Abstract
At least three phenotypically and morphologically distinguishable types of branched stromal cells are revealed in the human splenic white pulp by subtractive immunohistological double-staining. CD271 is expressed in fibroblastic reticulum cells of T-cell zones and in follicular dendritic cells of follicles. In addition, there is a third CD271− and CD271+/− stromal cell population surrounding T-cell zones and follicles. At the surface of follicles the third population consists of individually variable partially overlapping shells of stromal cells exhibiting CD90 (Thy-1), MAdCAM-1, CD105 (endoglin), CD141 (thrombomodulin) and smooth muscle α-actin (SMA) with expression of CD90 characterizing the broadest shell and SMA the smallest. In addition, CXCL12, CXCL13 and CCL21 are also present in third-population stromal cells and/or along fibres. Not only CD27+ and switched B lymphocytes, but also scattered IgD++ B lymphocytes and variable numbers of CD4+ T lymphocytes often occur close to the third stromal cell population or one of its subpopulations at the surface of the follicles. In contrast to human lymph nodes, neither podoplanin nor RANKL (CD254) were detected in adult human splenic white pulp stromal cells. The superficial stromal cells of the human splenic white pulp belong to a widespread cell type, which is also found at the surface of red pulp arterioles surrounded by a mixed T-cell/B-cell population. Superficial white pulp stromal cells differ from fibroblastic reticulum cells and follicular dendritic cells not only in humans, but apparently also in mice and perhaps in rats. However, the phenotype of white pulp stromal cells is species-specific and more heterogeneous than described so far.
Keywords: human spleen, immunohistology, splenic stromal cells, splenic white pulp
Introduction
In human and rodent spleens T or B lymphocytes inhabit different compartments of the white pulp.1–3 T lymphocytes are concentrated in elongated periarterial lymphatic sheaths (PALSs), which surround central arteries, while B lymphocytes prevail in round accumulations termed follicles. The stromal cells providing the scaffold for migrating T lymphocytes in the PALS are named fibroblastic reticulum cells (FRCs), whereas the follicles are supported by follicular dendritic cells (FDCs). The shape and phenotype of both stromal cell types is different.4–6 In rodents and humans the PALS and the follicles can be divided into sub-compartments. Hence, in rats the outer PALS is a migration area for B cells recirculating to or from the follicles and for plasmablasts.7,8 In mice and rats both PALS and follicles are delimited from the splenic red pulp by the marginal zone (MZ), which is most prominent in rats. In both rodent species this compartment is regarded as a separate part of the white pulp. It houses a special type of more or less sessile polyreactive B cells, which can be phenotypically differentiated from follicular B cells.9–11 In rodents and humans MZ B cells express surface IgM but only reduced amounts of IgD.12 Whether MZ B cells represent memory B cells or not is still debated in rodents.9–12 It is very likely that human MZ B cells consist of a heterogeneous B-cell population.13 The MZ is also inhabited by special macrophage populations in mice and rats.14–16
In contrast to mice and rats, the splenic MZ is difficult to localize in humans. Up to now, the human MZ has been associated with a superficial accumulation of CD27+ IgM+ IgD+/− B cells in and around follicles. The human MZ cannot be recognized as a separate compartment, because in contrast to mice and rats humans do not exhibit a marginal sinus or marginal metallophilic macrophages, which delimit the MZ from the follicles or the PALS.17–20 Hence, in humans the CD27+ MZ B cells partially intermingle with the small recirculating CD27− IgM+ IgD+ B cells of the follicular mantle zone. The human MZ has a complex structure, because the superficial follicular area is subdivided into an inner marginal zone (iMZ) and an outer marginal zone (oMZ).19,20 The inner part of the follicle including the iMZ is scaffolded by FDCs.21,22 The iMZ therefore belongs to the follicle proper, i.e. it is more appropriately described as an outer part of the mantle zone occupied by CD27+ IgM+ IgD+/− large B cells. In contrast, the stromal cells of the oMZ, which express smooth muscle α-actin (SMA), mucosal addressin cell adhesion molecule-1 (MAdCAM-1) or CD141, are continuous with the stromal cells of the superficial PALS.18–20 Part of the oMZ stromal cells is often accompanied by a crescent-shaped arrangement of CD4+ T cells. The oMZ is also inhabited by CD27+ B cells, but the staining intensity of these cells is less than in the iMZ and they are more widely spaced. In addition, scattered IgD++ B cells also occur in the oMZ.18–20 Hence, the superficial regions of human splenic follicles exhibit a complex and species-specific cell arrangement which is morphologically and phenotypically different from that in mice or rats.
Precisely defining the human MZ equivalent is of importance, because MZ B cells have been shown to fulfil decisive functions for combating bacteria carrying polysaccharide capsules.23 In addition, MZ B cells are regarded as pre-activated cells that are easily stimulated to differentiate into IgM-secreting plasma cells.12,24,25 Human MZ B cells therefore function in T-cell-independent immune reactions, but may also be recruited into T-cell-dependent antibody production involving somatic hypermutation and immunoglobulin isotype switching.12,23 It is still controversial, whether human MZ B cells represent a unique B-cell lineage with an a priori pre-diversified repertoire existing independent of germinal centre formation24,26–29 or whether they are a special population of post-germinal centre cells.30 Several recent reviews23 suppose that both opinions are correct, but that at least a subpopulation of human MZ B cells has a propensity to react like cells of the innate immune system due to immediate differentiation after co-stimulation by Toll-like receptors.
