Abstract
Leucocyte trafficking is vital for the immune defence. In adults, early tethering and rolling interactions between leucocytes and endothelial cells are mediated by P-, E- and L-selectins and their ligands. In contrast, the role of selectins in migration of mononuclear cells during fetal development in humans remains unknown. We studied the functions of endothelial E- and P-selectins and their counter-receptors during human ontogeny. Immunohistochemical stainings showed that P-selectin is expressed in megakaryocytes and endothelial cells starting from gestational weeks 7 and 11, respectively. Endothelial E-selectin appeared latest, at week 32. Real-time imaging using in vitro flow chamber assays showed that cord blood mononuclear leucocytes used E-, P- and L-selectin and PSGL-1 to roll on and adhere to endothelium under physiological shear stress. These data show that selectins are synthesized and functional before birth in humans and have the potential to mediate the emigration of mononuclear cells and inflammatory responses.
Keywords: adhesion molecules, cell recruitment/emigration, endothelium, ontogeny, selectins
Introduction
Leucocyte trafficking between blood and lymphatic organs is vital for immune surveillance. In adults, the three selectins (endothelial E- and P-selectins and leucocyte L-selectin; CD62E, CD62P and CD62L, respectively) mediate the initial tethering and rolling interactions between circulating leucocytes and vascular endothelial cells.1–3 E-selectin is normally absent from resting endothelium, with the possible exception of skin, but is transcriptionally induced to venules in multiple organs by inflammatory mediators such as tumour necrosis factor (TNF). P-selectin, in contrast, is constitutively synthesized both in endothelial cells and platelets and stored in Weibel–Palade bodies, from which it is rapidly translocated to the surface upon stimulation with inflammatory mediators like thrombin, histamine and platelet-activating factor.
All three selectins are type I transmembrane proteins, which bind to carbohydrate-based ligands.2,3 Sialyl Lewis x, a tetrasaccharide containing α1,3-fucose and α2,3-sialic acid and found as a capping group in certain cell-surface glycoproteins, is a prototype ligand for selectins. Additional sulphation of tyrosines and carbohydrates can improve the binding of P- and L-selectins to their ligands. The major physiological ligand for P-selectin is P-selectin glycoprotein ligand-1 (PSGL-1, CD162), which is expressed at the tips of leucocyte microvillae. However, in T cells the glycosyltransferases needed for production of functionally active PSGL-1 are induced only after activation.4 PSGL-1, together with CD44, is also a major leucocyte ligand for human E-selectin. In addition, several other molecules, including L-selectin, can bind to P- and E-selectins.
In contrast to the adult situation, very little is known about leucocyte trafficking during human ontogeny. Certain endothelial adhesion molecules are developmentally regulated. For instance, the dominant endothelial addressins mucosal cell adhesion molecule (MadCAM-1) and peripheral lymph node addressin (PNAd) controlling lymphocyte traffic to gut and peripheral lymph nodes are already functional at weeks 7 and 15, respectively, in humans.5 Vascular adhesion protein-1, an ecto-enzyme involved in leucocyte traffic is also produced early in the fetal vasculature.6 However, the expression of endothelial selectins during human ontogeny remains incompletely characterized.
Immune responses in fetuses and neonates are thought to be dominated by innate cells.7 It is known that the gestational age correlates with the ability of human cord blood neutrophils to bind to endothelium, and that P-selectin is important in this interaction.8,9 However, < 10% of leucocytes in fetal blood are neutrophils between weeks 18 and 30, whereas 85–88% are lymphocytes and about 3% are monocytes.10 Moreover, lymphocytes populate primary and secondary lymphoid organs early during fetal development.11 T-lymphocyte precursors colonize thymus at weeks 7–8, mature T cells are found in the periphery at weeks 10–12, and B cells in liver at week 9. The lymphocyte recirculation in fetuses is also relevant for maintenance of tolerance by enabling migration of regulatory T cells and T helper type 2 (Th2) cells. Moreover, although normally surrounded by a sterile environment, fetal lymphocytes can already mount adaptive T- and B-cell-dependent immune responses to microbes, such as cytomegalovirus or trypanosomes, early in development.11,12
The functional role of selectins and their counter-receptors in mediating shear-dependent interactions between human fetal mononuclear cells and endothelium have remained completely unexplored. Here, we found that P-selectin is present in fetal endothelial cells from week 11 onwards. Moreover, we show that neonatal mononuclear cells isolated from cord blood are functionally competent in using selectins for rolling under physiologically relevant laminar shear.
Materials and methods
Tissues and cells
Human fetal tissue samples were collected from aborted and stillborn fetuses (7, 11, 13, 15, 18, 23 and 40 weeks, n = 1 to n = 3).5,6 Samples from < 20 weeks of gestation fetuses were from spontaneous or induced abortions performed at the Turku University Central Hospital by the treating obstetricians according to Finnish law. Samples from 23 and 40 weeks of gestation were from stillbirths, who underwent autopsy for clinical reasons at the Department of Pathology. All samples were microscopically and macroscopically normal. The 7-week-old embryo was a whole mount and from the majority of later time-points heart, lung, liver, spleen, kidney, intestine (small or large bowel), skin, thymus, brain and lymph nodes were available. Inflamed tonsils were obtained from children undergoing tonsillectomy.
Cord blood was collected from the umbilical vein from healthy newborns and adult blood from healthy volunteers.5,6 Mononuclear cells (MC) were isolated using density gradient centrifugation over Ficoll. Human umbilical vein endothelial cells (HUVEC) were isolated from term and pre-term (32–36 weeks) deliveries using collagen digestion.13 The cells were cultured in Clonetics™ Endothelial Cell Medium (Lonza, Basel, Switzerland) supplemented with vascular endothelial growth factor A and were used for experiments at passages 1 and 2. All human samples were anonymously used and the Institutional Review Board of Medicolegal Affairs (Helsinki and Turku, Finland) approved the study protocol.
