Abstract
Interleukin-9 (IL-9) was initially thought to be a type 2 T helper (Th2)-associated cytokine involved in the regulation of autoimmune responses by affecting multiple cell types. However, it was recently shown that IL-9-producing CD4+ T cells represent a discrete subset of Th cells, designated Th9 cells. Although Th9 cells have been shown to be important in many diseases, their roles in myasthenia gravis (MG) are unclear. The aim of this study was to determine whether IL-9 and Th9 cells promote the progression of experimental autoimmune myasthenia gravis (EAMG). The results showed that the percentage of Th9 cells changed during the progression of EAMG, accompanied by an up-regulation of IL-9. Blocking IL-9 activity with antibodies against IL-9 inhibited EAMG-associated pathology in rats and reduced serum anti-acetylcholine receptor IgG levels. Neutralization of IL-9 altered the Th subset distribution in EAMG, reducing the number of Th1 cells and increasing the number of regulatory T cells. Administration of an anti-IL-9 antibody may represent an effective therapeutic strategy for MG-associated pathologies or other T-cell- or B-cell-mediated autoimmune diseases.
Keywords: B cells, experimental autoimmune myasthenia gravis, interleukin-9, T helper cells
Introduction
Experimental autoimmune myasthenia gravis (EAMG) is an autoimmune disease resulting from the generation of nicotinic acetylcholine receptor (AChR) -specific autoantibodies at the neuromuscular junctions. EAMG has been used as a model to study myasthenia gravis (MG).1–3 AChR-specific T helper (Th) cells are associated with autoreactive antibody production, leading to the autoimmune response in both MG and EAMG.4,5
In the late 1980s, interleukin-9 (IL-9) was described as one of a growing number of cytokines with pleiotropic immune functions.6 Initially thought to be a Th type 2 (Th2) -associated cytokine,7 IL-9 has been shown to play an important role in inflammatory diseases.8 The recent isolation of IL-9-producing CD4+ Th9 cells9 has improved the understanding of this cytokine's biological activities. Under specific conditions, IL-9 can be produced by regulatory T (Treg), Th1, Th17 and mast cells.
Interleukin-9-induced cell activation is mediated by a specific IL-9 receptor chain, which forms a heterodimeric receptor with the common γ chain associated with Janus kinase (JAK) 1 and JAK3 signalling. In the presence of IL-9, the receptor chain activates complexes containing signal transducer and activator of transcription 1 (STAT1), STAT3 and STAT5.10,11 The JAK/STAT pathway plays critical roles in the growth, survival and differentiation of many cell types, but is particularly important in controlling helper/effector T-cell differentiation.12 Interleukin-9 can cause pleiotropic effects in Treg, Th1, Th17 and Th2 cells.13–16 This cytokine has been shown to enhance immunoglobulin production by B cells, promote the proliferation and differentiation of mast cells and haematopoietic progenitors, and regulate immune responses differently depending on the microenvironment. Currently, IL-9-specific antibodies are being studied in models of asthma,17 human anaplastic large-cell lymphoma,18 Trichuris muris infection,19 collagen-induced arthritis,20 transplantation,21 and experimental autoimmune encephalomyelitis.22,23
In the present study, we investigated the role of IL-9 in the development of T and B cells in EAMG disease progression. We used anti-IL-9 antibodies, which have been shown to confer protective effects in other inflammatory diseases.19,24,25 Early administration of anti-IL-9 antibodies altered the CD4+ Th cell distribution and attenuated humoral immune responses associated with EAMG progression.
Materials and methods
Animals
Female Lewis rats (weight: 160–180 g, age: 6–8 weeks) were purchased from the Vital River Laboratory Animal Co. Ltd. (Beijing, China). Animal handling and experimental procedures were performed in accordance with the guidelines of the Care and Use of Laboratory Animals published by the China National Institute of Health.
Reagents
The peptide corresponding to the α-97–116 region (DGDFAIVKFTKVLLDYTGHI) of the rat AChR α subunit (R-AChR97–116) was synthesized by AC Scientific, Inc. (Xian, China). Incomplete Freund's adjuvant (IFA), anti-neurofilament (NF)-200, and FITC-conjugated anti-rabbit IgG were purchased from Sigma Aldrich (St Louis, MO). Mycobacterium tuberculosis strain H37RA was purchased from Difco (Detroit, MI). Complete Freund's adjuvant (CFA) was prepared by combining IFA with 2 mg of M. tuberculosis. FITC-conjugated anti-rat CD4 and phycoerythrin (PE)-conjugated anti-rat Foxp3 antibodies were purchased from eBioscience (San Diego, CA, USA). Peridinin chlorophyll protein complex (PerCP)-conjugated anti-rat CD25 and PE-conjugated anti-rat interferon-γ (IFN-γ) antibodies were purchased from BD Biosciences (San Jose, CA). The rabbit anti-rat IL-9 antibody was purchased from Santa Cruz Biologicals (Santa Cruz, CA). Cyanine dye 3 (Cy3) -conjugated goat anti-rabbit IgG and tetramethylrhodamine-labelled α-bungarotoxin (α-BTX) antibodies were purchased from Invitrogen (Carlsbad, CA). Anti-IL-9 antibodies were synthesized by Bioss Co., Ltd (Beijing, China).
Elicitation of EAMG
Rats were randomly divided into five groups (n = 6 rats/group). Experimental groups included an EAMG group, a control IgG-treated group, and low- and high-dose IL-9 neutralization groups. On day 0, rats in the experimental groups were anaesthetized and immunized subcutaneously at the tail base with the R-AChR97–116 peptide (50 μg/rat), emulsified in 100 μl of CFA and 100 μl PBS. On day 30, these rats were boosted with the same peptide emulsified in IFA. The control group was injected with CFA, emulsified in PBS instead of the R-AChR97–116 peptide, and was boosted with IFA/PBS at the same time-points as the experimental groups.26
Rats in the high- and low-dose IL-9 neutralization groups were given an intraperitoneal injection of 1 or 0.45 mg of anti-IL-9 antibodies (Bioss Co., Ltd.), respectively (diluted in 0.3 ml of PBS), on days −1, 0 and every 2 days thereafter. Rats in the IgG-treated group were similarly given 1 mg of IgG dissolved in 0.3 ml of PBS. All animals were weighed at the beginning of the experiment. Clinical progression of EAMG was scored every other day until the animals were killed.
