the two mAbs was assessed by flow cytometry and ELISA using FCRL1–5 proteins, expressed either as eukaryotic recombinant proteins (FCRL1, -3 and -5; R&D Systems), prokaryotic recombinant protein expressed in E. coli (FCRL2 produced in our laboratory) or stably expressed in transfected CHO cell lines. Our results showed that both mAbs specifically recognized FCRL2 without any cross-reactivity with the other FCRL proteins (Fig.1b–c).
Figure 1.
Reactivity of anti- Fc receptor like-2 (FCRL2) monoclonal antibodies (mAbs) with FCRL2 and other members of the FCRL family. (a) Western blot reactivity profile of anti-FCRL2 mAbs (3D8-G8) with recombinant FCRL2 protein. Lane 1: lysate of FCRL2-transfected Chinese hamster ovary (CHO) cells (15 μg); lane 2: lysate of CHO cells transfected with empty pCMV6-Neo (15 μg); lane 3: prokaryotic recombinant extracellular domain of FCRL2 (5 μg); M: protein size marker. (b) Flow cytometry analysis of the anti-FCRL2 mAbs using stable FCRL-transfected and empty vector transfected CHO cell lines. Shaded histograms represent background staining with an isotype-matched negative control mAb. 7G7 refers to a positive control commercial mAb obtained from Genentech Co. (c) ELISA results obtained for anti-FCRL2 mAbs showing specific binding to recombinant FCRL2 protein with no cross-reactivity to eukaryotic recombinant FCRL1, -3 and -5 proteins (R&D Systems). Recombinant FCRL4 protein was not available for this study. Commercial and home-made anti-FCRL polyclonal antibodies were included as positive controls in ELISA experiment as follows: reactivities of recombinant FCRL1 and -5 proteins were confirmed by commercial goat anti-human FCRL polyclonal antibodies (R&D Systems) and reactivity of recombinant FCRL3 was approved by polyclonal rabbit anti-FCRL antibody produced in our laboratory (data not shown). The latter antibody was produced using FCRL peptide and was not reactive by flow cytometry.
Influence of FCRL2 ligation on calcium influx induced by BCR stimulation
Given that calcium mobilization is an early event following the BCR ligation, we initially determined whether our anti-FCRL2 mAbs were able to influence mobilization of intracellular calcium following the BCR cross-linking. The BL cell line, CA46 was selected from a collection of B-cell lines for signalling analysis based on high level expression of membrane-bound FCRL2 and no expression of FcγRIIB (Fig.2).
Figure 2.
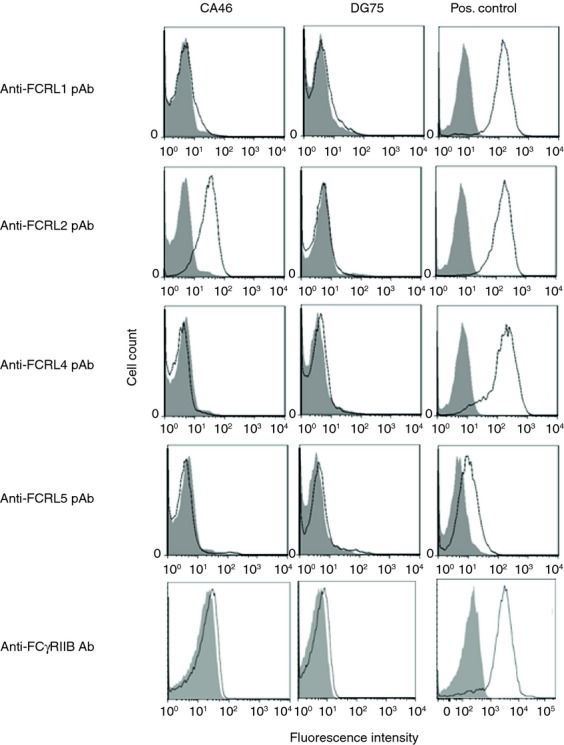
Fc receptor like-2 (FCRL2) and FcγRIIB expression profiles in CA46 and DG75 BL cell lines by flow cytometry. Shaded histograms represent background staining with biotinylated normal sheep immunoglobulin and irrelevant mouse IgG1 monoclonal antibody (mAb) as negative controls for FCRL and FcγRIIB, respectively. FCRL-transfected Chinese hamster ovary (CHO) cells were used as positive controls for FCRL1, -2, -4 detection. The Raji Burkitt's lymphoma cell line and the K562 cell line were used as FCRL5- and FcγRIIB-expressing cells, respectively.