Antibodies
L-Selectin was detected using Dreg-56 (mIgG1; ref. 14). Antibodies against P-selectin were WAPS12.2 (mIgG1; ref. 15), 1E3 (mIgG2a; DAKO, Glostrup, Denmark) and FITC-conjugated AK-6 (mIgG1; AbD Serotec, Oxford, UK). Antibodies against E-selectin were P2H3 (mIgG1, Developmental studies hybridoma bank) and 16G4 (mIgG1; Novocastra™, Leica Biosystems, Nussloch GmbH, Nussloch, Germany) and polyclonal goat serum (BBA18; R&D Systems, Minneapolis, MN). The antibody against CD49d was HP 2.1 (mIgG1; Meridian Life Science, Memphis, TN). Selectin ligands were detected using PL-1 (mIgG1; Ancell, Bayport, MN) against PSGL1, and Heca452 (rIgM; ref. 16) against cutaneous lymphocyte antigen (CLA). CD45-Cy-Chrome (mIgG1; Pharmingen, BD Biosciences, Franklin Lakes, NJ) and CD45-phycoerythrin (mIgG1; BD Bioscience) were used to stain CD45 cells. CD19-Alexa Fluor 700 (BD Bioscience, Franklin Lakes, NJ), CD8-FITC (BD Bioscience), CD4-FITC (eBioscience, San Diego, CA), CD14-Pacific blue (BD Bioscience), CD16-Peridinin chlorophyll protein-Cy5.5 (BD Bioscience) were used to stain leucocyte subpopulations. Negative non-binding control antibodies were 3G6 (mouse IgG1; ref. 17), AK-1 (mIgG1; In Vivo Biotech Services, Hennigsdorf, Germany) and NS-1 (mouse IgG1; ATCC TIB18) and binding, but adhesion non-inhibiting monoclonal antibody (mAb) was anti-HLA-classI mAb HB116 (ATCC, Manassas, VA).
Immunohistochemistry and immunofluorescence staining
Formalin-fixed paraffin-embedded sections were deparaffinized and antigen retrieval was performed using boiling in a citrate buffer or with Proteinase K treatment. Primary antibodies were used at 2 μg/ml or 1 : 50 dilution. The reaction was developed with Vectastain Ultra kits according to the manufacturer's instructions using diaminobenzidine as a chromogen. After counterstaining with haematoxylin and eosin, the slides were examined with an Olympus BX60 microscope (Hamburg, Germany), and micrographs were obtained using the Cell Analysis program. Anti-E-selectin antibodies (P2H3 or polyclonal) did not work with these or other tested (EDTA, pepsin, papain) antigen retrieval methods. Another anti-E-selectin mAb 16G4, which gave good staining with paraffin sections in our hands, was recently reported by the vendor to cross-react with P-selectin.
To stain P-selectin in HUVEC from preterm deliveries, endothelial cells were isolated, cultured to confluency and thereafter seeded onto glass coverslips. Non-stimulated cells were fixed with 4% paraformaldehyde and permeabilized with a 0·2% saponin solution. Cells were incubated with FITC-conjugated AK-6 or with FITC-conjugated negative control antibody 3G6 for 20 min. To intensify the signal, the cells were subjected to secondary antibody Alexa Fluor 488 anti-mouse IgG for 20 min, and finally mounted in Prolong Gold containing the nuclear stain DAPI. All staining and washing steps were performed in saponin-containing buffers. Samples were analysed using a Zeiss LSM510 Meta laser scanning microscope (Jena, Germany).
To stain E-selectin, HUVEC were activated with 100 U/ml TNF-α for 4 hr. Thereafter, the cells were detached using trypsin-EDTA and stained with P2H3 or negative control antibody 3G6 for 20 min on ice followed by a 20-min incubation with FITC-conjugated anti-mouse IgG secondary antibody. Samples were analysed on a FACSCalibur (BD Bioscience).
For selectin ligand analysis, cord blood and adult blood MC were first incubated with antibodies against PSGL-1 (PL-1), CLA (Heca 452), L-selectin (Dreg-56) or negative control (3G6) for 30 min. Thereafter, appropriate FITC anti-mouse IgG or FITC anti-rat IgG was added for 15 min, and after washings the cells were incubated with Cy-Chrome CD45 for 30 min. Samples were analysed on a FACSCalibur (BD Bioscience). To stain CD49d, cord blood and adult blood MC were sequentially incubated with antibodies against CD49d [(HP2.1) or negative control (AK-1)], FITC anti-mouse IgG and CD45-phycoerythrin. To stain for different leucocyte subtypes, CD19-Alexa Fluor 700, CD8-FITC, CD4-FITC, CD14-Pacific blue, CD16-Peridinin chlorophyll protein-Cy5.5 were used in combination with CD45-phycoerythrin. The samples were run using LSRII (BD Bioscience).
In vitro flow chamber assays
Adhesion assays under flow were modified from previously described protocols.13,18 HUVEC (passage 1–2) were grown to confluency on gelatine-coated glass capillaries and thereafter the endothelium was treated with 100 U/ml TNF-α for 4 hr and 5 μm histamine for 10 min. To study endothelial selectins, 20 μg/ml antibodies against E- and P-selectins (P2H3 or WAPS12.2) or negative control antibody (NS-1) were incubated on endothelium for 20 min. To study selectin ligands PSGL-1, L-selectin and CD49d integrin, cord blood and adult MC were treated with antibodies against PSGL-1 (PL-1), L-selectin (Dreg56), CD49d (HP2.1) or a negative control (binding, but non-inhibitory HB-116) at 2 μg/l × 106 peripheral blood mononuclear cells (PBMC) for 15 min before the flow assay. Thereafter, PBMC were washed and resuspended in RPMI-1640 containing 0·1% BSA.