A large proportion of human MZ B cells express the CD27 surface molecule belonging to the tumour necrosis factor-receptor family. In human blood, CD27+ B cells represent 15–20% of all circulating B cells.31 Hence, human MZ B cells most probably recirculate, a behaviour that has long been rejected for rodent MZ B cells.32 However, recent33 and previous34 findings in mice and rats indicate that MZ B cells are at least able to migrate to splenic follicles after appropriate stimulation or that they even permanently shuttle to and from the follicles.33,35
In humans, splenectomy or gut pathologies lead to elimination or to a dramatic decrease in blood CD27+ B cells and B memory cells.36–38 It is supposed that the loss of MZ B cells and of one of the largest phagocytic compartments of the body predisposes humans to overwhelming sepsis after splenectomy.39 An additional interpretation of the phenomenon is that splenectomy removes the decisive recirculation compartment, which maintains MZ B-cell vitality and function. MZ B-cell survival may depend on bacterial or viral components that are directly extracted from the blood, because the human spleen most likely has an entirely open microcirculation.40 This means, that capillaries of the human splenic red pulp do not join the venous sinuses, but pour their blood into the loose connective tissue of the splenic cords through open ends. This is especially evident in the perifollicular zone where erythrocytes, neutrophils and monocytes accumulate in spaces without endothelial lining superficial to the CD27+ MZ B cells.1,18,41 The human perifollicular zone is most likely perfused by special circumfollicular arterioles, which continue into an open perifollicular network of sheathed and non-sheathed capillaries. Capillary sheaths form a human-specific B-cell compartment at the follicular surface and in the entire splenic red pulp.22 It is well documented in humans and animals that at least erythrocytes finally enter the splenic sinuses from the surrounding connective tissue through openings between the sinus endothelial cells.42,43
Our present investigation is intended to more precisely define the stromal cells of the splenic superficial T- and B-cell zones in humans with emphasis on perifollicular stromal cells adjacent to CD27+ B cells. The two most crucial antigens detected are CD271 and MAdCAM-1. CD271 designates the low-affinity nerve growth factor receptor (NGFR, p75), which has been described to be present in neural crest-derived cells and mesenchymal stem cells in the bone marrow, in the dental pulp and in other locations in humans and mice.44 The function of CD271 in these cells is not clear. It has been speculated that CD271 is involved in inhibiting their differentiation.45 There have been several reports that reagents detecting NGFR p75 react with human FDCs.46–48 MAdCAM-1 is expressed in mouse spleens.49 It serves as an endothelial adhesion molecule mediating entry of lymphocytes into the gut wall and into other mucosa-associated lymphoid tissues in adult mice.50,51
We demonstrate a third population of stromal cells superficial to FRCs and FDCs. These cells are also present in red pulp periarteriolar lymphocyte sheaths, which represent neither T-cell nor B-cell regions.
Materials and methods
Specimens
All single-staining experiments were performed in paraffin sections of the spleen of a 22-year-old male who had died of an extra-abdominal trauma. The distribution of CD271 was tested in five additional spleen specimens of patients with splenic trauma. Most of the double-staining reactions shown in Table2 were performed in three to five different individuals aged 17–73 years. If necessary, cryosections of the spleen of a 17-year-old male with extra-abdominal trauma were used. Selected problems were also investigated in cryosections of three additional individuals aged 15–51 years.
Table 2.
Double staining combinations tested
| First antigen (DAB) | Antibody dilution | Second antigen (Fast Blue) | Antibody dilution and comment |
|---|---|---|---|