Clinical evaluation
Severity of EAMG was assessed by scoring muscular weakness in a blinded fashion. Clinical scoring was based on the presence of tremors, hunched posture, muscle weakness and fatigability after exercise (through repetitive paw grips on the cage grid for 30 s). Disease severity was graded as described by Lennon et al..27
Cell preparation
Mononuclear cells (MNCs) were obtained from the popliteal, inguinal, and para-aortic lymph nodes (LNs) of immunized rats. Cells were washed three times in RPMI-1640 and stimulated in vitro with 10 μg/ml R-AChR97–116 in RPMI-1640 medium (Sigma-Aldrich), supplemented with 10% fetal bovine serum (Gibco, Paisley, UK), 1% l-glutamine (Sigma-Aldrich), 1% sodium pyruvate, 1% non-essential amino acids, 2 × 10−5 m 2-mercaptoethanol (Amresco, Solon, OH), and 1% penicillin-streptomycin (Gibco).
Flow cytometric immunophenotyping
Different combinations of antibodies were used to characterize the cell populations for animals in the different groups. Intracellular cytokines and cell markers were detected through flow cytometry FACS, as described previously28 with some modifications. MNCs were harvested from LNs and prepared as described above. Before collection, cells were incubated with Brefeldin A (1 : 1000 dilution, eBioscience Inc.) for 4–6 hr in the presence of ionomycin (500 ng/ml) and PMA (50 ng/ml). After washing twice with staining buffer, T cells were stained extracellularly with FITC anti-rat CD4 antibodies for 30 min at 4°. PerCP anti-rat CD25 antibody staining was used to identify Treg cells. After fixation and permeabilization, cells were stained intracellularly with PE-conjugated anti-rat IFN-γ, PE-conjugated anti-rat Foxp3, and rabbit anti-rat IL-9 antibodies, followed by Cy3-conjugated goat anti-rabbit IgG. FITC-, PE- or PerCP-conjugated isotype-matched antibodies were used as negative controls. In vitro, MNCs were cultured with R-AChR97–116 peptide (10 μg/ml) without or with anti-IL-9 antibodies (10 μg/ml) for 72 hr, and then stained as described above. Stained cells were analysed on a BD FACS Calibur flow cytometer. Data were analysed with Cell Quest software (BD Biosciences).
Detection of anti-AChR IgG by ELISA
Serum samples collected from rats in all groups were analysed for IgG reactivity to AChR by ELISA. Briefly, 96-well flat-bottom polystyrene plates (Nunc, Copenhagen, Denmark) were coated with R-AChR97–116 peptide (2 μg/ml in 100 μl) overnight at 4°, washed with PBS-T (0.05% PBS in Tween 20), and blocked with 10% fetal calf serum at room temperature for 2 hr. Serum (1 : 5000, 100 μl) samples were incubated at room temperature for 2 hr and washed. Rabbit anti-rat IgG (1 : 2000, 100 μl) was added and incubated at room temperature for 2 hr. After washing, horseradish peroxidase-conjugated goat anti-rabbit IgG (1 : 5000, 100 μl) was added and incubated at 37° for 1 hr. After washing, substrate was added, and the reaction was allowed to progress at 37° in the dark. After adding stop solution, the optical density at 490 nm (OD490) was read. The results are expressed as mean OD values ± SD.
Histological staining
Changes to neuromuscular junctions were determined histologically by examining 7-μm-thick frozen sections of forelimb muscles, which were air-dried and blocked with 5% BSA at room temperature for 1 hr. Tetramethylrhodamine-labelled α-BTX (1 : 500 dilution in 1% BSA) and anti-NF-200 antibodies (1 : 400 dilution in 1% BSA) were added and incubated overnight at 4°. The sections were washed, followed by a second incubation with FITC-labelled anti-rabbit IgG for another 2 hr at room temperature. The sections were washed, viewed under an LSM700 confocal microscope (Zeiss, Germany), and photographed. Endplate areas were identified by NF-200.29,30 The mean intensity of AChR staining in the same area, considered to provide a relative measure of AChR expression, was analysed by Image-Pro Plus.
Quantitative real-time PCR
Total RNA was extracted from 1 × 107 MNCs with TRIzol reagent, as recommended by Invitrogen. Reverse transcription was performed on 1 μg total RNA with an RT-PCR kit from TaKaRa (Kyoto, Japan). Complementary DNA was generated on a 7500 real-time PCR system. The mRNA expression levels were estimated by real-time quantitative PCR with Light Cycler Fast Start DNA Master SYBR Green I (Genen Copoeia, Guangzhou, China). The following primer sequences were used: 18s sense: 5′-AGTCCCTGCC CTTTGTACAC A-3′, antisense: 5′-CGATCCGAGG GCCTCACTA-3′; T-bet sense: 5′-AAGGCAGTAT GCCAGGGAAC-3′, antisense: 5′-TTGGAAGCCC CCTTGTTGTT-3′; Foxp3 sense: 5′-CAGCTCCGGC AACTTTTCCT-3′, antisense: 5′-GGAGCCATAG GCTTAGCTGG-3′; STAT3 sense: 5′-GGAGGAGGCA TTCGGAAAGT-3′, antisense: 5′-GCACTACCTG GGTCAGCTTC A-3′; STAT5 sense: 5′-CCGTGGGATG CTATTGACTT-3′, and antisense: 5′-GGTGTTCTGC CTTCTTCTGC-3′. The 18s primers were used as internal controls to confirm equal loading of material into wells.
The mRNA expression levels of each gene were calculated by the 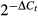 method, in which ΔCt represents the difference between the cycle threshold (Ct) of the target gene and Ct of the housekeeping gene. For comparison, animals in the CFA group were assigned an arbitrary value of 1. Data are represented as
method, in which ΔCt represents the difference between the cycle threshold (Ct) of the target gene and Ct of the housekeeping gene. For comparison, animals in the CFA group were assigned an arbitrary value of 1. Data are represented as  . The mRNA expression level of PU.1 was assessed by semi-quantitative RT-PCR, with the following primer sequences: sense: 5′-CCTTGATTGG TGGTGATGGA GAC-3′ and antisense: 5′-CAGCTCCATG TGGCGGTAGA-3′. The accepted bands were visualized and photographed under ultraviolet light.
. The mRNA expression level of PU.1 was assessed by semi-quantitative RT-PCR, with the following primer sequences: sense: 5′-CCTTGATTGG TGGTGATGGA GAC-3′ and antisense: 5′-CAGCTCCATG TGGCGGTAGA-3′. The accepted bands were visualized and photographed under ultraviolet light.