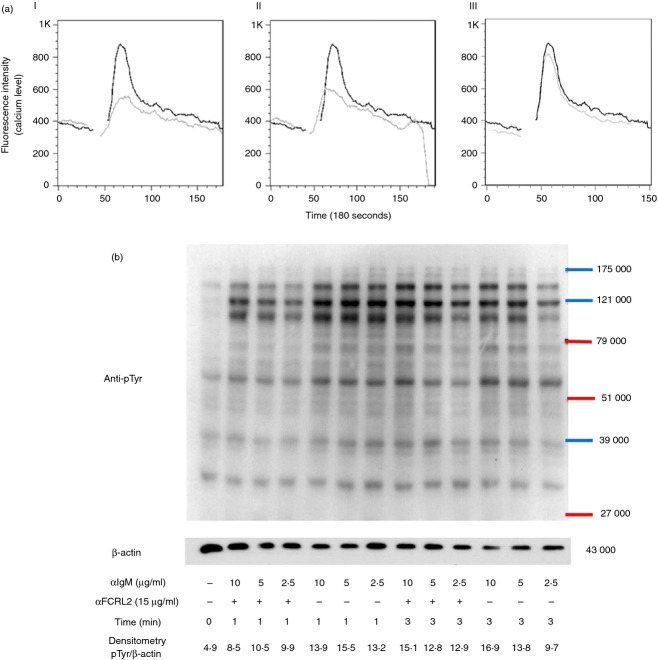
Application of goat anti-human IgM pAb alone did not cross-link BCR extensively and resulted in induction of a weak level of calcium influx (data not presented). To enhance the signalling process, a secondary antibody (anti-goat immunoglobulin) was employed that resulted in significant BCR cross-linking. The same rationale was applied for FCRL2 signalling through the anti-FCRL2 mAbs together with an anti-mouse immunoglobulin pAb, which resulted in FCRL cross-linking. Treatment of CA46 with both mAbs to FCRL2, particularly 5A7-E7, reduced the calcium influx induced by anti-IgM antibody as compared with the isotype-matched control mAb (Fig.3a). It is noteworthy that different concentrations of mAb 5A7-E7 (5, 10, 20, 30 μg/ml) induced a similar level of inhibition in calcium influx (data not presented).
Figure 3.
Influence of anti- Fc receptor like-2 (FCRL2) monoclonal antibodies (mAbs) on calcium mobilization (a) and protein tyrosine phosphorylation profiles (b) induced by anti-IgM antibody in the CA46 Burkitt's lymphoma (BL) cell line. (a) FCRL2-specific mAbs inhibit the calcium mobilization induced by anti-IgM antibody in the CA46 BL cell line. Calcium mobilization was analysed in Fluo-4FF loaded CA46 cells which were treated with saturating amounts of anti-IgM antibody together with (I) 5A7-E7, (II) 3D8-G8 and (III) negative control (anti-Env11, specific to HIV envelope protein, IgG1/k) mAbs. Baseline fluorescence was recorded for 30 seconds at 37° and subsequently the antibodies were added in appropriate tubes and fluorescence was continuously recorded in the FL1 channel by flow cytometry. Black lines represent anti-IgM and grey lines denote anti-IgM and anti-FCRL2 mAbs. The experiments were repeated twice. (b) Protein tyrosine phosphorylation profiles in the CA46 BL cell line stimulated with anti-FCRL2 mAb (5A7-E7) and different concentrations of anti-IgM antibody. The CA46 cell line was treated with different concentrations of anti-BCR antibody and constant concentration of anti-FCRL2 mAb for 1 and 3 min at 37°. Cells were lysed with 1% Triton X-100 and immunoblotted with phospho-tyrosine-specific antibody (clone 4G10). More obvious inhibition of some phosphoproteins was seen especially at 1 min after treatment and also at lower concentrations of anti-IgM antibody.