| SMA | 1 : 500 | MAdCAM-1 | 1 : 10, cryosections |
| SMA | 1 : 50 | CD27 | 1 : 20, special protocol, tyramide amplification19 |
| SMA | 1 : 500 | CD20 | 1 : 800 |
| SMA | 1 : 500 | CD141 | 1 : 500 |
| SMA | 1 : 500 | CD271 | 1 : 80 |
| SMA | 1 : 500 | CD3 | 1 : 200 |
| SMA | 1 : 500 | CD90 | 1 : 1000, cryosections |
| CD105 | 1 : 1000 | MAdCAM-1 | 1 : 10, cryosections |
| CD105 | 1 : 1000 | SMA | 1 : 100, cryosections |
| CD105 | 1 : 500 | CD141 | 1 : 800, cryosections |
| CD105 | 1 : 3000 | CD271 | 1 : 100, cryosections |
| CD90 | 1 : 3000 | MAdCAM-1 | 1 : 10, cryosections |
| CD90 | 1 : 3000 | SMA | 1 : 100, cryosections |
| CD90 | 1 : 3000 | CD271 | 1 : 100, cryosections |
| CD271 | 1 : 500 | SMA | 1 : 100 |
| CD271 | 1 : 400 | CCL21 | 1 : 1000, special antigen retrieval |
| CD271 | 1 : 100 | CD141 | 1 : 200 |
| CD271 | 1 : 500 | MAdCAM-1 | 1 : 10, cryosections |
| CD271 | 1 : 500 | IgD | 1 : 800 |
| CD271 | 1 : 800 | IgM | 1 : 2000–1 : 4000 |
| CD271 | 1 : 800 | CD20 | 1 : 800 |
| CD271 | 1 : 500 | CD27 | 1 : 50, Fast Blue, then DAB, tyramide amplification |
| CD141 | 1 : 1500 | CD271 | 1 : 80 |
| CD141 | 1 : 800 | MAdCAM-1 | 1 : 10, cryosections |
| CXCL13 | 1 : 10000 | CD271 | 1 : 200 |
| CD20 | 1 : 400 | CD271 | 1 : 500 |
| CD3 | 1 : 400 | CD271 | 1 : 500 |
| CD3 | 1 : 500 | CD20 | 1 : 800 |
| CD4 | 1 : 200 | CD271 | 1 : 100 |
| IgM | 1 : 1000 | CD20 | 1 : 800 |
| IgM | 1 : 1000 | IgD | 1 : 200 |
| IgD | 1 : 2500 | CD20 | 1 : 800 |
| IgD | 1 : 2000 | CD271 | 1 : 100 |
| IgD | 1 : 2000 | SMA | 1 : 100 |
| CNA.42 | 1 : 40 | MAdCAM-1 | 1 : 10, cryosections |
| MAdCAM-1 | 1 : 10 | CNA.42 | 1 : 20, cryosections |
| MAdCAM-1 | 1 : 20 | CD271 | 1 : 200, cryosections |
| MAdCAM-1 | 1 : 20 | CD141 | 1 : 500, cryosections |
| MAdCAM-1 | 1 : 10 | SMA | 1 : 100, cryosections |
The specimens were obtained by cooperation with members of the Clinics for Visceral, Thoracic and Vascular Surgery and of the Institute of Pathology of Marburg University Hospital between 1994 and 2000. The regulations for patients’ consent valid at that time were followed.
Pretreatment of sections
Paraffin sections were dewaxed and treated with glucose oxidase (Sigma, St Louis, MO; No. G-6641 at 100 U/ml in PBS, pH 7·2, containing 20 mm β-d-glucose and 2 mm NaN3) for 1 hr at 37° to remove endogenous peroxidase activity. The standard antigen retrieval was autoclaving in citrate buffer pH 6·0. The polyclonal anti-CCL21 reagent was applied after autoclaving at pH 8·0 with 1 mm EDTA/H2O as published.52 Monoclonal antibody (mAb) TM1009 (anti-CD141) could be used without antigen retrieval. The mAbs AS02 (anti-CD90), 10A6 (anti-MAdCAM-1), 18H5 (anti-podoplanin), 4D5aE5E6 (anti-podoplanin), polyclonal anti-receptor activator of nuclear factor-κB ligand (RANKL) and anti-CD105 gave optimal staining only in cryosections. These sections were fixed in 100% isopropanol for 10 min at 4° before glucose oxidase treatment and staining.
Single staining
The antibodies (Table1) were diluted as indicated for the first antigen in Table2 using PBS/1% BSA/0·1% NaN3 containing 0·003 mg/ml avidin. They were applied overnight at 4°. Then the sections were incubated with the biotinylated secondary anti-mouse or anti-rabbit antibody of the Vectastain Elite Kit for Peroxidase (Vector Laboratories, Burlingame, CA; No. BA-9200, via Alexis, Grünberg, Germany) for 30 min at room temperature as recommended by the supplier with a final concentration of 0·02 mg/ml biotin in the solution. The mAb CNA.42 was detected with a biotinylated anti-mouse IgM (Vector Laboratories No. BA-2020). Subsequently, the avidin-biotinylated peroxidase complex was prepared according to the instructions and also applied for 30 min at room temperature. Peroxidase activity was finally visualized in brown colour by a diaminobenzidine reaction. With the exception of the controls the sections were counterstained with Mayer's haemalum if necessary, dehydrated and coverslipped in Eukitt. A negative control using the Vectastain kit without a primary antibody was included in each experiment.
Table 1.