Western blot analysis
Cellular protein extracts were subjected to 10% SDS–PAGE and transferred onto PVDF membranes (Millipore, Bedford, MA) via wet transfer. Membranes were blocked with 5% non-fat milk at room temperature for 1 hr, followed by probing with primary antibodies diluted in Tris-buffered saline with 0.1% Tween 20 at 4° overnight. Primary antibodies used were specific to GAPDH (1 : 1000, Beyotime, Shanghai, China), STAT3 (1:500, Cell Signaling, Beverly, MA), and p-STAT3 (1 : 500, Cell Signaling). After washing, blots were probed with alkaline phosphatase-conjugated secondary antibodies for 2 hr at room temperature to detect respective protein bands.
Statistical analyses
Data are reported as means ± standard deviations (SDs). Differences were analysed by the two-tailed Student's t-test for comparisons of paired and unpaired data between groups, by analysis of variance for comparisons between various groups, and by Fisher's exact test for comparisons among clinical incidences. Statistical analyses were performed with the GraphPad software program (GraphPad Software, San Diego, CA). Values of P < 0.05 were considered statistically significant.
Results
The percentage of IL-9-producing CD4+ T cells increases during EAMG progression
EAMG presents in two clinical phases: the early phase, at around 8–14 days post-immunization;31,32 and the chronic phase, beginning approximately 40 days after primary immunization. To determine the percentage of Th9 cells present during the different stages of EAMG, we isolated MNCs from the LNs of rats in the CFA and EAMG groups during the early and chronic phases. During the early phase, the percentage of Th9 cells was similar in both groups (Fig.1a). During the chronic phase, the EAMG group showed a decrease in the percentage of CD4+ T cells, with a higher percentage of Th9 cells in the CD4+ T-cell population, compared with the CFA group (Fig.1b, P < 0.05).
Figure 1.
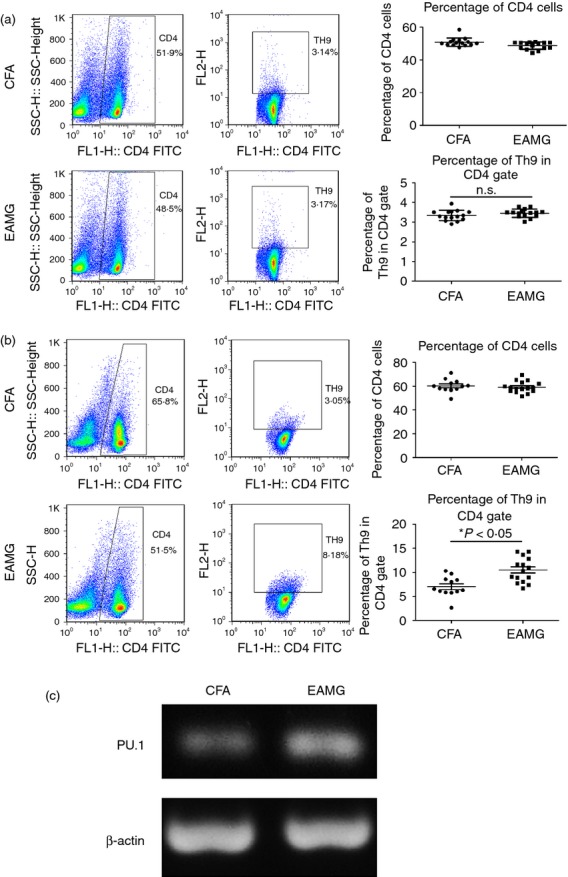
T helper type 9 (Th9) subset of the CD4+ T-cell population increased during experimental autoimmune myasthenia gravis (EAMG) progression. Mononuclear cells (MNCs) from the lymph nodes (LNs) of rats in the CFA and EAMG groups were isolated in the early phase (a) and the chronic phase (b) after primary immunization. Th9 subset in the CD4+ T-cell population was detected by FACS. Data are expressed as the mean ± SD of four separate experiments, *P < 0.05. (c) LN MNCs from rats in the CFA and EAMG groups in the chronic phase were cultured in the presence of R-AChR97–116 for 72 hr. Total RNA was obtained, and the mRNA expression level of PU.1 was assessed by semi-quantitative RT-PCR.
PU.1 is an E-26 transformation-specific family transcription factor that is required for the development of IL-9-secreting Th cells. PU.1 binds directly to the IL-9 promoter to promote specific chromatin modifications.33,34 We obtained MNCs from the LNs of rats in the CFA and EAMG groups during the chronic phase, and cultured these cells in the presence of R-AChR97–116 for 72 hr. We extracted the total RNA from the MNCs, and assessed the PU.1 mRNA expression levels by semi-quantitative RT-PCR. The PU.1 mRNA expression level was significantly higher in the EAMG group compared with the CFA group (Fig.1c). Taken together, the increased percentage of Th9 cells and the up-regulation of PU.1 suggest that Th9 cells may participate in EAMG pathogenesis, especially during the chronic phase.
Interleukin-9 neutralization ameliorates EAMG symptoms
To determine the role of IL-9 in EAMG disease progression, IL-9 was neutralized by administering anti-IL-9 antibodies before primary immunization. Rats treated with a high dose of anti-IL-9 antibodies showed lower average clinical scores and less weight loss than untreated rats during the chronic phase. The IgG-treated group, EAMG group, and low-dose IL-9 neutralization group showed similar results in terms of disease severity (Fig.2a,b).
Figure 2.
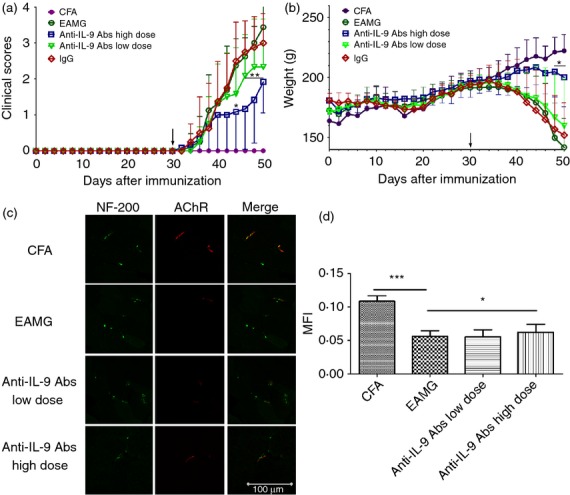
Interleukin-9 (IL-9) neutralization ameliorates experimental autoimmune myasthenia gravis (EAMG) symptoms. Clinical manifestations (a) and body weight (b) were measured in rats from the complete Freund's adjuvant (CFA) group (•), EAMG group (○), IgG-treated group (◇), and the high-dose (□) and low-dose (▽) IL-9 neutralization groups. Arrows indicate the day that R-AChR97–116 boosters were injected. Data are expressed as the mean ± SD of four different experiments. (c) Sections from rats in the CFA, EAMG, low-dose and high-dose IL-9 neutralization groups were examined for the presence of AChR (red fluorescence) at the endplates (NF-200, green fluorescence) to assess changes to the neuromuscular junctions (bar = 100 μm) during the chronic phase. (d) Mean fluorescence intensities (MFIs) of AChR staining were measured from more than 10 fields from each group. Data are expressed as the mean ± SD, ***P < 0.001, *P < 0.05.