One end of the capillary was connected via tubing to a reservoir of cells and the other end to a syringe pump (Model 22; Harvard Apparatus, Holliston, MA). The RPMI-1640 containing 0·1% BSA was perfused over the endothelium for 1 min at a defined laminar shear stress of 1 dyn/cm2. Thereafter, cord blood or adult MC (1 × 106 cells/ml in RPMI-1640 containing 0·1% BSA) was perfused over the endothelium, and the cells were allowed to interact with endothelium for 5 min at 1 dyn/cm2. Thereafter, 15 fields (each 0·3072 mm2) were recorded for 15 seconds each using an inverted microscope (Olympus IX70, Olympus Optics) and × 100 magnification (HMC10 Plan UIS objective, NA 0,25, Modulation Optics) equipped with a Hoffman modulator (Hoffman modulation contrast and model G3 NA 0,6 condenser, Modulation optics) and a CCD camera (C5405-01, Hamamatsu Photonics, Hamamatsu) connected to a digital video recorder (Panasonic NV-DV10000, Matsushita Electrical Industrial) for later analysis.
All analyses were performed off-line in a blinded manner using analysis 3.0 (Soft Imaging System GmbH, Munster, Germany) software. The cell was counted as rolling when it moved slowly into a direction of flow and it rolled for ≥ 10 μm. Leucocytes rolling on top of already bonded leucocytes (secondary rolling) were excluded. Cells stably bound for the whole 15-second recording period were scored as adherent. On average in one experiment the number of rolling cord blood MC in the presence of control antibody was 60 ± 10 and that of adherent cord blood MC was 537 ± 60 (mean ± SEM, n = 8). The rolling velocity was obtained by measuring the distance travelled by a rolling cell manually frame-by-frame and the time it travelled. For measuring the rolling velocities, 10 rolling cells were counted in negative control-treated HUVEC capillary per experiment (in total eight experiments were analysed).
For the micrographs in Fig.2(a) the original frames from videos were captured using adobe premiere elements 9 software (Adobe Systems Incorporated, San Jose, CA). The representative fields were cut using Corel Photo Paint ×5 and arrows and bars were added using Corel Draw ×5 software. The selected representative video clips were captured and edited (a representative field was cropped and arrows and bars were added) using adobe premiere pro software.
Figure 2.
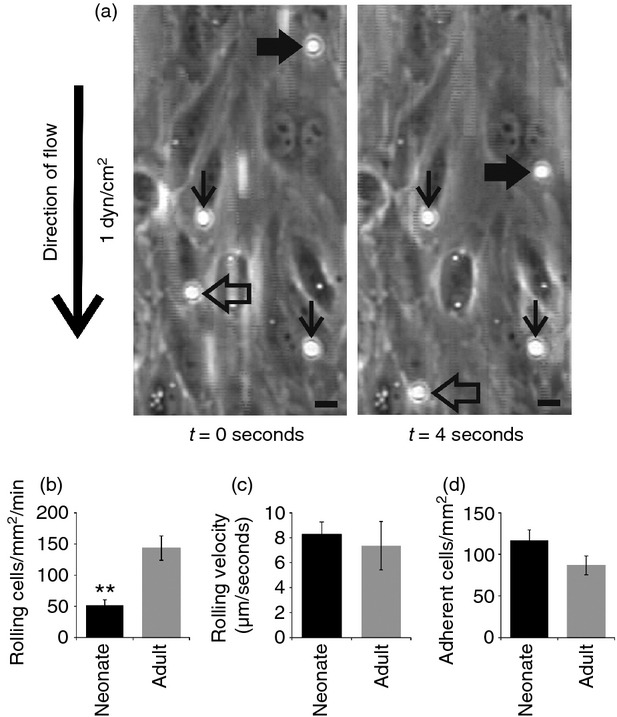
Cord blood mononuclear cells (MC) interact with endothelium under flow conditions. (a) Two representative video frames (4 seconds apart) showing two rolling (thick black and open arrows) and two firmly adherent cord blood MC (thin black arrows) on activated human umbilical vein endothelial cells in a flow assay (1 dyn/cm2). (b–d) Comparison of rolling (b, c) and firm adherence (d) of cord blood and adult blood MC under the flow. **P < 0·01.
Statistical analysis
Two-sided Student's t-test was used for statistical analyses.
Results
P-selectin is already expressed in megakaryocytes, platelets and endothelial cells during the first trimester
To study induction of P-selectin during human ontogeny, we immunohistochemically stained multiple tissue samples from major organs and lymphoid tissues from several different developmental time-points. In normal, non-inflamed fetal samples P-selectin was present in megakaryocytes located in liver already in our earliest specimen from gestational week 7 (Fig.1a; Table1). These cells continued to express P-selectin for the whole intrauterine development period, and platelets seen in the red pulp of spleen became P-selectin positive at week 13.
Figure 1.