Antibodies used
| Specificity (human) | Reagent name | Distributor (Germany) | Product no. | Type of section |
|---|---|---|---|---|
| Podoplanin | 4D5aE5E6 | Reliatech, Braunschweig | 101-M41 | Cryo + paraffin |
| Podoplanin | 18H5 | Reliatech, Braunschweig | 101-M40 | Only cryo |
| RANKL (CD254) | Polyclonal | Peprotech, Hamburg | 500-P133 | Only cryo |
| RANKL (CD254) | 2.1_3C12-2D11 | Peprotech, Hamburg | 500-M46 | Cryo, weak in paraffin |
| CD105 | Polyclonal | Reliatech, Braunschweig | 102-PA60 | Only cryo |
| CD20 | L26 | DAKO, Hamburg | M0755 | Cryo + paraffin |
| CD3 | Polyclonal | DAKO, Hamburg | A 0452 | Cryo + paraffin |
| CD4 | 4B12 | DAKO, Hamburg | M 7310 | Cryo + paraffin |
| CD90 | AS02 | Dianova, Hamburg | Dia 100 | Only cryo |
| Smooth muscle α-actin | Asm-1 | Progen, Heidelberg | 61001 | Cryo + paraffin |
| MAdCAM-1 | 10A6 | M. Briskin | Reference 51 | Only cryo |
| CD271 | EP1039Y | Abcam/Epitomics | ab 52987 (Abcam), 1812-1 (Epitomics) | Cryo and paraffin |
| CXCL13 | Polyclonal | Peprotech, Hamburg | 500-P141 | Cryo + paraffin |
| CCL21 | Polyclonal | Peprotech, Hamburg | 500-P109 | Paraffin, cryo not tested |
| IgD | Polyclonal | DAKO, Hamburg | A0093 | Cryo + paraffin |
| IgM | Polyclonal | DAKO, Hamburg | A425 | Cryo + paraffin |
| CD27 | 137B4 | Quartett, Berlin | 30410901 | Cryo + paraffin |
| CD141 | TM1009 | DAKO, Hamburg | M0617 | Cryo + paraffin |
| Antigen unknown | CNA.42 | DAKO, Hamburg | M7157 | Cryo + paraffin |
Double staining
The antibodies were applied as depicted in Table2. The first primary antibody was revealed as described above. After washing, the sections were then covered with the second primary antibody overnight, which was revealed by blue coloration using either the Vectastain Elite Kit for alkaline phosphatase with biotinylated anti-mouse or anti-rabbit immunoglobulin (Vector Laboratories, No. AK-5000, via Alexis, Grünberg, Germany) or the ultravision LP kit (Thermo Scientific, Schwerte, Germany, No. TL-060-AL) according to the manufacturer's instructions. The presence of alkaline phosphatase was shown by a standard reaction using Fast Blue in AS-MX phosphate buffer containing 0·24 mg/ml levamisole. For cryosections the concentration of levamisole was increased to 6 mg/ml. Double-stained specimens were coverslipped with Mowiol (polyvinyl alcohol). MAdCAM-1 was double-stained versus CD27 in cryosections using the nitroblue tetrazolium/bromochloro-indolylphosphate procedure published previously for visualization of CD3 and CD27 in paraffin sections.19
Results
Stromal cells associated with T-cell regions
CD271 proved decisive for differentiating stromal cells in the human splenic white pulp, both in paraffin sections and in cryosections.22 It was strongly expressed in FDCs of follicles and somewhat more weakly in FRCs of the PALS.22 In addition, strong staining for CD271 occurred in certain adventitial stromal cells and in capillary sheaths of the red pulp (Fig.1a). In addition, diffusely arranged branched stromal cells of the red pulp were more weakly stained. A CD271− area was observed between the CD271+ FRCs of the PALS and the red pulp stromal cells and also between the CD271++ FDCs of the follicles and the red pulp (Fig.1a).
Figure 1.
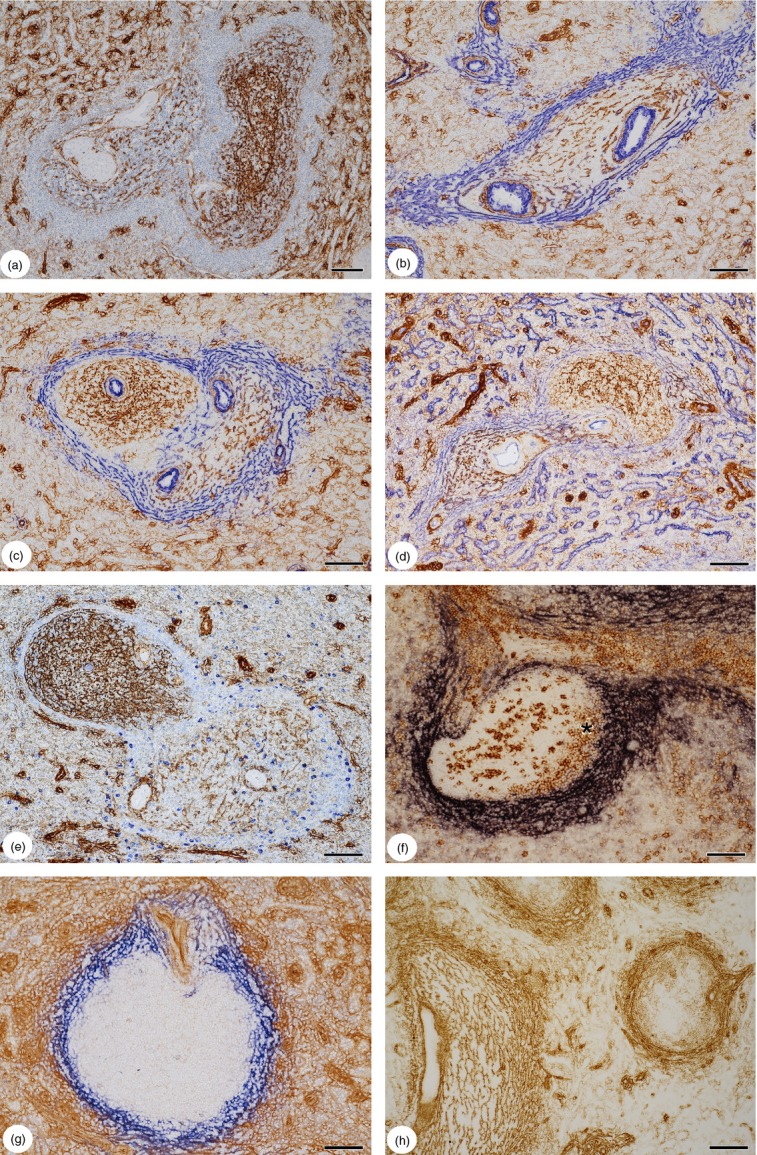
(a) Expression of CD271 in the human splenic white pulp. Fibroblastic reticulum cells (FRCs) in a periarterial lymphatic sheath (PALS) with a central artery and a trabecula (left) and follicular dendritic cells (FDCs) in an elongated secondary follicle (right) are positive. Both regions of the white pulp are delimited from the red pulp by a CD271− area. The red pulp contains ubiquitous CD271+/− branched cells in the splenic cords and CD271++ capillary sheath cells. (b) Sequential double-staining of a PALS for CD271 (brown) and smooth muscle α-actin (SMA) (blue). SMA+ cells are located in the CD271− area at the surface of the PALS. There is no unstained region between the most superficial CD271+ FRCs and the SMA+ cells; some cells appear to be double-positive. Note the periarteriolar SMA+ stromal cells around a smaller arterial vessel in the red pulp (upper left). (c) Double-staining for CD271 (brown) and SMA (blue) in a follicle (left) and a PALS (right). The follicle shows