A typical pathological change during EAMG development is loss of AChR. To examine this aspect, we performed a histological examination of forelimb muscles from rats in the CFA, EAMG, low-dose and high-dose IL-9 neutralization groups during the chronic phase. Muscle sections were incubated with fluorescently labelled α-BTX and anti-NF-200 antibodies, which bind AChR and NF, respectively, at the neuromuscular junctions. Rats in the EAMG and low-dose IL-9 neutralization groups showed significant AChR loss and endplate damage, associated with weaker and elongated AChR staining, compared with the CFA group. Findings in the EAMG group were reversed after treatment with anti-IL-9 antibodies (Fig.2c,d). The high-dose IL-9 neutralization group had a lower disease incidence than the EAMG group (Table.1).
Table 1.
Disease incidence of different treatment groups after immunization
| Days afer immunization | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 30 | 32 | 34 | 36 | 38 | 40 | 42 | 44 | 46 | 48 | 50 | |
| CFA | 0/20 | 0/20 | 0/20** | 0/20** | 0/20*** | 0/20*** | 0/20*** | 0/20*** | 0/20*** | 0/20*** | 0/20*** |
| EAMG | 1/20 | 3/20 | 7/20 | 7/20 | 12/20 | 13/20 | 17/20 | 19/20 | 19/20 | 19/20 | 19/20 |
| Anti-IL-9 Abs, Low dose | 0/19 | 4/19 | 4/19 | 7/19 | 10/19 | 11/19 | 14/19 | 15/19 | 16/19 | 16/19 | 16/19 |
| Anti-IL-9 Abs, High dose | 0/19 | 1/19 | 2/19 | 3/19 | 4/19* | 5/19* | 7/19** | 7/19*** | 8/19*** | 8/19*** | 10/19** |
Abbreviations: CFA, complete Freund's adjuvant; EAMG, experimental autoimmune myasthenia gravis; IL-9 Abs, interleukin-9 antibodies.
Incidences were compared with the experimental autoimmune myasthenia gravis (EAMG) group.
Statistical comparisons among the clinical incidences were made by Fisher's exact test.
P < 0.05,
P < 0.01,
P < 0.001.
Neutralization of IL-9 attenuates humoral immune responses in EAMG rats
The efficacy of anti-IL-9 antibody treatment was further evaluated by assessing the levels of anti-AChR IgG titres. Sera were collected from rats in the CFA, EAMG and high-dose IL-9 neutralization groups during the chronic phase. Antibody responses were assessed by ELISA (Fig.3a). Compared with the EAMG group, the anti-AChR IgG level was significantly reduced in the high-dose IL-9 neutralization group. Sera from rats in the CFA group were used as a control.
Figure 3.

Effects of interleukin-9 (IL-9) neutralization on anti-AChR IgG production and B-cell differentiation. (a) Anti-AChR IgG titres were determined during the chronic phase and compared with responses in sera from rats in the experimental autoimmune myasthenia gravis (EAMG) group, ***P < 0.001. (b, c) Total lymphocytes were collected during the chronic phase. (b) Signal transducer and activator of transcription 3 (STAT3) mRNA expression levels were determined by quantitative real-time PCR. (c) pSTAT3/STAT3 protein expressions were detected by Western blot analysis. Data are expressed as the mean ± SD of six rats/group, representative of four independent experiments, *P< 0.05, ***P < 0.001.
Interleukin-9 stimulation can induce JAK1 and JAK3 kinase activation, resulting in tyrosine phosphorylation of STAT3 (p-STAT3).11,35 STAT3 has been shown to up-regulate BLIMP1 gene expression and to promote murine plasma cell differentiation.36 Examination of STAT3 mRNA expression in MNCs harvested during the chronic phase and cultured for 0 hr revealed that rats in the high-dose IL-9 neutralization group showed reduced STAT3 expression levels compared with the EAMG group (Fig.3b). The splenic p-STAT3/STAT3 protein ratio was lower in the high-dose IL-9 neutralization group compared with the EAMG group (Fig.3c). These results suggest that the neutralization of IL-9 affected B-cell differentiation and decreased the production of AChR antibodies.
Neutralization of IL-9 alters the Th subset distribution in EAMG in vivo
We previously demonstrated that EAMG progression is associated with imbalances between pro-inflammatory (Th1, Th17) and anti-inflammatory (Th2, Treg) T-cell populations.4,37,38 Interleukin-9 plays critical roles in the differentiation of Th1 and Treg cells,13,14 and its neutralization ameliorates experimental autoimmune encephalomyelitis by decreasing effector T-cell populations.25 Therefore, we examined the effects of IL-9 neutralization on AChR-specific Th cells in the respective treatment groups. Lymphocytes from rats in the CFA, EAMG, and high-dose IL-9 neutralization groups were harvested and cultured in the presence of R-AChR97–116. As EAMG progressed, the percentages of Th1 and Treg cells decreased and increased, respectively, after neutralization of IL-9, compared with the T-cell profiles observed in the EAMG group (Fig.4a,b). There were no differences between the groups in terms of the numbers of Th17 and Th2 cells (data not shown).
Figure 4.
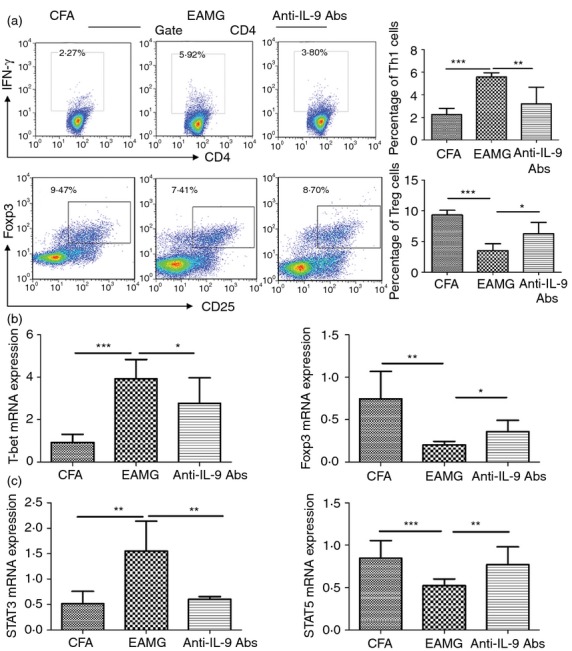
Neutralization of interleukin-9 (IL-9) altered the T helper (Th) subset distribution in vivo. Mononuclear cells (MNCs) were isolated from lymph nodes (LNs) in treatment groups during the chronic phase and cultured with 10 μg/ml R-AChR97–116 peptide for 72 hr. Expression levels were determined for (a) interferon-γ (IFN-γ) and CD25-Foxp3 in CD4+ T cells by FACS, and (b) T-bet/Foxp3 and (c) signal transducer and activator of transcription 3 (STAT3)/STAT5 mRNA by quantitative real-time PCR. Data are expressed as the mean ± SD of six rats/group, representative of four independent experiments, *P < 0.05, **P < 0.01, ***P < 0.001.