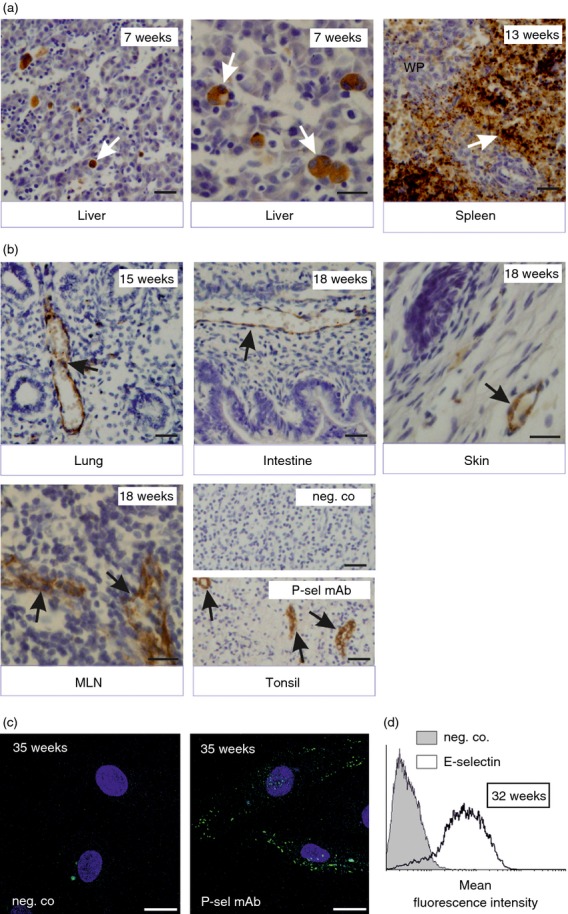
Endothelial selectins are expressed during fetal development in humans. (a–b) Expression of P-selectin in (a) megakaryocytes and platelets and (b) vascular endothelium in fetal organs. Gestational age is indicated, and white arrows point to megakaryocytes and platelets and black arrows point to vessels; WP, white pulp. Scale bar, 50 μm. (c, d) Human umbilical vein endothelial cells (HUVEC) isolated from preterm deliveries were (c) left untreated and stained for negative control and P-selectin and analysed by microscopy (blue is DAPI, scale bar, 20 μm) or (d) stimulated with tumour necrosis factor-α for 4 hr and stained for E-selectin using FACS. Representative data from at least three independent experiments with different donors are shown.
Table 1.
P-selectin expression in human fetuses
| Tissue1 | 7 weeks | 13 weeks | 15 weeks | 18 weeks | 23 weeks | 40 weeks |
|---|---|---|---|---|---|---|
| Lung | − | + | + | − | − | + |
| Intestine | − | ND | + | ND | ND | + |
| Skin | − | − | + | + | ND | + |
| Lymph node | ND | ND | ND | + | ND | + |
| Heart | ND | − | ND | − | − | + |
| Brain | − | ND | − | − | ND | − |
| Kidney | ND | ND | − | − | − | − |
| Spleen | ND | + | + | + | + | + |
| Liver | + | + | + | + | + | + |
+, present; −, absent; ND, not determined (no tissue available).
Expression seen in vessels, except in spleen and liver in which P-selectin is in megakaryocytes/platelets.
On vascular endothelial cells, P-selectin was not present at week 7 (Table1). It first became visible in intestinal vessels at week 11, and endothelial cells at this location remained P-selectin-positive in all available samples from later time-points (Fig.1b; Table1 and data not shown). Vessels in skin were P-selectin negative at weeks 7 and 13, but positive from week 15 onwards. In lung, P-selectin was variably seen in vessels after week 13. High endothelial venules (available in the lymph nodes of 11-, 18- and 40-week samples) also prominently expressed P-selectin. Notably, at each time-point only a few individual vessels were P-selectin positive, whereas the majority of the adjacent vessels remained negative. Representative micrographs of P-selectin expression in lung, intestine, skin and mesenteric lymph nodes are shown in Fig.1(b). Resolution of the immunohistochemistry did not allow separate analyses of luminal and intracellular expression of P-selectin. P-selectin was not found in vessels of brain, kidney or heart during embryonic development (Table1). Confocal analyses of non-stimulated HUVEC isolated from pre-term (32–36 weeks of gestation) umbilical cords also revealed constitutive (cytoplasmic) P-selectin expression in endothelial cells before birth (Fig.1c). Hence, collectively these data show that P-selectin is synthesized from week 7 onwards during the fetal development and that it is localized to platelets and their precursors and focally to vessels both in lymphoid and in certain non-lymphoid tissues.
E-selectin is inducible prenatally
We found no E-selectin antibody that would work with paraffin-embedded sections and would not cross-react with P-selectin (see Materials and methods). Therefore, the developmental expression of E-selectin was studied using FACS and pre-term HUVEC [week 32 (n = 2) and week 36 (n = 2)]. At both 32 and 36 weeks of gestation, HUVEC E-selectin was readily triggered by a short 4-hr pro-inflammatory TNF stimulus (Fig.1d and data not shown). Hence, E-selectin can be induced on fetal endothelial cells at least 2 months before birth.
Mononuclear cord blood leucocytes roll on vascular endothelium under shear
We then wanted to study whether neonatal MC were mature enough to interact with vascular endothelium and dissect the potential role of endothelial selectins in this process. We found that cord blood MC rolled on HUVEC, which were treated with TNF and histamine to induce surface expression of E- and P-selectin (Fig.2a; see Supporting information, Video S1). Cord blood MC also adhered firmly to the endothelium under shear conditions (Fig.2a; see Supporting information, Video S1). In direct comparisons fewer cord blood MC than adult PBMC rolled on HUVEC, but no statistically significant differences between cord blood and adult MC were observed in the rolling velocity (Fig.2b,c). The numbers of firmly adherent cord blood and adult MC were comparable (Fig.2d). Hence, the adhesion efficacy (fraction of firmly adherent cells from all accumulating rolling cells) was higher in cord blood MC than in adult MC (0·46 ± 0·06 versus 0·12 ± 0·02, P < 0·01, n = 6).