Influence of FCRL2 ligation on BCR-mediated protein phosphorylation
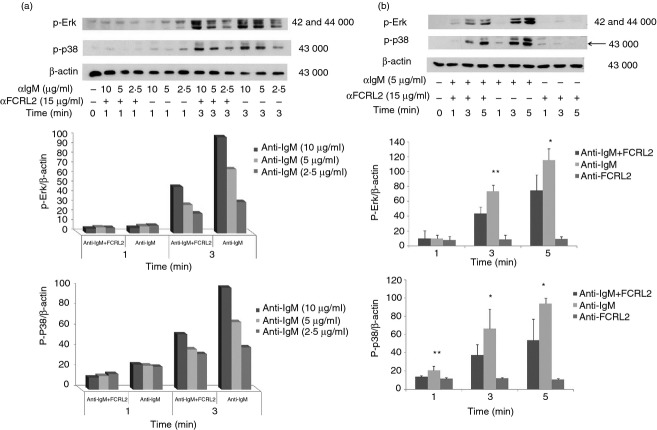
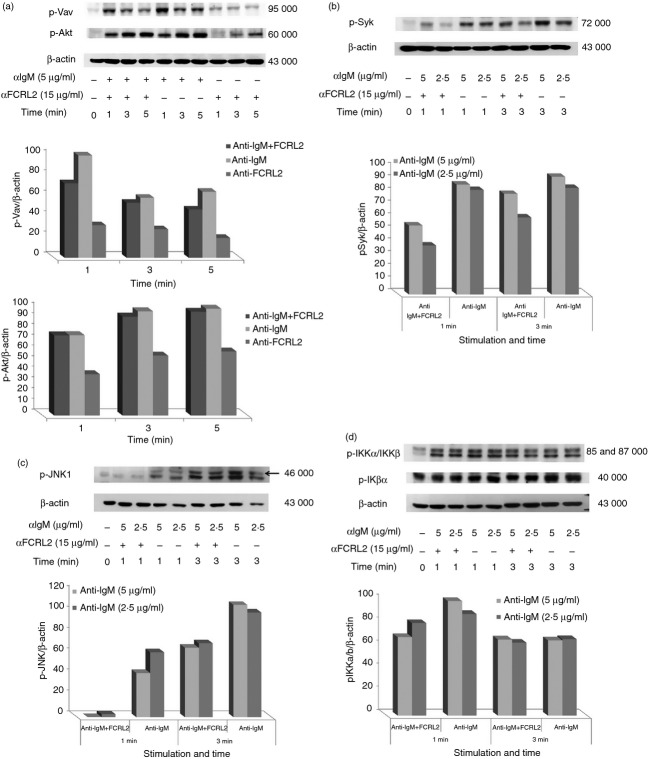
BCR signalling promotes tyrosine phosphorylation of many proteins in the activated B cells. FCRL2 cross-talk with BCR signalling mediators can be detected by alterations in whole tyrosine protein phosphorylation induced by a BCR activator. In these experiments CA46 cells were stimulated with anti-IgM antibody, anti-FCRL2 mAb (5A7-E7) and the combination of anti-IgM and anti-FCRL2 antibodies for different time-points. Anti-IgM antibody was used at 10, 5 and 2·5 μg/ml and anti-FCRL2 mAb was employed at a single concentration (15 μg/ml). The duration of the stimulation and concentration of anti-IgM antibody were optimized for each experiment. The results showed that anti-FCRL2 mAb did not have any inhibitory or stimulatory effects on the whole protein tyrosine phosphorylation level observed in anti-IgM untreated cells (data not presented). However, mAb to FCRL2 inhibited the whole protein tyrosine phosphorylation in the CA46 cell line stimulated through BCR (Fig.3b). The inhibitory effects were more pronounced at lower concentrations of anti-IgM antibody. Based on the band size of the inhibited phosphoproteins by FCRL2 signalling, we analysed the most important mediators of BCR signalling pathways. We initially checked the inhibitory effect of a fixed concentration of anti-FCRL2 mAb (15 μg/ml) on phosphorylation of both Erk and p38 molecules induced by different concentrations of anti-IgM antibody (2·5, 5 and 10 μg/ml) (Fig.4a). Substantial inhibition was observed in cells stimulated with both 5 and 10 μg/ml of anti-IgM antibody. Next we tested the effects of anti-FCRL2 mAb on Erk, p38, Vav and Akt phosphorylation induced by 5 μg/ml of anti-IgM antibody at different time-points. Significant inhibitions of both Erk (P = 0·002 and P = 0·02) and p38 (P = 0·017 and P = 0·024) phosphorylations were observed, particularly after 3-min and 5-min stimulations, respectively, by anti-IgM and anti-FCRL2 antibodies (Fig.4b). However, BCR-induced phosphorylation of Akt was not significantly influenced and Vav phosphorylation was slightly reduced by FCRL2 ligation (Fig.5a). Marginal enhancement of phosphorylation of Akt, and to a lesser extent Vav, were observed in CA46 cells stimulated with mAb to FCRL2 alone (Fig.5a). According to our protocol to decrease the phosphorylation background, the cells were cultured in fetal bovine serum-free medium for 2 hr before stimulation. However, p-Akt had a slightly high background. In this condition the background of p-Erk was completely negative. Finally, the phosphorylation profiles of Syk, Jnk1, IKKα/IKKβ and IKβα were assessed. Results showed inhibition of Syk and Jnk1, but not IKKα/IKKβ and IKβα phosphorylations (Fig.5b–d). In parallel, we employed a surface IgM-positive BL cell line (DG75) which neither expresses FCRL2 nor FCRL1, 3, 4 or 5 either at the mRNA (data not presented) or at the protein levels (Fig.2) to assess the effect of anti-FCRL2 mAb on phosphorylation of whole protein tyrosine, Erk and p38. Furthermore, our results showed that FcγRIIB was not expressed on DG75 cells (Fig.2). This cell line was stimulated in parallel with CA46 by anti-IgM antibody alone and together with anti-FCRL2 mAb at different time-points. No substantial inhibitory effect was observed in the anti-IgM induced phosphorylation pattern, as anticipated in the DG75 cell line (Fig.6a–c). Phosphorylation of Erk was not inhibited by FCRL2 either with 10 or with 5 μg/ml of anti-IgM antibody in the DG75 cell line.