an unstained area between the CD271+ FDCs and the SMA+ CD271− superficial stromal cells. This area is not present around the PALS. (d) Sequential double-staining of a PALS (left) and a follicle (right) for CD271 (brown) and CD141 (blue). CD141+ CD271− stromal cells occur both at the surface of the PALS and of the follicle. Red pulp sinus endothelia are also CD141+ CD271−. The elongated CD271++ structures in the red pulp represent capillary sheaths. (e) Sequential double-staining of a follicle (upper left) and a PALS (lower right) for CD271 (brown) and IgM (blue). In this patient the CD271− region at the surface of the PALS, but not at the surface of the follicle, contains IgM+ plasmablasts. High dilution of anti-IgM to visualize intracellular IgM only. (f) Double-staining for mucosal addressin cell adhesion molecule-1 (MAdCAM-1) (dark blue) and CD27 (brown) in a PALS and follicle. CD27+ B cells occur in the outer mantle zone inside the shell of MAdCAM-1+ superficial stromal cells (asterisk). The strongly CD27+ cells in the follicle interior are plasmablasts and follicular T cells.19 T cells in the PALS are also CD27+. (g) Double-staining for CD105 (brown) and MAdCAM-1 (blue) in a follicle with a mixed periarterial region or small PALS (upper area). The innermost superficial stromal cells of the follicle are CD105− MAdCAM-1+. Most stromal cells of the red pulp including capillary sheath cells are CD105+. (h) Single-staining for CD90 in a PALS (left) and a follicle (right). CD90 is present in FRCs and the superficial stromal cells of PALS and follicles. It weakly stains stromal cells of the red pulp and is also present in capillary sheaths. FDCs of mantle zones are CD90+, but the FDCs of germinal centres appear unstained. (a–d) 22-year-old male patient with extraabdomial trauma, (e–h) 17-year-old male patient with extraabdominal trauma. (a–e) paraffin sections, (f–h) cryosections. (a) ABC-DAB single staining with haemalum nuclear counterstain. (b–e, g) ABC-DAB staining for first primary antibody followed by Ultravision-AP with Fast Blue chromogen for second primary antibody. (f) ABC-AP staining with NBT/BCIP colour reaction for MAdCAM-1 followed by ABC-DAB with tyramide amplification for CD27. (h) ABC-DAB single staining. Scale bars: (a–g) = 100 μm, (h) = 200 μm.
Subtractive double-staining for CD271 and SMA revealed that the SMA+ branched cells in the red pulp cords described in previous publications1,18 were all CD271+/− (Fig.1b). At the surface of the PALS, however, SMA and CD271 exhibited a more or less complementary staining pattern. The CD271− areas were filled with a network of SMA+ stromal cells (Fig.1b). It was clearly visible, that these cells continued into the CD271− areas at the surface of the follicles (Fig.1c). In larger T-cell zones the FRCs in the inner PALS tended to be SMA+/− and were more loosely arranged than the stromal cells at the surface of the PALS. However, there was a strong interindividual variation in the intensity of SMA expression in FRCs. In contrast, FRCs of the inner PALS were uniformly CD271+ in all individuals investigated. Interestingly, double staining for CD271 and CD141 showed that both the CD271+ FRCs in the inner PALS and the superficial CD271− stromal cells were uniformly CD141+. The CD141+ stromal cells also continued at the surface of the follicles (Fig.1d). The distribution of CD271 in combination with SMA and CD141 therefore showed that there were at least two types of stromal cells in the human PALS, an inner CD271+ type and an outer CD271− type. In some, but not in all, specimens the outer CD271− stromal cells were associated with IgM+ plasmablasts, which were much more frequent at the superficial PALS than in the red pulp stromal cords or at the surface of follicles (Fig.1e).
As described previously,18 MAdCAM-1+ stromal cells also occurred in association with the PALS of human spleens. The distribution of MAdCAM-1 resembled that of SMA as it was more intensely expressed in stromal cells of the superficial PALS (Fig.1f). The most intense expression of MAdCAM-1 occurred in stromal cells of the CD271− region. Similar to SMA, the intensity of MAdCAM-1 staining in FRCs was individually variable. MAdCAM-1 or SMA single-positive FRCs were not detected.