Cord blood MC use endothelial E- and P-selectins for rolling
The rolling of neonate MC on HUVEC was significantly diminished when E- or P-selectin was blocked with mAbs (Fig.3a). Simultaneous inhibition of both endothelial selectins did not further augment the reduction of rolling. Blockade of endothelial E- or P-selectin alone showed a trend of impaired firm adhesion of cord blood MC, which became statistically significant when both selectins were blocked simultaneously (Fig.3c). This effect appeared to be secondary to the reduced rolling, as the adhesion efficacy (defined as the fraction of firmly adherent cells from all rolling cells) was not affected (Fig.3e). In the same flow assays (HUVEC from the same donors) adult MC also used selectins mainly for rolling, as expected (Fig.3b,d,f). These functional data suggest that neonate MC can use endothelial selectins for rolling at the time of birth.
Figure 3.
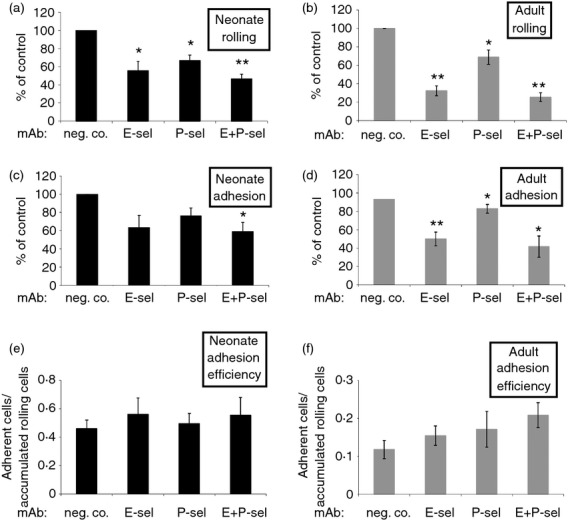
Endothelial E- and P-selectins support rolling of cord blood mononuclear cells (MC) under flow conditions. The effect of inhibition (mean ± SEM, n = 4 to n = 8 independent experiments using MC and activated human umbilical vein endothelial cells from different individuals) of E-selectin and P-selectin with monoclonal antibodies on (a, b) rolling, (c, d) firm adhesion and (e, f) adhesion efficacy (fraction of adherent cells from all rolling cells) of neonatal and adult MC under the flow. *P < 0·05; **P < 0·01.
Cord blood MC express PSGL-1 and L-selectin, and reduced levels of CLA
L-Selectin and PSGL-1 are potential leucocyte receptors, which could be involved in selectin-dependent rolling. We found that both L-selectin and PSGL-1 protein were expressed on the majority of CD45-positive cord blood MC (Fig.4a–c). The surface levels of these two adhesion receptors were similar on cord blood MC and on adult PBMC. Cutaneous lymphocyte-associated antigen CLA, a unique glycoform of PSGL-119 mediating leucocyte homing to skin, was also found on a small subpopulation of cord blood MC. However, CLA was present on clearly fewer cord blood than adult MC. Hence, PSGL-1 protein, the major leucocyte ligand of endothelial selectins, is expressed on cord blood MC, but its glycosylation status may be different when compared with adult MC.
Figure 4.
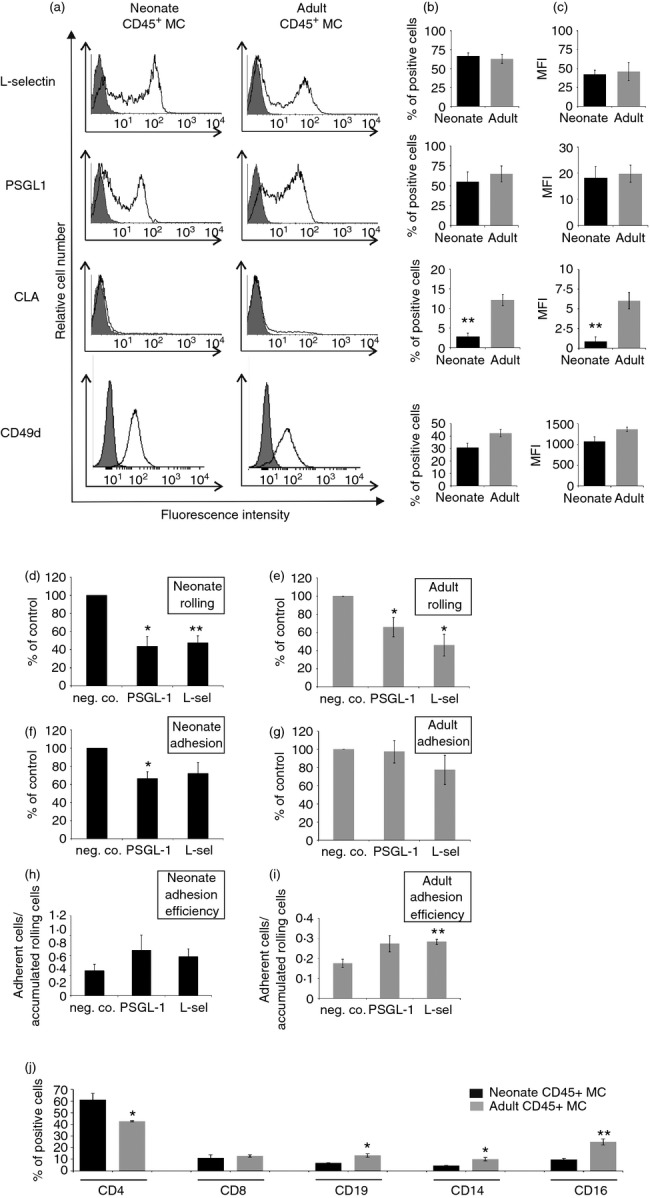
Cord blood mononuclear cell (MC) P-selectin glycoprotein ligand-1 (PSGL-1) and L-selectin mediate rolling under laminar shear. Cord blood MC and adult peripheral blood mononuclear cells (PBMC) were double stained for CD45 and the indicated adhesion molecules, and analysed using FACS. (a) Representative histograms, (b) percentage of positive cells and (c) mean fluorescence intensities are shown (mean ± SEM; n = 3 to n = 5 different individuals). (d, e) Rolling, (f, g) firm adherence and (h, i) adhesion efficacy of cord and adult blood MC in the flow assay (1 dyn/cm2) was determined in the presence of monoclonal antibodies against PSGL-1, L-selectin, or negative control. The results are (mean ± SEM, n = 4 or 5 independent experiments using MC and activated human umbilical vein endothelial cells from different individuals). (j) Distribution of different leucocyte subpopulations among CD45+ cord blood and adult MC was determined using FACS [percentage of positive cells (mean ± SEM; n = 3 different individuals/group)]. *P < 0·05; **P < 0·01.