Interleukin-9-mediated signalling transduction has been shown to be dependent on the JAK–STAT pathway. The STAT family plays a major role in the signalling pathways of Th cell subsets. We characterized the STAT3 and STAT5 mRNA expression levels in MNCs harvested from the LNs during the chronic phase. After cells were cultured in the presence of R-AChR97–116 for 72 hr, anti-IL-9 antibody treatment reduced the STAT3 and increased the STAT5 mRNA expression levels compared with levels in EAMG rats (Fig.4c). Neutralization of IL-9 had potent regulatory properties, with the potential to shift the Th cell balance in rats with EAMG, probably by affecting the STAT3/STAT5 signalling. These results suggest that blocking IL-9 suppressed Th1 subset differentiation and promoted Treg subset development, contributing to the amelioration of EAMG symptoms.
Effect of anti-IL-9 on CD4+ Th cell distribution in EAMG rats in vitro
Because in vivo treatment with anti-IL-9 antibodies altered the Th subset distribution, we investigated whether anti-IL-9 antibody treatment would affect the Th cell distribution in vitro. Lymphocytes were harvested from EAMG rats during the chronic phase and cultured in the presence of R-AChR97–116 (10 μg/ml) without or with anti-IL-9 antibodies (10 μg/ml) for 72 hr before FACS analysis. The percentage of Th1 cells was decreased after incubation with anti-IL-9 antibodies compared with the untreated group, while the percentage of Treg cells was increased (Fig.5a). The T-bet mRNA level was reduced after anti-IL-9 antibody treatment, but there was no significant difference compared with EAMG rats. The Foxp3 mRNA level was significantly increased after anti-IL-9 antibody treatment compared with EAMG rats (Fig.5b). After treatment with anti-IL-9 antibodies, AChR-specific cells showed lower STAT3 and higher STAT5 expression levels (Fig.5c). These data further confirm that IL-9 neutralization can suppress the differentiation of AChR-specific Th cells during the chronic phase of EAMG.
Figure 5.
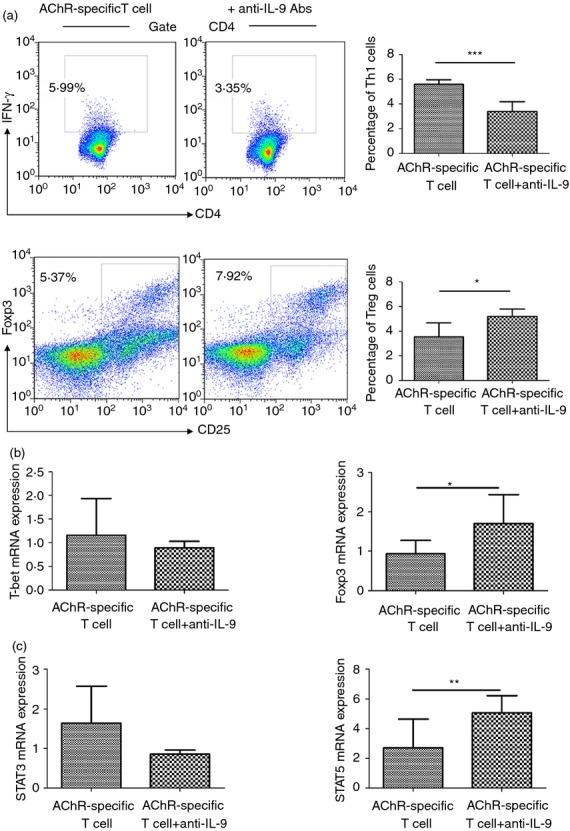
Neutralization of interleukin-9 (IL-9) affected the distribution of AChR-specific T-cell subsets in vitro. Mononuclear cells (MNCs) were isolated from the lymph nodes (LNs) of rats in the experimental autoimmune myasthenia gravis (EAMG) group during the chronic phase and cultured in the presence of 10 μg/ml R-AChR97–116 peptide for 72 hr with anti-IL-9 antibodies (10 μg/ml). (a) FACS analysis of interferon-γ (IFN-γ) and CD25-Foxp3 expression by CD4+ T cells. Quantitative real-time PCR analysis of (b) T-bet/Foxp3 and (c) signal transducer and activator of transcription 3 (STAT3)/STAT5 mRNA expression levels. Data are expressed as mean ± SD, *P< 0.05, **P < 0.01, ***P < 0.001. Figures are representative of four independent experiments with six rats/group.
Discussion
In this study, the percentage of the Th9 subset of T cells was increased during EAMG progression. By neutralizing IL-9, the disease incidence and severity of EAMG could be suppressed. The beneficial effects of anti-IL-9 antibody treatment were associated with diminished humoral responses and alterations of the Th1 : Treg cell ratio.
Results from murine disease models and human patients have suggested that Th9 cells can play both protective and pathogenic roles in different diseases. Mice with impaired Th9 cell development that could not expel parasitic worms were used as a model to demonstrate the protective effect of IL-9-induced mastocytosis against intestinal nematodes.15,39 Th9 cells inhibited subcutaneous melanoma growth through mast cells, CD8+ T cells, or dendritic cells, and, in this context, neutralization of IL-9 accelerated tumour growth.40,41 Studies in multiple mouse models have indicated a role for Th9 cells in allergic pathologies. Neutralization of IL-9 reduced allergic and airway hyper-reactivity symptoms in an ovalbumin-induced model of airway inflammation and in a chronic model of airway hyper-reactivity induced by intranasal Aspergillus fumigatus lysates.33,42 In addition, Th9 cell-derived IL-9 was shown to exacerbate experimental autoimmune encephalomyelitis.13,23
Although Th9 cells and IL-9 are thought to have roles in many diseases, their effects in EAMG are not clear. In the present study, the effects of Th9 cells and IL-9 on EAMG progression were examined. EAMG progression is associated with the presence of autoantibodies against the nicotinic AChR at the post-synaptic membrane.43 B cells serve as antigen-presenting cells for autoreactive T-cell priming and as effector cells that secrete anti-AChR antibodies. Rats treated with a high dose of anti-IL-9 antibodies developed a milder form of EAMG and lost less weight than untreated rats. Their levels of anti-AChR IgG were significantly reduced compared with the EAMG group. The high-dose IL-9 neutralization group had a significantly lower disease incidence than the EAMG group from 38 days after the first immunization (chronic phase).