CD105 and CD90 were also found in stromal cells of the white pulp. Anti-CD105 stained FRCs and the stromal cells at the surface of the PALS and the follicles, but no FDCs. It was also present in the majority of red pulp stromal cells. Hence, CD105 expression could not be used to delimit the white pulp from the red pulp (Fig.1g). In contrast, CD90 was only weakly detected in red pulp stromal cells. It represented the most widespread stromal cell antigen of the white pulp, because it occurred in FRCs as well as in FDCs (Fig.1h).
A polyclonal reagent detecting CCL21 also reacted with FRCs, certain superficial stromal cells of the PALS and a shell of branched cells and/or fibres at the follicular surface, as described elsewhere.52 Using mAb 79018 against CXCL12 in cryosections, a similar pattern of stromal cell and/or fibre reactivity was revealed (Fig.2a). Interestingly, strong staining for CXCL13 was associated not only with stromal cells at the surface of the PALS and follicles, but also with smooth muscle cells and with stromal cells and/or adjacent fibres surrounding red pulp arterioles and arterial vessels with mixed T-cell/B-cell sheaths (Fig.2b,c).22 Whether the superficial CXCL13+ stromal cells and/or fibres of the white pulp were identical to those structures strongly staining for SMA and/or CCL21 and/or CXCL12 at the follicular surface, was not investigated in detail. In cryosections, where intracellular CXCL13 is not preserved due to the short fixation time with isopropanol, CXCL13 was primarily detected at the surface of fibres in the superficial PALS and surrounding the follicles. In addition, single long fibre-like CXCL13+ strands of extracellular material were present inside follicles. CXCL13 was also associated with extracellular structures in the walls of arterial vessels.
Figure 2.
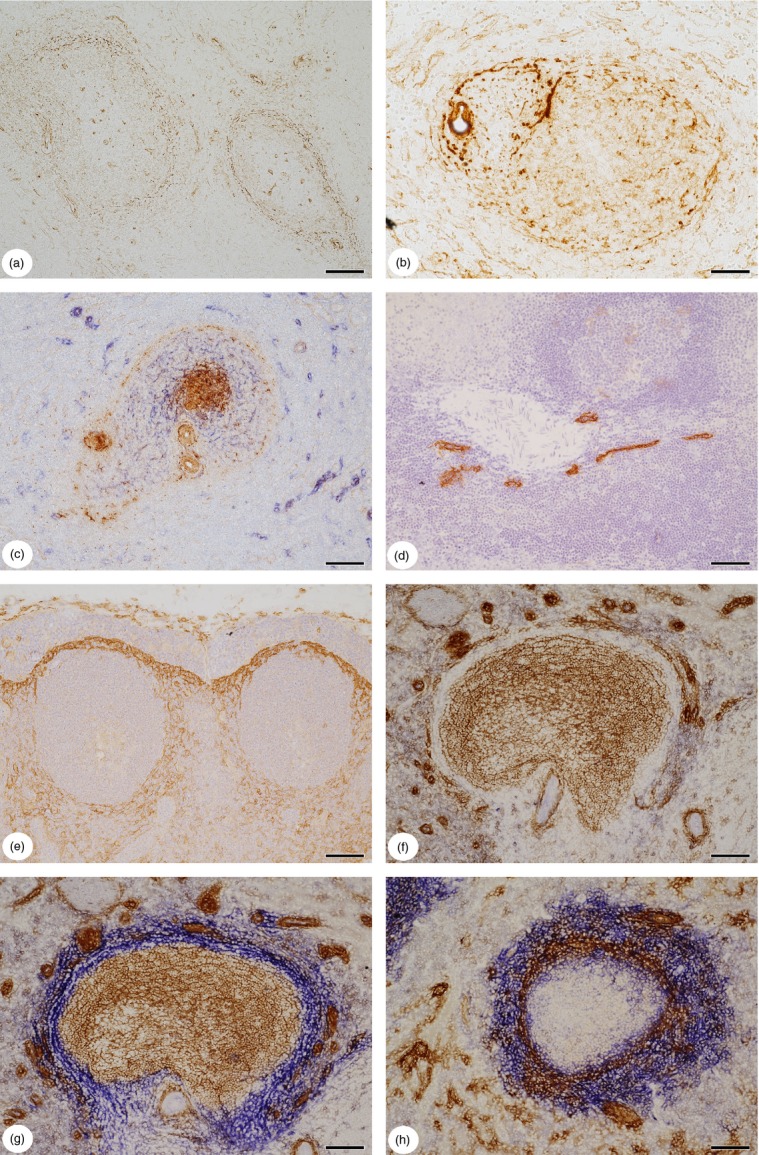
(a) Distribution of CXCL12 in the human splenic white pulp. Cells and/or fibres surrounding periarterial lymphatic sheath (PALS) and follicles are positive. (b) Distribution of CXCL13 in the human splenic white pulp. A small mixed periarterial region or a PALS (left) with a follicle (right) are surrounded by CXCL13+ stromal cells and/or fibres. Follicular dendritic cells (FDCs) in the mantle zone of the follicle are weakly positive. Smooth muscle cells and adventitial stromal cells or fibres surrounding an arteriole (left) are also stained. (c) Double-staining for CXCL13 (brown) and CD271 (blue) in a small periarterial region or PALS (left) and a secondary follicle (right). The FDCs in the germinal centre are strongly CXCL13+, whereas FDCs in the mantle zone and cells and/or fibres at the surface of the periarterial region and the follicle stain more weakly. Adventitial stromal cells or fibres and smooth muscle cells of arterial walls are also CXCL13+. (d) Distribution of podoplanin in the adult human splenic white pulp. Podoplanin is only visualized in endothelia of a lymphatic vessel network accompanying a large artery. Fibroblastic reticulum cells (FRCs) are not stained. Single ABC-DAB staining with monoclonal antibody 4D5aE5E6 and haemalum nuclear counterstain. (e) Expression of receptor activator of nuclear factor-κB ligand (RANKL) (CD254) in a lymph node situated at the splenic hilum (most probably a gastric lymph node). RANKL is most strongly expressed in branched stromal cells of the subcapsular sinus floor and more weakly in stromal cells surrounding two secondary follicles. Single staining for RANKL with polyclonal antibody and haemalum nuclear counterstain. (f) Control section of a follicle stained for CD271 (brown) with omission of the second primary antibody (mucosal addressin cell adhesion molecule-1; MAdCAM-1), but with Ultravision and AP-reaction. The CD271− region is especially well visible, because of