PSGL-1 and L-selectin mediate fetal MC interactions with endothelium under shear
To study the functional importance of different ligands of endothelial selectins in mediating cord blood MC–endothelial interactions under shear conditions, antibody inhibition studies were performed. Blocking of either PSGL-1 or L-selectin on cord blood MC reduced the number of rolling cells by > 50 % (Fig.4d,e). PSGL-1 blockade also diminished the number of firmly adherent cord blood MC, but not the adhesion efficacy, whereas L-selectin blockade had no significant effect (Fig.4f–i). In adult PBMC, rolling was also L-selectin and PSGL-1 dependent. The role of CLA could not be tested because the anti-CLA antibody is not antagonistic.
Finally, the effect of simultaneous inhibition of all three selectins (E- and P-selectin on endothelium and L-selectin on MC) was analysed. The results showed that combined blockade of all selectins reduced cord blood MC rolling by 63·9 ± 3·4% (mean ± SEM, n = 4, P = 0·00003; see Supporting information, Video S2) compared with control mAb treatments, like that of adult MC [52·2 ± 10·4% (mean ± SEM), n = 4, P = 0·015]. Residual rolling and adhesion were largely mediated by CD49d–vascular cell adhesion molecule-1 interactions, because simultaneous blocking of CD49d in addition to E- and P-selectin further reduced adult and cord blood MC rolling (by 52·1 ± 3·4%, n = 3, P = 0·019 and 49·1 ± 23·4%, n = 4, P = 0·13, respectively) and adhesion (52,1 ± 1·8%, n = 3, P = 0·001 and 74·2 ± 7·3%, n = 4, P = 0·002, respectively). Hence, cord blood MC display functionally active PSGL-1, L-selectin, and integrin α4, which mediate rolling/adhesion on endothelium under laminar shear.
Discussion
We show here that endothelial selectins are expressed already during fetal development in humans. Under normal conditions P-selectin was present in embryos from week 11 onwards both in megakaryocytes and endothelial cells and E-selectin became detectable at least 2 months before birth. L-selectin, PSGL-1 and CLA were expressed on cord blood MC at the time of birth, and the selectins and their ligands were functional in mediating the rolling of cord blood MC on inflamed endothelium under physiologically relevant shear stress.
The dominant endothelial addressins MadCAM-1 and PNAd controlling lymphocyte traffic to gut and peripheral lymph nodes are functional in humans already at weeks 7 and 15, respectively, in mediating lymphocyte adhesion to high endothelial venules in in vitro assays.5 Moreover, vascular adhesion protein-1 is expressed from week 7 onwards and is active in mediating lymphocyte binding in vitro.6 Here we found P-selectin from week 11 onwards in endothelial cells in different major organs. It was also expressed in certain high endothelial venules already at week 11, which is relatively early after the appearance of these venules, specialized for lymphocyte extravasation.20 Previously, only cutaneous and intestinal expression of P-selectin has been analysed at selected time-points.21–23 It was found in dermal vessels at weeks 11–16, whereas 18–22 weeks mesenteric vessels were P-selectin negative. Collectively these data imply that P-selectin, if it is present or inducible also at the luminal surface of endothelial cells, may support lymphocyte (and other leucocyte) trafficking very early during human development.
Analyses of E-selectin expression during ontogeny was hampered by the lack of specific antibodies that can be used on paraffin-embedded archived material. However, our in vitro induction experiments with pre-term HUVEC implied that E-selectin can be induced at least 2 months before full-term birth, which is consistent with another study.8 In analyses of frozen sections from skin and mesentery, E-selectin was found to be practically absent at weeks 11–22.21–23 Hence, E-selectin can be induced on fetal endothelial cells at least 2 months before birth, but it is probably not physiologically present on non-inflamed vessels during human ontogeny.
The major leucocyte ligand for endothelial selectins, PSGL-1, was also expressed and functional on cord blood MC, indicating that it carried the appropriate post-translational modifications, which are developmentally regulated in myeloid cells.24 We observed no significant differences between PSGL-1 and L-selectin expression between neonatal and adult MC, which is in contrast to cord blood granulocytes, on which PSGL-1 and L-selectin are significantly lower than in adult granulocytes.8,9,25 We also found CLA on cord blood MC, albeit at reduced levels when compared with adult PBMC. This is consistent with the observation that the CLA epitope in endothelium and macrophages is found at week 11 in developing lymph nodes.26 These data suggest that MC in fetal blood are equipped with a repertoire of selectin ligands allowing interactions with endothelial selectins.
