Abstract
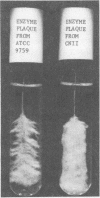
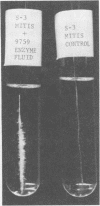
The effect of extracellular products from Streptococcus salivarius on sucrose-dependent adherence to smooth surfaces by other oral bacteria was studied in vitro. Strains of Streptococcus mitis, Streptococcus pyogenes, and Veillonella parvula without innate ability to adhere to a steel wire were able to do so when incubated with sucrose and cell-free culture fluid from S. salivarius strains 9759, 25975, CNII, and MEPI. These culture fluids synthesized more adherent material and water-insoluble glucan than those from Streptococcus mutans C67-1 and seven other S. salivarius strains. Among the S. salivarius strains, glucosyltransferase (GT; dextransucrase, EC 2.4.1.5) activity varied more than 100-fold. Cells of Veillonella and S. mitis S3 that had been incubated in culture fluids from S. salivarius 25975 and 9759, respectively, and then washed adhered upon subsequent incubation with sucrose. This was due to adsorbed GT because (i) the adherence was sensitive to dextranase; (ii) it was observed only with the high-GT culture fluids; (iii) it was dependent on sucrose; and (iv) the washed Veillonella cells synthesized glucan, but not fructan, from sucrose. These results suggest that sucrose-dependent adherence of bacteria without such innate ability can be mediated by (i) entrapment in insoluble glucan synthesized by S. salivarius culture fluids, and (ii) prior adsorption of GT from S. salivarius culture fluids. The possibility that GT formed by high-yield strains of S. salivarius is distributed through the mouth by the action of salivary flow and contributes to sucrose-dependent adherence and plaque formation is considered.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowden G. H., Hardie J. M., Slack G. L. Microbial variations in approximal dental plaque. Caries Res. 1975;9(4):253–277. doi: 10.1159/000260162. [DOI] [PubMed] [Google Scholar]
- Carlsson J. Effect of diet on presence of Streptococcus salivarius in dental plaque and saliva. Odontol Revy. 1965;16(4):336–347. [PubMed] [Google Scholar]
- Carlsson J., Grahnén H., Jonsson G. Lactobacilli and streptococci in the mouth of children. Caries Res. 1975;9(5):333–339. doi: 10.1159/000260166. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Newbrun E., Krasse B. Purification and properties of dextransucrase from Streptococcus sanguis. Arch Oral Biol. 1969 May;14(5):469–478. doi: 10.1016/0003-9969(69)90140-x. [DOI] [PubMed] [Google Scholar]
- Carlsson J. Presence of various types of non-haemolytic streptococci in dental plaque and in other sites of the oral cavity in man. Odontol Revy. 1967;18(1):55–74. [PubMed] [Google Scholar]
- Chaiet L., Kempf A. J., Harman R., Kaczka E., Weston R., Nollstadt K., Wolf F. J. Isolation of a pure dextranase from Penicillium funiculosum. Appl Microbiol. 1970 Sep;20(3):421–426. doi: 10.1128/am.20.3.421-426.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassy B. M., Beall J. R., Bielawski R. M., Porter E. V., Donkersloot J. A. Occurrence and distribution of sucrose-metabolizing enzymes in oral streptococci. Infect Immun. 1976 Aug;14(2):408–415. doi: 10.1128/iai.14.2.408-415.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewar M. D., Walker G. J. Metabolism of the polysaccharides of human dental plaque. I. Dextranase activity of streptococci, and the extracellular polysaccharides synthesized from sucrose. Caries Res. 1975;9(1):21–35. doi: 10.1159/000260139. [DOI] [PubMed] [Google Scholar]
- Donkersloot J. A., Robrish S. A., Krichevsky M. I. Fluorometric determination of deoxyribonucleic acid in bacteria with ethidium bromide. Appl Microbiol. 1972 Aug;24(2):179–183. doi: 10.1128/am.24.2.179-183.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerlad R. J. Dental caries research in gnotobiotic animals. Caries Res. 1968;2(2):139–146. doi: 10.1159/000259552. [DOI] [PubMed] [Google Scholar]