Figure 4.
Inhibitory influence of anti-Fc receptor like-2 (FCRL2) monoclonal antibody (mAb; 5A7-E7) on phosphorylation status of Erk1/2 and p38 mitogen-activated protein kinase (MAPK) in the CA46 Burkitt's lymphoma (BL) cell line. (a) Stimulation was done with different concentrations of anti-IgM antibody (10, 5 and 2·5 μg/ml) for 1 and 3 min. (b) Stimulation was performed with 5 μg/ml of anti-IgM antibody for 1, 3 and 5 min. The FCRL2-positive CA46 cell line was stimulated with anti-FCRL2 and different concentrations of anti-IgM antibodies for 1, 3 and/or 5 min. The cells were lysed and immunoblotted with phospho-specific anti-p-Erk1/2 and anti-p-p38 mAbs then stripped filters were immunoblotted with anti-β-actin antibodies as loading control. The band density was quantified by laser densitometry and the results are shown relative to β-actin. The error bars represent the standard deviations obtained from three independent experiments. * and ** denote significant inhibition of Erk and p38 phosphorylation induced by anti-FCRL2 mAb relative to anti-IgM antibody alone at different stimulation time intervals (*< 0·05 and **< 0·01).
Figure 5.
Inhibitory influence of anti- Fc receptor like-2 (FCRL2) monoclonal antibody (mAb; 5A7-E7) on phosphorylation status of B-cell receptor (BCR) signalling mediators in the CA46 Burkitt's lymphoma (BL) cell line. The FCRL2-positive CA46 cell line was stimulated with anti-FCRL2 and anti-IgM antibodies for 1, 3 and 5 min. The cells were lysed and immunoblotted with phospho-specific mAbs then stripped filters were immunoblotted with anti-β-actin antibodies as loading control. The band density was quantified by laser densitometry. (a) Immunoblotting with anti-p-Vav and anti-p-Akt and densitometry results related to p-Vav/β-actin and Akt/β-actin indicated that BCR-induced phosphorylation of Akt was not significantly influenced and Vav phosphorylation was slightly reduced by FCRL2 ligation. The phosphorylation status of Syk (b), Jnk1 (c) and IKK (d) in the CA46 BL cell line stimulated with anti-IgM antibody and anti-FCRL2 mAb (5A7-E7) showed that Syk and Jnk1 phosphorylation was inhibited by FCRL2 signalling, however, IKK was not affected by FCRL2 stimulation. The experiments were performed twice.
Figure 6.
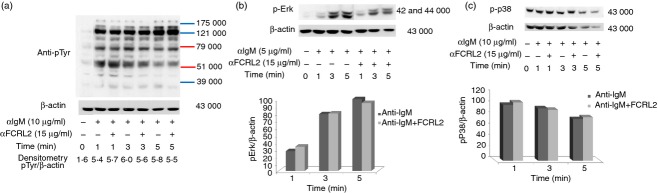
Anti- Fc receptor like-2 (FCRL2) monoclonal antibody (mAb; 5A7-E7) has no inhibitory influence on the phosphorylation status of whole protein tyrosine (a), Erk (b) and p38 (c) in the anti-IgM antibody stimulated DG75 cell line. The FCRL2-negative DG75 cell line was treated with anti-BCR antibody and anti-FCRL2 mAb for 1, 3 and 5 min at 37°. Cells were lysed with 1% Triton X-100 and immunoblotted with phospho-tyrosine-specific (clone 4G10), p-Erk1/2 and p-p38 antibodies. Stripped filters were immunoblotted with anti-β-actin antibodies as loading control and the band density was quantified by laser densitometry. These experiments were done as control in parallel under the same conditions adapted for the CA46-treated cell line. Phosphorylation of whole tyrosine protein, p-Erk1/2 and p-p38 was not altered by anti-FCRL2 mAb. Phosphorylation of Erk1/2 was assessed at 5 μg/ml of anti-IgM antibody and phosphorylation of whole tyrosine protein and p38 was checked at 10 μg/ml of anti-IgM antibody.
Discussion
In the present study, the FCRL2 signalling was investigated using two novel anti-FCRL2 mAbs (3D8-G8 and 5A7-E7). These mAbs were established against recombinant FCRL2 protein. Characterization of these mAbs showed that both of them recognized native FCRL2 by flow cytometry, ELISA and/or immunoblotting techniques, with no cross-reactivity to the other members of the FCRL family, despite 45–83% amino acid sequence homology between FCRL2 and the other FCRL molecules.16
To study FCRL2-mediated signalling, CA46 and DG75 BL cell lines were selected as FCRL2 positive and negative cell lines, respectively. Both cell lines were negative for expression of the inhibitory FcγRIIB receptor (Fig.2). Our results clearly demonstrated that FCRL2 was highly expressed on CA46 cells, however, the other FCRL proteins were negative in both CA46 and DG75 cells. Lack of expression of the other FCRL family members together with the fact that these two cell lines do not express the FcγRIIB (CD32) (Fig.2),21,22 indicate that the signals induced by our mAbs are delivered by FCRL2.