Interleukin-9 has effects on B-cell development, and it enhances IgE and IgG production.44–46 Although autoantibodies are products of B cells, STAT3 has been shown to be required for T-cell-dependent IgG plasma cell differentiation in mice47 and to act upstream of genes associated with IL-9 signalling events.40 In this study, the high-dose IL-9 neutralization group presented a lower STAT3 mRNA expression level and lower p-STAT3/STAT3 protein ratio than the EAMG group. During development of the anti-AChR immune response, IL-9 neutralization inhibited anti-AChR IgG produced by B cells by reducing expression of STAT3. Finally, IL-9 neutralization attenuated the disease presentation.
Interleukin-9 is a pleiotropic cytokine with direct and indirect effects on multiple cell types that affect immune response and inflammation.35 It has been shown to promote the proliferation and differentiation of mast cells,48 to induce goblet cell metaplasia, to enhance chemokine production by epithelial cells,49 to support erythroid colony formation, and to promote the maturation of haematopoietic stem/progenitor cells.50 As a T-cell growth factor, IL-9 can increase IFN-γ production and promote Th17 development, but it has variable effects on Treg cell development.13,14,51 AChR-specific T cells are necessary for EAMG development. Th1 cells secrete the pro-inflammatory cytokine IFN-γ, which is required during the initiation and development of an organ-specific autoimmune disease. Administration of IFN-γ exacerbates EAMG severity.52 Th17 responses are associated with the production of the inflammatory cytokine IL-17 and promote EAMG development.4 Interleukin-4 plays an anti-inflammatory role and can prevent the development of EAMG.53 Finally, Treg cells are associated with the tolerance and suppression of EAMG.54,55
Considering the effects of IL-9 on Th cells and the important roles of Th cells in EAMG, we blocked IL-9 to detect its influence on the differentiation of Th subsets in EAMG. Neutralization of IL-9 affected the profile of Th subsets in EAMG both in vitro and in vivo. Functional blocking of IL-9 inhibited the differentiation of Th1 cells in vivo during the chronic phase of disease. Anti-IL-9 antibodies significantly suppressed IFN-γ production in culture. The T-bet mRNA expression was significantly reduced in vivo, and a similar trend was observed in vitro. Comparing the developmental suppression of Th1 cells after anti-IL-9 antibody treatment in vivo or in vitro, the frequency of Treg cells was significantly increased during the chronic phase. Interleukin-9 exerts its action on Treg cells through STAT3 and STAT5 signalling. STAT5 binds directly to the FoxP3 gene56 and is required for optimal induction of Foxp3 in vitro. However, STAT3 and STAT5 can sometimes play opposing roles.57 Anti-IL-9 antibody treatment increased STAT5 and suppressed STAT3 mRNA expression levels, so influencing the balance between Th1/Treg cells and altering the EAMG disease presentation.
In conclusion, our results demonstrate that the protective effect of anti-IL-9 antibody treatment in EAMG was mediated not only by suppressing humoral immune responses, but also by inhibiting AChR-specific Th1 cells and expanding the Treg cell population. These data suggest that IL-9 plays an important pathogenic role in EAMG, and that anti-IL-9 antibody treatment might represent a promising therapy for MG and other autoimmune diseases.
Acknowledgments
This research was supported by the National Nature Science Foundation for Youth of China (81000511; 81000536; 81000512), a Project funded by the China Postdoctoral Science Foundation (20100480062), a Special Financial Grant from the China Postdoctoral Science Foundation (2012T50356), The Harbin Science & Technology Bureau Creative Talent Fund (2012RFQXS049), the Science and Technology Study project of the Education Department of Heilongjiang Province (12521213), the Open Topic of Key Laboratory of Neurobiology, General Colleges and Universities in Heilongjiang Province of China (2013HLJKLNT-10), Natural Science Foundation of Heilongjiang Province (LC201431) and the National Nature Science Foundation of China (81301023).
Glossary
- α-BTX
α-bungarotoxin
- AChR
acetylcholine receptor
- CFA
complete Freund's adjuvant
- Cy3
cyanine dye 3
- EAMG
experimental autoimmune myasthenia gravis
- IFA
incomplete Freund's adjuvant
- IFN
interferon
- IL
interleukin
- JAK
janus kinase
- LN
lymph node
- MG
myasthenia gravis
- MNC
mononuclear cells
- NF
neurofilament
- OD
optical density
- PE
phycoerythrin
- PerCP
peridinin chlorophyll protein complex
- R-AChR97–116
rat AChR α subunit
- STAT
signal transducer and activator of transcription
- Th
T helper cells
- Treg
regulatory T cells
Disclosures
The author has no potential conflicts of interest.