weak background staining for endogenous AP in perifollicular cells. (g) Double-staining for CD271 (brown) and MAdCAM-1 (blue) in a follicle. The section shows the same follicle as (f). The CD271+ FDCs (brown) and the MAdCAM-1+ CD271− superficial stromal cells join one another without any unstained area in between. As far as recognizable after subtractive staining, the two stromal cell types do not overlap at the border of the mantle zone. The round or elongated dark brown structures are capillary sheaths. (h) Double-staining for SMA (brown) and MAdCAM-1 (blue) at the follicular surface. In this patient the staining for MAdCAM-1 is much more widespread than that for SMA. MAdCAM-1 stains many stromal cells located outside the SMA+ shell, but also some stromal cells located inside. (a, d, f–h) 17-year-old male patient, (b, c) 22-year-old male patient, (e) 15-year-old male patient. All patients had suffered an extraabdominal trauma. (b, c) Paraffin sections, (a, d–h) cryosections. (a, b, d, e) Single ABC-DAB staining. (c, g, h) ABC-DAB staining for first primary antibody followed by Ultravision-AP with Fast Blue chromogen for second primary antibody. (f) Omission of second primary antibody. Scale bars: a, c–h = 100 μm, b = 50 μm.
Although podoplanin expression has been described as a hallmark of mouse splenic and lymph node FRCs,4,53 neither of two monoclonal reagents against human podoplanin (Table1) reacted with stromal cells in human spleen paraffin sections or in cryosections. The only structures detected were endothelia in lymphatic vessels accompanying larger arteries and veins (Fig.2d). Interestingly, the findings in ‘normal’ spleens differed from those in human lymph nodes, where podoplanin occurred in FRCs and other branched stromal cells (not shown). CD254 (RANKL), which is present in mouse marginal reticular cells (MRCs),4,54 was also not detected in human spleens using a monoclonal and a polyclonal antibody (Table1) in paraffin sections and cryosections. Again, CD254 was demonstrated in human lymph nodes, where it occurred in branched stromal cells beneath the subcapsular sinus, around follicles and in cells lining the medullary sinuses (Fig.2e).
Follicle-associated non-FDC stromal cells
SMA+, MAdCAM-1+ and CD141+ branched stromal cells were not only present within and superficial to the PALS, but also occupied the follicular surface, as described previously.18–20 Similar to the PALS, an unstained region occurred between the CD271+/− red pulp stromal cells and the CD271+ FDCs at the surface of follicles (Fig.2f). We first tried to further analyse the stromal cells in this unstained region. Detailed phenotyping of FDCs was not intended, but it immediately turned out that CD271 was superior to CNA.42, CD21 and CD35 used so far to visualize these cells.21 CD271 seemed to be expressed in all FDCs (in contrast to CNA.42) and did not occur in B lymphocytes (in contrast to CD21 and CD35). The areas occupied by SMA+, by MAdCAM-1+ and by CD141+ stromal cells were broader around the follicles than around the PALS. Subtractive double staining for CD271 and MAdCAM-1 in cryosections revealed that the CD271− region around follicles contained MAdCAM-1+ stromal cells (Fig.2f,g). The MAdCAM-1+ superficial stromal cells directly bordered the CD271+ FDCs (Fig.2g). Double staining in both combinations showed that there was no unstained region between both cell types at the follicular surface. The expression of MAdCAM-1 and CD271 did not seem to overlap, although this is difficult to establish in a subtractive system. The relation of MAdCAM-1+ cells to FDCs expressing CNA.42 needs further investigation, because it could not be entirely excluded that a small population of double-positive stromal cells was present at the outer border of the FDC region in some specimens.