Ligation of the FCRL2 expressed on a BL cell line (CA46) by these mAbs down-regulated anti-IgM antibody-mobilized calcium influx (Fig.3a). In particular, clone 5A7-E7 induced a higher level of inhibition compared with 3D8-G8 mAb. Total protein tyrosine phosphorylation was also inhibited, though the inhibitory effect was more evident at suboptimal concentrations of anti-IgM antibody. It seems that FCRL2 inhibitory function is not strong enough to inhibit signalling from the BCR at higher concentration of anti-IgM antibody. Under this condition, phosphorylation of Erk, p38, Jnk, Vav and Syk was also inhibited after stimulation of CA46 cells with anti-FCRL2 and anti-IgM antibodies. However, no alteration was observed in phosphorylation of Akt, IKKα/IKKβ and IKβα in the stimulated cells. The anti-FCRL2 mAbs were not able to induce apoptosis in the CA46 cell line (data not presented). It seems that the downstream signalling mediators induced through FCRL2 could not trigger apoptosis in the CA46 cell line.
It needs to be considered that an irrelevant isotype-matched mouse IgG1 mAb (anti-Env11) was employed in the calcium mobilization experiment (Fig.3a III). Since addition of this control antibody did not show any alteration in BCR-induced calcium influx, as opposed to the anti-FCRL2 mAbs, it was not included in our subsequent phosphorylation experiments. Instead, an FCRL2-negative BL cell line (DG75) was employed as a negative control cell line in all our experiments to account for non-specific binding of primary and secondary antibodies and their influence on the signalling process. Stimulation of DG75 by anti-IgM antibody alone and together with anti-FCRL2 mAb had no substantial inhibitory effect on the anti-IgM-induced phosphorylation pattern of Erk1/2 and p38 (Fig.6b–c).
It is worthy of mention that the first step pAb (goat anti-IgM) indeed triggers stimulation. However, this antibody alone does not strongly cross-link BCR and so results in induction of weak stimulatory signals in the level of calcium influx and phosphorylation. To enhance the signalling process, a secondary antibody (anti-goat immunoglobulin) needs to be employed that augments the stimulatory signals induced by anti-IgM antibody. Although the second antibody may cross-react with BCR (human IgM) and/or mouse anti-FCRL2 mAbs, this cross-reaction does not influence the inhibitory signals induced by anti-FCRL2 mAbs for the following reasons. Cross-reaction of the second antibody with BCR may result in enhancement of the stimulatory signals induced by the first antibody (anti-IgM) which is in parallel with its stimulatory effect induced through cross-linking of the anti-BCR antibody. On the other hand, cross-reaction with the mouse anti-FCRL2 mAbs may cross-link the BCR with FCRL2. This cross-linking, in essence, is in favour of the aim of this study, which investigates the effect of FCRL2 signalling on the stimulatory function of anti-BCR antibody. The use of isotype-matched irrelevant mouse mAb (anti-Env11) in the calcium mobilization experiment did not show any inhibitory effect on the stimulatory signals induced through BCR cross-linking using the combination of goat anti-IgM and anti-goat immunoglobulin pAbs (Fig.3a III). This, together with the fact that the combination of these two pAbs induced strong stimulatory signals in both FCRL2-positive and -negative cell lines, which are both FcγRIIB negative (Fig.2), and that the addition of anti-FCRL2 mAbs to this combination induced inhibitory signals only in FCRL2-positive cells, suggest that the use of these antibodies most likely does not interfere with the signals delivered by anti-FCRL2 mAbs.
In the only published report on FCRL2 signalling in B cells, Jackson et al. employed a cell line transfected with a chimeric construct containing the intracytoplasmic domain of FCRL2 and the extracellular and transmembrane regions of FcγRIIB to study the functionality of the putative ITAM-like and ITIM sequences of FCRL2.6 This model has been widely used as a standard method to study FCRL2–5 signalling in which cross-linking of FCRL-hybrid protein and BCR occurs using intact anti-IgG antibody.6,7,13,27 When the FCRL2-hybrid protein was co-ligated with the BCR, tyrosine residues in the ITIMs of FCRL2 became phosphorylated and recruited the SHP-1. The recruitment of SHP-1 was accompanied by inhibition of BCR-triggered calcium mobilization and dephosphorylation of a number of proteins, including the p38 and Erk MAPKs.6 Phosphorylation of p38 was completely inhibited at 1, 3, 5, 10 and 20 min after co-ligation of FCRL2 and BCR. However, the phosphorylation of Erk was partially inhibited, and the highest inhibition level was observed at 1 min post-stimulation.6 Inhibition of Erk phosphorylation was also detected in peripheral blood memory B cells isolated from healthy donors.6 Employment of the FCRL2-FcγRIIB fusion protein allows researchers to investigate the biochemical properties of the intracellular domain of FCRL2 on BCR signalling. However, cross-linking of FCRL2 with BCR may never occur in the physiological condition, because no immunoglobulin binding activity has been observed for FCRL2. In favour of this hypothesis no charged residues exist in the transmembrane portion of the FCRL2 molecule, hence it could not pair with charged residues of the other transmembrane proteins to associate with the BCR complex.