References
- 1.Vincent A. Unravelling the pathogenesis of myasthenia gravis. Nat Rev Immunol. 2002;2:797–804. doi: 10.1038/nri916. [DOI] [PubMed] [Google Scholar]
- 2.Jayam Trouth A, Dabi A, Solieman N, Kurukumbi M, Kalyanam J. Myasthenia gravis: a review. Autoimmune Dis. 2012;2012:874680. doi: 10.1155/2012/874680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Baets M, Stassen M, Losen M, Zhang X, Machiels B. Immunoregulation in experimental autoimmune myasthenia gravis – about T cells, antibodies, and endplates. Ann N Y Acad Sci. 2003;998:308–17. doi: 10.1196/annals.1254.033. [DOI] [PubMed] [Google Scholar]
- 4.Mu L, Sun B, Kong Q, et al. Disequilibrium of T helper type 1, 2 and 17 cells and regulatory T cells during the development of experimental autoimmune myasthenia gravis. Immunology. 2009;128:e826–36. doi: 10.1111/j.1365-2567.2009.03089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang H, Zhang Y, Wu M, et al. Suppression of ongoing experimental autoimmune myasthenia gravis by transfer of RelB-silenced bone marrow dendritic cells is associated with a change from a T helper Th17/Th1 to a Th2 and FoxP3+ regulatory T-cell profile. Inflamm Res. 2010;59:197–205. doi: 10.1007/s00011-009-0087-6. [DOI] [PubMed] [Google Scholar]
- 6.Goswami R, Kaplan MH. A brief history of IL-9. J Immunol. 2011;186:3283–8. doi: 10.4049/jimmunol.1003049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stassen M, Schmitt E, Bopp T. From interleukin-9 to T helper 9 cells. Ann N Y Acad Sci. 2012;1247:56–68. doi: 10.1111/j.1749-6632.2011.06351.x. [DOI] [PubMed] [Google Scholar]
- 8.Hultner L, Kolsch S, Stassen M, et al. In activated mast cells, IL-1 up-regulates the production of several Th2-related cytokines including IL-9. J Immunol. 2000;164:5556–63. doi: 10.4049/jimmunol.164.11.5556. [DOI] [PubMed] [Google Scholar]
- 9.Tan C, Gery I. The unique features of Th9 cells and their products. Crit Rev Immunol. 2012;32:1–10. doi: 10.1615/critrevimmunol.v32.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demoulin JB, Uyttenhove C, Lejeune D, Mui A, Groner B, Renauld JC. STAT5 activation is required for interleukin-9-dependent growth and transformation of lymphoid cells. Cancer Res. 2000;60:3971–7. [PubMed] [Google Scholar]
- 11.Demoulin JB, Uyttenhove C, Van Roost E, DeLestre B, Donckers D, Van Snick J, Renauld JC. A single tyrosine of the interleukin-9 (IL-9) receptor is required for STAT activation, antiapoptotic activity, and growth regulation by IL-9. Mol Cell Biol. 1996;16:4710–6. doi: 10.1128/mcb.16.9.4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adamson AS, Collins K, Laurence A, O'Shea JJ. The Current STATus of lymphocyte signaling: new roles for old players. Curr Opin Immunol. 2009;21:161–6. doi: 10.1016/j.coi.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elyaman W, Bradshaw EM, Uyttenhove C, et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc Natl Acad Sci USA. 2009;106:12885–90. doi: 10.1073/pnas.0812530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finiasz MR, Franco MC, de la Barrera S, et al. IL-9 promotes anti-Mycobacterium leprae cytotoxicity: involvement of IFNγ. Clin Exp Immunol. 2007;147:139–47. doi: 10.1111/j.1365-2249.2006.03241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veldhoen M, Uyttenhove C, van Snick J, et al. Transforming growth factor-β ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–6. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 16.Wu B, Huang C, Kato-Maeda M, Hopewell PC, Daley CL, Krensky AM, Clayberger C. IL-9 is associated with an impaired Th1 immune response in patients with tuberculosis. Clin Immunol. 2008;126:202–10. doi: 10.1016/j.clim.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Renauld JC. [Role of interleukin-9 in asthma and allergic reactions] Bull Mem Acad R Med Belg. 2007;162:275–82. discussion 83-5. [PubMed] [Google Scholar]
- 18.Qiu L, Lai R, Lin Q, et al. Autocrine release of interleukin-9 promotes Jak3-dependent survival of ALK+ anaplastic large-cell lymphoma cells. Blood. 2006;108:2407–15. doi: 10.1182/blood-2006-04-020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richard M, Grencis RK, Humphreys NE, Renauld JC, Van Snick J. Anti-IL-9 vaccination prevents worm expulsion and blood eosinophilia in Trichuris muris-infected mice. Proc Natl Acad Sci USA. 2000;97:767–72. doi: 10.1073/pnas.97.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsuji F, Yoshimi M, Katsuta O, Takai M, Ishihara K, Aono H. Point mutation of tyrosine 759 of the IL-6 family cytokine receptor, gp130, augments collagen-induced arthritis in DBA/1J mice. BMC Musculoskelet Disord. 2009;10:23. doi: 10.1186/1471-2474-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu LF, Lind EF, Gondek DC, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 22.Nowak EC, Weaver CT, Turner H, et al. IL-9 as a mediator of Th17-driven inflammatory disease. J Exp Med. 2009;206:1653–60. doi: 10.1084/jem.20090246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jager A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol. 2009;183:7169–77. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng G, Arima M, Honda K, et al. Anti-interleukin-9 antibody treatment inhibits airway inflammation and hyperreactivity in mouse asthma model. Am J Respir Crit Care Med. 2002;166:409–16. doi: 10.1164/rccm.2105079. [DOI] [PubMed] [Google Scholar]
- 25.Li H, Nourbakhsh B, Ciric B, Zhang GX, Rostami A. Neutralization of IL-9 ameliorates experimental autoimmune encephalomyelitis by decreasing the effector T cell population. J Immunol. 2010;185:4095–100. doi: 10.4049/jimmunol.1000986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baggi F, Annoni A, Ubiali F, et al. Breakdown of tolerance to a self-peptide of acetylcholine receptor α-subunit induces experimental myasthenia gravis in rats. J Immunol. 2004;172:2697–703. doi: 10.4049/jimmunol.172.4.2697. [DOI] [PubMed] [Google Scholar]
- 27.Lennon VA, Lindstrom JM, Seybold ME. Experimental autoimmune myasthenia: a model of myasthenia gravis in rats and guinea pigs. J Exp Med. 1975;141:1365–75. doi: 10.1084/jem.141.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JB, Jang JE, Song MK, Chang J. Intranasal delivery of cholera toxin induces Th17-dominated T-cell response to bystander antigens. PLoS ONE. 2009;4:e5190. doi: 10.1371/journal.pone.0005190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marques MJ, Pertille A, Carvalho CL, Santo Neto H. Acetylcholine receptor organization at the dystrophic extraocular muscle neuromuscular junction. Anat Rec. 2007;290:846–54. doi: 10.1002/ar.20525. [DOI] [PubMed] [Google Scholar]