Our data show that selectins are functional at the time of birth to mediate rolling of cord blood MC on stimulated endothelium. The percentage of rolling of cord blood MC on endothelium under shear conditions was somewhat lower than that of adult cells. However, there was no difference in the rolling velocity or number of adherent cells, and hence the transition of rolling cells to firmly adherent cells was more effective in cord blood MC. Our aim was to study whether immature cord blood MC as a population can use the selectin adhesion system, and therefore we performed the rolling assays and FACS stainings with the total pool of MC. Since many different leucocyte subpopulations, including CD4+ T helper cells, CD8+ T cytotoxic cells, CD19+ B cells, CD14+ monocytes and CD16+ natural killer cells are included in MC (Fig.4j), the rolling and staining data could be refined in future studies to dissect the behaviour of individual subpopulations. For instance, in mouse Th1, Th2 and Th17 cells seem to use endothelial selectins differently for extravasation.27,28 However, cord blood mainly contains undifferentiated CD4 cells, and it was not possible to isolate sufficient numbers of highly purified other subpopulations of cord blood MC (CD4, CD8, B cells or monocytes) for the functional flow assays, and therefore the dissection of the contribution of each selectin–ligand pair in different subpopulations for MC–endothelial interaction awaits future experimentation. Nevertheless, our data show that selectin-mediated rolling is likely to be functional during human ontogeny to direct MC migration to non-inflamed lymphoid tissues as well as to sites of inflammation. Moreover, the fact that substantial numbers of cord blood MC still rolled on endothelium when all three selectins were blocked, strongly suggests that alternative molecules, such as α4-containing integrins, which support rolling in adults,29 are also relevant for early interactions between these two cell types under shear.
Analyses of lymphocyte trafficking during the development in humans is essential, as it differs dramatically from that in rodents. For instance, mature lymphocytes appear in periphery only during the third trimester in mice, whereas they are already present during the first trimester in humans.11 Hence, the present study adds to our knowledge of the mechanisms by which lymphocytes can populate lymphoid organs during ontogeny and how adaptive immunity can be brought into the play to protect the fetus against intrauterine infections.
In conclusion, endothelial selectins are expressed and functional in humans during gestation. Cord blood MC can roll on and adhere to vascular endothelium under physiological shear stress using selectins. Together these data imply that cells of adaptive immune system already have the potential to use a selectin-dependent extravasation cascade for dispersing lymphocytes to lymphoid organs and areas of infection before birth.
Acknowledgments
This work was supported by the Academy of Finland. We thank Ms Riikka Sjöroos, Etta Väänänen and Pirjo Heinilä for expert technical help and Ms Anne Sovikoski-Georgieva for secretarial help.
Glossary
- CLA
cutaneous lymphocyte antigen
- HUVEC
human umbilical vein endothelial cell(s)
- mAb
monoclonal antibody
- MC
mononuclear cell(s)
- PSGL-1
P-selectin glycoprotein ligand-1
- Th1
T helper type 1
- TNF
tumour necrosis factor
Author contributions
All authors have been involved in the conception and design of the study, acquisition of data, and analysis and interpretation of data, in drafting the article and they have given final approval of the version to be submitted.
Disclosures
The authors declare no conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Video S1. Rolling and adhesion of cord blood mononuclear cells to histamine- and tumour necrosis factor-α-activated human umbilical vein endothelial cell monolayer under flow in the presence of negative control antibodies.
Video S2. Selectin blockade inhibits cord-blood mononuclear cell rolling and adhesion under flow.
References
- 1.Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nat Rev Immunol. 2013;13:309–20. doi: 10.1038/nri3442. [DOI] [PubMed] [Google Scholar]
- 2.Zarbock A, Ley K, McEver RP, Hidalgo A. Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood. 2011;118:6743–51. doi: 10.1182/blood-2011-07-343566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–89. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 4.Vachino G, Chang X-J, Veldman GM, Kumar R, Sako D, Fouser LA, Berndt MC, Cumming DA. P-selectin glycoprotein ligand-1 is the major counter-receptor for P-selectin on stimulated T cells and is widely distributed in non-functional form on many lymphocytic cells. J Biol Chem. 1995;270:21966–74. doi: 10.1074/jbc.270.37.21966. [DOI] [PubMed] [Google Scholar]
- 5.Salmi M, Alanen K, Grenman S, Briskin M, Butcher EC, Jalkanen S. Immune cell trafficking in uterus and early life is dominated by the mucosal addressin madcam-1 in humans. Gastroenterology. 2001;121:853–64. doi: 10.1053/gast.2001.27968. [DOI] [PubMed] [Google Scholar]
- 6.Salmi M, Jalkanen S. Developmental regulation of the adhesive and enzymatic activity of vascular adhesion protein-1 (VAP-1) in humans. Blood. 2006;108:1555–61. doi: 10.1182/blood-2005-11-4599. [DOI] [PubMed] [Google Scholar]