In the present study the BCR and FCRL2 molecules were first triggered separately by their specific antibodies and then cross-linked using different secondary antibodies (goat anti-human IgM Fcμ antibody and goat anti-mouse immunoglobulin antibody). Compatible with the previous report on the inhibitory function of FCRL2 ligation on BCR downstream pathways,6 our anti-FCRL2 mAbs induced a similar inhibitory effect on BCR signalling using the CA46 BL cell line. It has been demonstrated that both ITIM motifs of FCRL2 are phosphorylated after its co-ligation with BCR and subsequent recruitment of SHP-1.6 SHP-1 affects Syk phosphorylation/activation, which is essential to couple the BCR to distal signal transduction elements. In turn, Syk phosphorylates and activates some adaptor molecules such as the B-cell linker protein and the downstream Rac and Ras MAPK family pathways.2 Based on these findings, Syk and members of the MAPK family are expected to be negatively affected by FCRL2 signalling. We did not check the mechanisms underlying FCRL2-mediated BCR regulation, though the ITIM residues located within the cytoplasmic tail of FCRL2 are probably phosphorylated and SHP-1 tyrosine phosphatase is recruited as proposed by Jackson et al.6 This proposition is supported by inhibition of Erk, p38 and Jnk phosphorylation (Figs4 and 5c). Hence, it seems that FCRL2 signalling influences BCR signalling mainly through the MAPK pathway. However, no inhibition of Akt phosphorylation and other downstream proteins such as IKKα/IKKβ and IKβα was observed after FCRL2 stimulation. It seems that the phosphoinositide 3-kinase/Akt pathway, which promotes B-cell proliferation and survival, is not affected by FCRL2 ligation using anti-FCRL2 mAb. Similar to FCRL2, ligation of FCRL4 or FCRL5 down-regulates BCR signalling by inhibition of Erk MAPK activation.7,13 These findings imply that the FCRL inhibitory molecules cross-talk with BCR signalling mediators through the MAPK pathway. Inhibition of BCR-mediated calcium mobilization is another common event observed following triggering in all the inhibitory FCRL molecules.6,7,13,14
Since FCRL2 is not expressed in pro-B and pre-B cells of bone marrow,17,28 and its expression is shown only in memory B cells of peripheral blood and other lymphoid tissues (tonsil and spleen),17 this pattern of expression implies an important regulatory role for this molecule in the memory B-cell response. Analysis of FCRL2 signalling in memory B cells using these mAbs will extend our knowledge on the regulatory function of this molecule in B-cell-mediated autoimmune diseases as well as B-cell malignancies. Down-regulation of FcγRIIb expression on memory B cells has been described in autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis,29–32 which suggests the critical importance of negative regulatory receptors on B-cell tolerance. As explained, FCRL2 is expressed on memory B cells, which constitute a small population of the total peripheral B cells. Polson et al.17 showed that FCRL2 is expressed on most peripheral blood CD20-positive isolated cells. Our mAbs to FCRL2, as well as the commercial mAb employed by Polson et al., detect on average 2–3% of normal peripheral blood mononuclear cells in an indirect staining flow cytometry (data not presented). However, we could not show whether the stained cells are memory B cells. Over-expression of FCRL2 in some B-cell malignancies, such as chronic lymphocytic leukaemia and mantle cell lymphoma indicates the potential implication of this molecule for targeted immunotherapy of cancer.
Acknowledgments
We would like to thank Sara Gharib for her valuable help in producing mAbs to FCRL2, as well as Giulia Masi, Laura Patrussi, Francesca Finetti and Nagaja Capitani (Department of Life Sciences, University of Siena) for their technical support and suggestions. We also thank Dr Andrew G. Polson from Genentech, Inc. for providing the mAb specific to the FCRL2 protein (clone 7G7). This work was partly supported by a grant from the Food and Drug Administration of the Ministry of Health, Treatment and Medical Education of Iran (grant number S87P/3/414). MS was also supported by EFIS-IL fellowship grant.