- 30.Nagaoka T, Hotta S, Chiba T, Utsunomiya I, Abe K, Yoshino H, Koshikawa C, Taguchi K. IgG anti-Galnac-GD1a antibodies bind to neuromuscular junctions of rat hemidiaphragm. Muscle Nerve. 2012;46:705–10. doi: 10.1002/mus.23385. [DOI] [PubMed] [Google Scholar]
- 31.Gu D, Wogensen L, Calcutt NA, et al. Myasthenia gravis-like syndrome induced by expression of interferon γ in the neuromuscular junction. J Exp Med. 1995;181:547–57. doi: 10.1084/jem.181.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mu L, Zhang Y, Sun B, et al. Activation of the receptor for advanced glycation end products (RAGE) exacerbates experimental autoimmune myasthenia gravis symptoms. Clin Immunol. 2011;141:36–48. doi: 10.1016/j.clim.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Chang HC, Sehra S, Goswami R, et al. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol. 2010;11:527–34. doi: 10.1038/ni.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang HC, Han L, Jabeen R, Carotta S, Nutt SL, Kaplan MH. PU.1 regulates TCR expression by modulating GATA-3 activity. J Immunol. 2009;183:4887–94. doi: 10.4049/jimmunol.0900363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li H, Rostami A. IL-9: basic biology, signaling pathways in CD4+ T cells and implications for autoimmunity. J Neuroimmune Pharmacol. 2010;5:198–209. doi: 10.1007/s11481-009-9186-y. [DOI] [PubMed] [Google Scholar]
- 36.Diehl SA, Schmidlin H, Nagasawa M, et al. STAT3-mediated up-regulation of BLIMP1 Is coordinated with BCL6 down-regulation to control human plasma cell differentiation. J Immunol. 2008;180:4805–15. doi: 10.4049/jimmunol.180.7.4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kong QF, Sun B, Bai SS, et al. Administration of bone marrow stromal cells ameliorates experimental autoimmune myasthenia gravis by altering the balance of Th1/Th2/Th17/Treg cell subsets through the secretion of TGF-β. J Neuroimmunol. 2009;207:83–91. doi: 10.1016/j.jneuroim.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Kong QF, Sun B, Wang GY, et al. BM stromal cells ameliorate experimental autoimmune myasthenia gravis by altering the balance of Th cells through the secretion of IDO. Eur J Immunol. 2009;39:800–9. doi: 10.1002/eji.200838729. [DOI] [PubMed] [Google Scholar]
- 39.Licona-Limon P, Henao-Mejia J, Temann AU, et al. Th9 cells drive host immunity against gastrointestinal worm infection. Immunity. 2013;39:744–57. doi: 10.1016/j.immuni.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu Y, Hong S, Li H, et al. Th9 cells promote antitumor immune responses in vivo. J Clin Invest. 2012;122:4160–71. doi: 10.1172/JCI65459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Purwar R, Schlapbach C, Xiao S, et al. Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells. Nat Med. 2012;18:1248–53. doi: 10.1038/nm.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kerzerho J, Maazi H, Speak AO, et al. Programmed cell death ligand 2 regulates TH9 differentiation and induction of chronic airway hyperreactivity. J Allergy Clin Immunol. 2013;131:1048–57. doi: 10.1016/j.jaci.2012.09.027. 57 e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang HB, Li H, He B, et al. The role of B-cells in experimental myasthenia gravis in mice. Biomed Pharmacother. 1999;53:227–33. doi: 10.1016/S0753-3322(99)80093-6. [DOI] [PubMed] [Google Scholar]
- 44.Petit-Frere C, Dugas B, Braquet P, Mencia-Huerta JM. Interleukin-9 potentiates the interleukin-4-induced IgE and IgG1 release from murine B lymphocytes. Immunology. 1993;79:146–51. [PMC free article] [PubMed] [Google Scholar]
- 45.Vink A, Warnier G, Brombacher F, Renauld JC. Interleukin 9-induced in vivo expansion of the B-1 lymphocyte population. J Exp Med. 1999;189:1413–23. doi: 10.1084/jem.189.9.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fawaz LM, Sharif-Askari E, Hajoui O, Soussi-Gounni A, Hamid Q, Mazer BD. Expression of IL-9 receptor alpha chain on human germinal center B cells modulates IgE secretion. J Allergy Clin Immunol. 2007;120:1208–15. doi: 10.1016/j.jaci.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 47.Fornek JL, Tygrett LT, Waldschmidt TJ, Poli V, Rickert RC, Kansas GS. Critical role for Stat3 in T-dependent terminal differentiation of IgG B cells. Blood. 2006;107:1085–91. doi: 10.1182/blood-2005-07-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Townsend JM, Fallon GP, Matthews JD, Smith P, Jolin EH, McKenzie NA. IL-9-deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity. 2000;13:573–83. doi: 10.1016/s1074-7613(00)00056-x. [DOI] [PubMed] [Google Scholar]
- 49.Steenwinckel V, Louahed J, Orabona C, et al. IL-13 mediates in vivo IL-9 activities on lung epithelial cells but not on hematopoietic cells. J Immunol. 2007;178:3244–51. doi: 10.4049/jimmunol.178.5.3244. [DOI] [PubMed] [Google Scholar]
- 50.Holbrook ST, Ohls RK, Schibler KR, Yang YC, Christensen RD. Effect of interleukin-9 on clonogenic maturation and cell-cycle status of fetal and adult hematopoietic progenitors. Blood. 1991;77:2129–34. [PubMed] [Google Scholar]
- 51.Singh TP, Schon MP, Wallbrecht K, Gruber-Wackernagel A, Wang XJ, Wolf P. Involvement of IL-9 in Th17-associated inflammation and angiogenesis of psoriasis. PLoS ONE. 2013;8:e51752. doi: 10.1371/journal.pone.0051752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang HB, Shi FD, Li H, van der Meide PH, Ljunggren HG, Link H. Role for interferon-γ in rat strains with different susceptibility to experimental autoimmune myasthenia gravis. Clin Immunol. 2000;95:156–62. doi: 10.1006/clim.2000.4850. [DOI] [PubMed] [Google Scholar]
- 53.Milani M, Ostlie N, Wu H, Wang W, Conti-Fine BM. CD4+ T and B cells cooperate in the immunoregulation of experimental autoimmune myasthenia gravis. J Neuroimmunol. 2006;179:152–62. doi: 10.1016/j.jneuroim.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 54.Liu R, La Cava A, Bai XF, et al. Cooperation of invariant NKT cells and CD4+CD25+ T regulatory cells in the prevention of autoimmune myasthenia. J Immunol. 2005;175:7898–904. doi: 10.4049/jimmunol.175.12.7898. [DOI] [PubMed] [Google Scholar]
- 55.Liu R, Zhou Q, La Cava A, Campagnolo DI, Van Kaer L, Shi FD. Expansion of regulatory T cells via IL-2/anti-IL-2 mAb complexes suppresses experimental myasthenia. Eur J Immunol. 2010;40:1577–89. doi: 10.1002/eji.200939792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yao Z, Kanno Y, Kerenyi M, et al. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109:4368–75. doi: 10.1182/blood-2006-11-055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laurence A, Tato CM, Davidson TS, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–81. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]