- Gaffar A., Coleman E. J., Marcussen H., Kestenbaum R. C. Effects of inoculating a levan-forming cariogenic streptococcus on experimental caries in hamsters. Arch Oral Biol. 1970 Dec;15(12):1393–1396. doi: 10.1016/0003-9969(70)90030-0. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Banghart S. Induction of dental caries in gnotobiotic rats with a levan-forming streptococcus and a streptococcus isolated from subacute bacterial endocarditis. Arch Oral Biol. 1968 Mar;13(3):297–308. doi: 10.1016/0003-9969(68)90128-3. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Banghart S. Variation in extracellular polysaccharide synthesis by cariogenic streptococci. Arch Oral Biol. 1968 Jun;13(6):697–701. doi: 10.1016/0003-9969(68)90147-7. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Depaola P. F., Spinell D. M., Skobe Z. Interdental localization of Streptococcus mutans as related to dental caries experience. Infect Immun. 1974 Mar;9(3):481–488. doi: 10.1128/iai.9.3.481-488.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Synthesis of insoluble dextran and its significance in the formation of gelatinous deposits by plaque-forming streptococci. Arch Oral Biol. 1968 Oct;13(10):1249–1262. doi: 10.1016/0003-9969(68)90081-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. On the formation of dental plaques. J Periodontol. 1973 Jun;44(6):347–360. doi: 10.1902/jop.1973.44.6.347. [DOI] [PubMed] [Google Scholar]
- Green R. M., Drucker D. B., Blackmore D. K. The reproducibility of experimental caries studies within and between two inbred strains of gnotobiotic rat. Arch Oral Biol. 1974 Nov;19(11):1049–1054. doi: 10.1016/0003-9969(74)90094-6. [DOI] [PubMed] [Google Scholar]
- Guggenheim B., Newbrun E. Extracellular glucosyltransferase activity of an HS strain of Streptococcus mutans. Helv Odontol Acta. 1969 Oct;13(2):84–97. [PubMed] [Google Scholar]
- Guggenheim B., Schroeder H. E. Biochemical and morphological aspects of extracellular polysaccharides produced by cariogenic streptococci. Helv Odontol Acta. 1967 Oct;11(2):131–152. [PubMed] [Google Scholar]
- Guggenheim B. Streptococci of dental plaques. Caries Res. 1968;2(2):147–163. doi: 10.1159/000259553. [DOI] [PubMed] [Google Scholar]
- Hardie J. M., Bowden G. H. Physiological classification of oral viridans streptococci. J Dent Res. 1976 Jan;55:A166–A176. doi: 10.1177/002203457605500108011. [DOI] [PubMed] [Google Scholar]
- Hillman J. D., Van Houte J., Gibbons R. J. Sorption of bacteria to human enamel powder. Arch Oral Biol. 1970 Sep;15(9):899–903. doi: 10.1016/0003-9969(70)90163-9. [DOI] [PubMed] [Google Scholar]
- Jablon J. M., Zinner D. D. Differentiation of cariogenic streptococci by fluorescent antibody. J Bacteriol. 1966 Dec;92(6):1590–1596. doi: 10.1128/jb.92.6.1590-1596.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan H. V., Englander H. R., Engler W. O., Kulczyk S. Observations on the implantation and transmission of Streptococcus mutans in humans. J Dent Res. 1972 Mar-Apr;51(2):515–518. doi: 10.1177/00220345720510024501. [DOI] [PubMed] [Google Scholar]
- Kelstrup J., Gibbons R. J. Induction of dental caries and alveolar bone loss by a human isolate resembling Streptococcus salivarius. Caries Res. 1970;4(4):360–377. doi: 10.1159/000259657. [DOI] [PubMed] [Google Scholar]
- Krasse B., Carlsson J. Various types of streptococci and experimental caries in hamsters. Arch Oral Biol. 1970 Jan;15(1):25–32. doi: 10.1016/0003-9969(70)90142-1. [DOI] [PubMed] [Google Scholar]
- Liljemark W. F., Gibbons R. J. Ability of Veillonella and Neisseria species to attach to oral surfaces and their proportions present indigenously. Infect Immun. 1971 Sep;4(3):264–268. doi: 10.1128/iai.4.3.264-268.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCARTHY C., SNYDER M. L., PARKER R. B. THE INDIGENOUS ORAL FLORA OF MAN. I. THE NEWBORN TO THE 1-YEAR-OLD INFANT. Arch Oral Biol. 1965 Jan-Feb;10:61–70. doi: 10.1016/0003-9969(65)90058-0. [DOI] [PubMed] [Google Scholar]