Glossary
- BCR
B-cell receptor
- BL
Burkitt's lymphoma
- CHO
Chinese hamster ovary
- FCRL2
Fc receptor like-2
- ITAMs
immunoreceptor tyrosine-based activation motifs
- ITIMs
immunoreceptor tyrosine-based inhibitory motifs
- mAb
monoclonal antibody
- pAb
polyclonal antibody
- SHP-1
Src homology region 2 domain-containing phosphatase-1
Disclosures
The authors declare that they have no competing interests to disclose.
Authorship contributions
MS performed the experiments, analysed data and wrote the manuscript. AAB and MHF performed experiments, MJT advised on mAb production, HR advised on molecular experiments, CU and CTB advised on signalling studies, ZA supervised the study and FS designed and supervised the study, analysed data and wrote the manuscript.
References
- 1.Gauld SB, Dal Porto JM, Cambier JC. B cell antigen receptor signaling: roles in cell development and disease. Science. 2002;296:1641–2. doi: 10.1126/science.1071546. [DOI] [PubMed] [Google Scholar]
- 2.Dal Porto JM, Gauld SB, Merrell KT, et al. B cell antigen receptor signaling 101. Mol Immunol. 2004;41:599–613. doi: 10.1016/j.molimm.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Weinstein E, Peeva E, Putterman C, et al. B-cell biology. Rheum Dis Clin North Am. 2004;30:159–74. doi: 10.1016/S0889-857X(03)00109-1. [DOI] [PubMed] [Google Scholar]
- 4.Kurosaki T. Regulation of BCR signaling. Mol Immunol. 2011;48:1287–91. doi: 10.1016/j.molimm.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Leu CM, Davis RS, Gartland LA, et al. FcRH1: an activation coreceptor on human B cells. Blood. 2005;105:1121–6. doi: 10.1182/blood-2004-06-2344. [DOI] [PubMed] [Google Scholar]
- 6.Jackson TA, Haga CL, Ehrhardt GR, et al. FcR-like 2 Inhibition of B cell receptor-mediated activation of B cells. J Immunol. 2010;185:7405–12. doi: 10.4049/jimmunol.1002305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehrhardt GR, Davis RS, Hsu JT, et al. The inhibitory potential of Fc receptor homolog 4 on memory B cells. Proc Natl Acad Sci USA. 2003;100:13489–94. doi: 10.1073/pnas.1935944100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis RS. Fc receptor-like molecules. Annu Rev Immunol. 2007;25:525–60. doi: 10.1146/annurev.immunol.25.022106.141541. [DOI] [PubMed] [Google Scholar]
- 9.Hatzivassiliou G, Miller I, Takizawa J, et al. IRTA1 and IRTA2, novel immunoglobulin superfamily receptors expressed in B cells and involved in chromosome 1q21 abnormalities in B cell malignancy. Immunity. 2001;14:277–89. doi: 10.1016/s1074-7613(01)00109-1. [DOI] [PubMed] [Google Scholar]
- 10.Davis RS, Wang YH, Kubagawa H, et al. Identification of a family of Fc receptor homologs with preferential B cell expression. Proc Natl Acad Sci USA. 2001;98:9772–7. doi: 10.1073/pnas.171308498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakayama Y, Weissman SM, Bothwell AL. BXMAS1 identifies a cluster of homologous genes differentially expressed in B cells. Biochem Biophys Res Commun. 2001;285:830–7. doi: 10.1006/bbrc.2001.5231. [DOI] [PubMed] [Google Scholar]
- 12.Xu MJ, Zhao R, Zhao ZJ. Molecular cloning and characterization of SPAP1, an inhibitory receptor. Biochem Biophys Res Commun. 2001;280:768–75. doi: 10.1006/bbrc.2000.4213. [DOI] [PubMed] [Google Scholar]
- 13.Haga CL, Ehrhardt GR, Boohaker RJ, et al. Fc receptor-like 5 inhibits B cell activation via SHP-1 tyrosine phosphatase recruitment. Proc Natl Acad Sci USA. 2007;104:9770–5. doi: 10.1073/pnas.0703354104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kochi Y, Myouzen K, Yamada R, et al. FCRL3, an autoimmune susceptibility gene, has inhibitory potential on B-cell receptor-mediated signaling. J Immunol. 2009;183:5502–10. doi: 10.4049/jimmunol.0901982. [DOI] [PubMed] [Google Scholar]
- 15.Sohn HW, Krueger PD, Davis RS, et al. FcRL4 acts as an adaptive to innate molecular switch dampening BCR signaling and enhancing TLR signaling. Blood. 2011;118:6332–41. doi: 10.1182/blood-2011-05-353102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller I, Hatzivassiliou G, Cattoretti G, et al. IRTAs: a new family of immunoglobulinlike receptors differentially expressed in B cells. Blood. 2002;99:2662–9. doi: 10.1182/blood.v99.8.2662. [DOI] [PubMed] [Google Scholar]