- 7.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–90. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 8.Nussbaum C, Gloning A, Pruenster M, et al. Neutrophil and endothelial adhesive function during human fetal ontogeny. J Leukoc Biol. 2013;93:175–84. doi: 10.1189/jlb.0912468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mariscalco MM, Tcharmtchi MH, Smith CW. P-Selectin support of neonatal neutrophil adherence under flow: contribution of L-selectin, LFA-1, and ligand(s) for P-selectin. Blood. 1998;91:4776–85. [PubMed] [Google Scholar]
- 10.Forestier F, Daffos F, Catherine N, Renard M, Andreux JP. Developmental hematopoiesis in normal human fetal blood. Blood. 1991;77:2360–3. [PubMed] [Google Scholar]
- 11.Mold JE, McCune JM. Immunological tolerance during fetal development: from mouse to man. Adv Immunol. 2012;115:73–111. doi: 10.1016/B978-0-12-394299-9.00003-5. [DOI] [PubMed] [Google Scholar]
- 12.Dauby N, Goetghebuer T, Kollmann TR, Levy J, Marchant A. Uninfected but not unaffected: chronic maternal infections during pregnancy, fetal immunity, and susceptibility to postnatal infections. Lancet Infect Dis. 2012;12:330–40. doi: 10.1016/S1473-3099(11)70341-3. [DOI] [PubMed] [Google Scholar]
- 13.Koskinen K, Vainio PJ, Smith DJ, Pihlavisto M, Yla-Herttuala S, Jalkanen S, Salmi M. Granulocyte transmigration through endothelium is regulated by the oxidase activity of vascular adhesion protein-1 (VAP-1) Blood. 2004;103:3388–95. doi: 10.1182/blood-2003-09-3275. [DOI] [PubMed] [Google Scholar]
- 14.Kishimoto TK, Jutila MA, Butcher EC. Identification of a human peripheral lymph node homing receptor: a rapidly down-regulated molecule. Proc Natl Acad Sci USA. 1990;87:2244–8. doi: 10.1073/pnas.87.6.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jutila MA, Bargatze RF, Kurk S, Warnock RA, Ehsani N, Watson SR, Walcheck B. Cell surface P- and E-selectin support shear-dependent rolling of bovine g/d T cells. J Immunol. 1994;153:3917–28. [PubMed] [Google Scholar]
- 16.Picker LJ, Michie SA, Rott LS, Butcher EC. A unique phenotype of skin-associated lymphocytes in humans. Preferential expression of the HECA-452 epitope by benign and malignant T cells at cutaneous sites. Am J Pathol. 1990;136:1053–68. [PMC free article] [PubMed] [Google Scholar]
- 17.Salmi M, Jalkanen S. A 90-kilodalton endothelial cell molecule mediating lymphocyte binding in humans. Science. 1992;257:1407–9. doi: 10.1126/science.1529341. [DOI] [PubMed] [Google Scholar]
- 18.Salmi M, Koskinen K, Henttinen T, Elima K, Jalkanen S. CLEVER-1 mediates lymphocyte transmigration through vascular and lymphatic endothelium. Blood. 2004;104:3849–57. doi: 10.1182/blood-2004-01-0222. [DOI] [PubMed] [Google Scholar]
- 19.Fuhlbrigge RC, Kieffer JD, Armerding D, Kupper TS. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature. 1997;389:978–81. doi: 10.1038/40166. [DOI] [PubMed] [Google Scholar]
- 20.Bailey RP, Weiss L. Light and electron microscopic studies of postcapillary venules in developing human fetal lymph nodes. Am J Anat. 1975;143:43–58. doi: 10.1002/aja.1001430103. [DOI] [PubMed] [Google Scholar]
- 21.Dogan A, MacDonald TT, Spencer J. Ontogeny and induction of adhesion molecule expression in human fetal intestine. Clin Exp Immunol. 1993;91:532–7. doi: 10.1111/j.1365-2249.1993.tb05937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davies JR, Dyson M, Mustafa Y, Compton F, Perry ME. The ontogeny of adhesion molecules expressed on the vascular endothelium of the developing human skin. J Anat. 1996;189(Pt 2):373–82. [PMC free article] [PubMed] [Google Scholar]
- 23.Lorant DE, Li W, Tabatabaei N, Garver MK, Albertine KH. P-selectin expression by endothelial cells is decreased in neonatal rats and human premature infants. Blood. 1999;94:600–9. [PubMed] [Google Scholar]
- 24.Hidalgo A, Frenette PS. Enforced fucosylation of neonatal CD34+ cells generates selectin ligands that enhance the initial interactions with microvessels but not homing to bone marrow. Blood. 2005;105:567–75. doi: 10.1182/blood-2004-03-1026. [DOI] [PubMed] [Google Scholar]
- 25.Tcharmtchi MH, Smith CW, Mariscalco MM. Neonatal neutrophil interaction with P-selectin: contribution of P-selectin glycoprotein ligand-1 and sialic acid. J Leukoc Biol. 2000;67:73–80. doi: 10.1002/jlb.67.1.73. [DOI] [PubMed] [Google Scholar]
- 26.Horst E, Meijer CJLM, Duijvestijn AM, Hartwig N, Van der Harten HJ, Pals ST. The ontogeny of human lymphocyte recirculation: high endothelial cell antigen (HECA-452) and CD44 homing receptor expression in the development of the immune system. Eur J Immunol. 1990;20:1483–9. doi: 10.1002/eji.1830200712. [DOI] [PubMed] [Google Scholar]
- 27.Austrup F, Vestweber D, Borges E, et al. P-and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflamed tissues. Nature. 1997;385:81–3. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 28.Alcaide P, Maganto-Garcia E, Newton G, Travers R, Croce KJ, Bu DX, Luscinskas FW, Lichtman AH. Difference in Th1 and Th17 lymphocyte adhesion to endothelium. J Immunol. 2012;188:1421–30. doi: 10.4049/jimmunol.1101647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alon R, Kassner PD, Carr MW, Finger EB, Hemler ME, Springer TA. The integrin VLA-4 supports tethering and rolling in flow on VCAM-1. J Cell Biol. 1995;128:1243–53. doi: 10.1083/jcb.128.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Rolling and adhesion of cord blood mononuclear cells to histamine- and tumour necrosis factor-α-activated human umbilical vein endothelial cell monolayer under flow in the presence of negative control antibodies.
Video S2. Selectin blockade inhibits cord-blood mononuclear cell rolling and adhesion under flow.