- McCabe M. M., Smith E. E. Origin of the cell-associated dextransucrase of Streptococcus mutans. Infect Immun. 1973 Jun;7(6):829–838. doi: 10.1128/iai.7.6.829-838.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe R. M., Keyes P. H., Howell A., Jr An in vitro method for assessing the plaque forming ability of oral bacteria. Arch Oral Biol. 1967 Dec;12(12):1653–1656. doi: 10.1016/0003-9969(67)90200-2. [DOI] [PubMed] [Google Scholar]
- McGaughey C., Field B. D., Stowell E. C. Effects of salivary proteins on the adsorption of cariogenic streptococci by hydroxyapatite. J Dent Res. 1971 Jul-Aug;50(4):917–922. doi: 10.1177/00220345710500042201. [DOI] [PubMed] [Google Scholar]
- Niven C. F., Smiley K. L., Sherman J. M. The Production of Large Amounts of a Polysaccharid by Streptococcus salivarius. J Bacteriol. 1941 Apr;41(4):479–484. doi: 10.1128/jb.41.4.479-484.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson G. A., Bleiweis A. S., Small P. A., Jr Adherence inhibition of Streptococcus mutans: an assay reflecting a possible role of antibody in dental caries prophylaxis. Infect Immun. 1972 Apr;5(4):419–427. doi: 10.1128/iai.5.4.419-427.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orstavik D., Kraus F. W., Henshaw L. C. In vitro attachment of streptococci to the tooth surface. Infect Immun. 1974 May;9(5):794–800. doi: 10.1128/iai.9.5.794-800.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON R. L., JONES M. A bacteriologic census of human saliva. J Dent Res. 1958 Aug;37(4):697–709. doi: 10.1177/00220345580370041701. [DOI] [PubMed] [Google Scholar]
- ROGOSA M. THE GENUS VEILLONELLA. I. GENERAL CULTURAL, ECOLOGICAL, AND BIOCHEMICAL CONSIDERATIONS. J Bacteriol. 1964 Jan;87:162–170. doi: 10.1128/jb.87.1.162-170.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogosa M. The Genus Veillonella IV. Serological Groupings, and Genus and Species Emendations. J Bacteriol. 1965 Sep;90(3):704–709. doi: 10.1128/jb.90.3.704-709.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen S. Comparison of sucrose and glucose in the causation of dental caries in gnotobiotic rats. Arch Oral Biol. 1969 May;14(5):445–450. doi: 10.1016/0003-9969(69)90137-x. [DOI] [PubMed] [Google Scholar]
- SNYDER M. L., HACKEDORN H. M., MARTIN D. O., JOHNSTON D. D. The synthesis of mucinous polysaccharides from sucrose by oral bacteria. J Dent Res. 1955 Jun;34(3):368–379. doi: 10.1177/00220345550340031101. [DOI] [PubMed] [Google Scholar]
- Schachtele C. F., Loken A. E., Schmitt M. K. Use of specifically labeled sucrose for comparison of extracellular glucan and fructan metabolism by oral streptococci. Infect Immun. 1972 Feb;5(2):263–266. doi: 10.1128/iai.5.2.263-266.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky S. S., Manganiello S. D. The oral microbiota of man from birth to senility. J Periodontol. 1971 Aug;42(8):485–496. doi: 10.1902/jop.1971.42.8.485. [DOI] [PubMed] [Google Scholar]
- Tanzer J. M., McCabe R. M. Selection of plaque-forming streptococci by the serial passage of wires through sucrose-containing broth. Arch Oral Biol. 1968 Jan;13(1):139–143. doi: 10.1016/0003-9969(68)90045-9. [DOI] [PubMed] [Google Scholar]
- Van Houte J., Gibbons R. J., Banghart S. B. Adherence as a determinant of the presence of Streptococcus salivarius and Streptococcus sanguis on the human tooth surface. Arch Oral Biol. 1970 Nov;15(11):1025–1034. doi: 10.1016/0003-9969(70)90115-9. [DOI] [PubMed] [Google Scholar]
- Van Houte J., Gibbons R. J., Pulkkinen A. J. Adherence as an ecological determinant for streptococci in the human mouth. Arch Oral Biol. 1971 Oct;16(10):1131–1141. doi: 10.1016/0003-9969(71)90042-2. [DOI] [PubMed] [Google Scholar]
- Van Houte J., Green D. B. Relationship between the concentration of bacteria in saliva and the colonization of teeth in humans. Infect Immun. 1974 Apr;9(4):624–630. doi: 10.1128/iai.9.4.624-630.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Stoppelaar J. D., König K. G., Plasschaert A. J., van der Hoeven J. S. Decreased cariogenicity of a mutant of Streptococcus mutans. Arch Oral Biol. 1971 Aug;16(8):971–975. doi: 10.1016/0003-9969(71)90186-5. [DOI] [PubMed] [Google Scholar]