- 17.Polson AG, Zheng B, Elkins K, et al. Expression pattern of the human FcRH/IRTA receptors in normal tissue and in B-chronic lymphocytic leukemia. Int Immunol. 2006;18:1363–73. doi: 10.1093/intimm/dxl069. [DOI] [PubMed] [Google Scholar]
- 18.Huttmann A, Klein-Hitpass L, Thomale J, et al. Gene expression signatures separate B-cell chronic lymphocytic leukaemia prognostic subgroups defined by ZAP-70 and CD38 expression status. Leukemia. 2006;20:1774–82. doi: 10.1038/sj.leu.2404363. [DOI] [PubMed] [Google Scholar]
- 19.Kazemi T, Asgarian-Omran H, Hojjat-Farsangi M, et al. Fc receptor-like 1-5 molecules are similarly expressed in progressive and indolent clinical subtypes of B-cell chronic lymphocytic leukemia. Int J Cancer. 2008;123:2113–9. doi: 10.1002/ijc.23751. [DOI] [PubMed] [Google Scholar]
- 20.Li FJ, Ding S, Pan J, et al. FCRL2 expression predicts IGHV mutation status and clinical progression in chronic lymphocytic leukemia. Blood. 2008;112:179–87. doi: 10.1182/blood-2008-01-131359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magrath IT, Freeman CB, Pizzo P, et al. Characterization of lymphoma-derived cell lines: comparison of cell lines positive and negative for Epstein–Barr virus nuclear antigen. II. Surface markers. J Natl Cancer Inst. 1980;64:477–83. [PubMed] [Google Scholar]
- 22.Ben-Bassat H, Goldblum N, Mitrani S, et al. Establishment in continuous culture of a new type of lymphocyte from a “Burkitt like” malignant lymphoma (line D.G.-75) Int J Cancer. 1977;19:27–33. doi: 10.1002/ijc.2910190105. [DOI] [PubMed] [Google Scholar]
- 23.Shabani M, Hemmati S, Hadavi R, et al. Optimization of gene transfection in murine myeloma cell lines using different transfection reagents. AJMB. 2010;2:123–30. [PMC free article] [PubMed] [Google Scholar]
- 24.Asgarian-Omran H, Amirzargar AA, Arjmand M, et al. Expression, purification and characterization of three overlapping immunodominant recombinant fragments from Bordetella pertussis filamentous hemagglutinin. Avicenna J Med Biotechnol. 2013;5:20–8. [PMC free article] [PubMed] [Google Scholar]
- 25.Kazemi T, Tahmasebi F, Bayat AA, et al. Characterization of novel murine monoclonal antibodies directed against the extracellular domain of human HER2 tyrosine kinase receptor. Hybridoma (Larchmt) 2011;30:347–53. doi: 10.1089/hyb.2011.0023. [DOI] [PubMed] [Google Scholar]
- 26.Ghittoni R, Patrussi L, Pirozzi K, et al. Simvastatin inhibits T-cell activation by selectively impairing the function of Ras superfamily GTPases. Faseb J. 2005;19:605–7. doi: 10.1096/fj.04-2702fje. [DOI] [PubMed] [Google Scholar]
- 27.Won WJ, Foote JB, Odom MR, et al. Fc receptor homolog 3 is a novel immunoregulatory marker of marginal zone and B1 B cells. J Immunol. 2006;177:6815–23. doi: 10.4049/jimmunol.177.10.6815. [DOI] [PubMed] [Google Scholar]
- 28.Kochi Y, Yamada R, Suzuki A, et al. A functional variant in FCRL3, encoding Fc receptor-like 3, is associated with rheumatoid arthritis and several autoimmunities. Nat Genet. 2005;37:478–85. doi: 10.1038/ng1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mackay M, Stanevsky A, Wang T, et al. Selective dysregulation of the FcγIIB receptor on memory B cells in SLE. J Exp Med. 2006;203:2157–64. doi: 10.1084/jem.20051503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su K, Yang H, Li X, et al. Expression profile of FcγRIIb on leukocytes and its dysregulation in systemic lupus erythematosus. J Immunol. 2007;178:3272–80. doi: 10.4049/jimmunol.178.5.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isaak A, Gergely P, Jr, Szekeres Z, et al. Physiological up-regulation of inhibitory receptors FcγRII and CR1 on memory B cells is lacking in SLE patients. Int Immunol. 2008;20:185–92. doi: 10.1093/intimm/dxm132. [DOI] [PubMed] [Google Scholar]
- 32.Catalan D, Aravena O, Sabugo F, et al. B cells from rheumatoid arthritis patients show important alterations in the expression of CD86 and FcγRIIb, which are modulated by anti-tumor necrosis factor therapy. Arthritis Res Ther. 2010;12:R68. doi: 10.1186/ar2985. [DOI] [PMC free article] [PubMed] [Google Scholar]