In contrast to CD271 and MAdCAM-1, double-staining for CD271 and SMA revealed an unstained rim of tissue between the SMA+ superficial stromal cells and the outermost CD271+ FDCs (Fig.1c). This was different from the surface of the adjacent PALS (Fig.1b,c), where no SMA− region was present. When SMA and MAdCAM-1 were stained sequentially at the follicular surface, MAdCAM-1+ cells were located both inside and outside the shell of SMA+ stromal cells in most individuals (Fig.2h). However, in one specimen staining for SMA almost prevented subsequent visualization of MAdCAM-1 (Fig.3a). Using a reversed staining sequence, no SMA+ cells were left after staining for MAdCAM-1 in all specimens. Hence, MAdCAM-1 tended to be more widely expressed in stromal cells around follicles than SMA. The MAdCAM-1+ stromal cells appeared to comprise not only all stromal cells of the follicular CD271− region, including the innermost SMA− CD271− cells, but also a substantial number of the CD271+/− stromal cells at the outermost follicular surface bordering the red pulp (Fig.2h).
Figure 3.
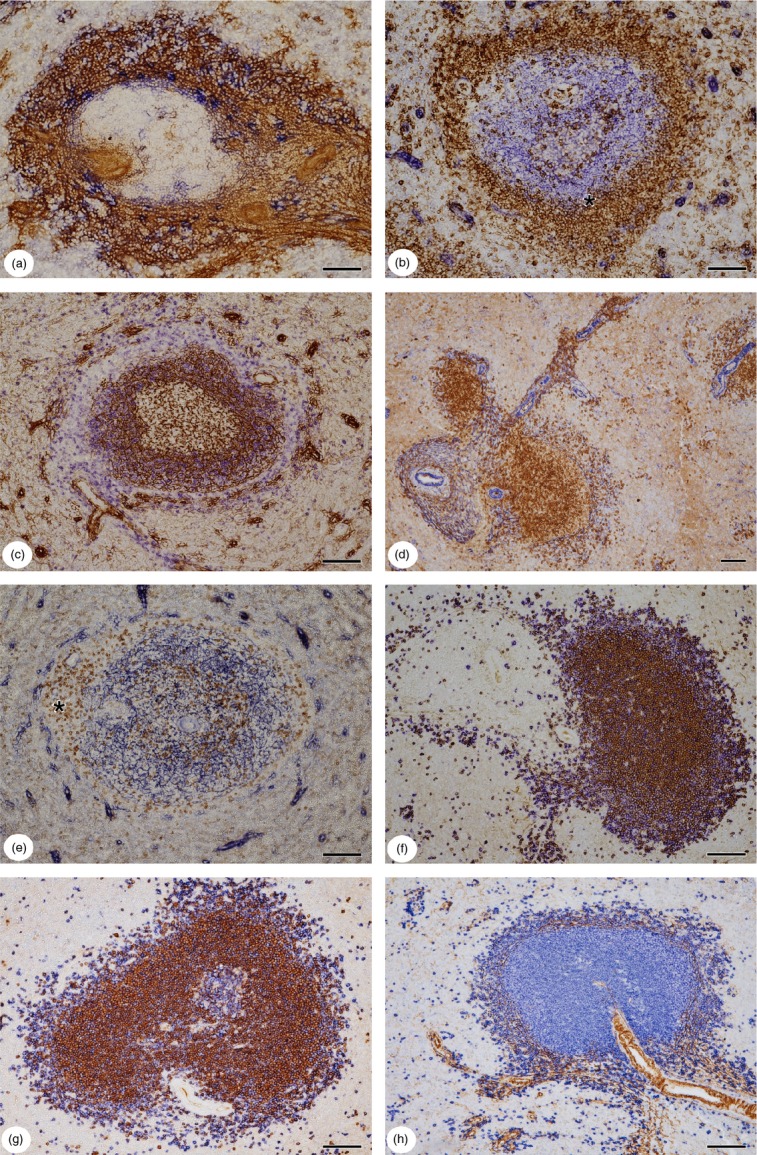
(a) Double-staining for smooth muscle α-actin (SMA) (brown) and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) (blue) at the follicular surface (left) and a periarterial region or a periarterial lymphatic sheath (PALS) (right) of another patient. The amount of SMA+ stromal cells is individually variable. In this patient most of the circumfollicular stromal cells are SMA+. MAdCAM-1 alone is only present in few scattered cells most of which are located adjacent to the follicle interior. (b) Double-staining for CD271 (blue) and CD27 (brown) in a secondary follicle. All follicular dendritic cells (FDCs) including those of the germinal centre (GC) are CD271+. The outermost FDCs of the mantle zone intermingle with the CD27+ perifollicular B cells (asterisk). The CD271− area and the area beyond are occupied by CD27+ B cells. (c) Double-staining for CD271 (brown) and IgD (blue) in a secondary follicle. Most of the IgD+ B cells occur in the inner part of the mantle zone adjacent to the GC. The more superficial B cells in the mantle zone express reduced amounts of IgD. Scattered IgD++ B cells are also present in the CD271− area and beyond. (d) Double-staining for IgD (brown) and SMA (blue) showing a PALS or a mixed B-cell/T-cell region (left), two follicles and branching arterioles, most likely with mixed lymphocyte sheaths. The outer mantle zone (or iMZ) of the right follicle is occupied by a large number of B cells with reduced IgD expression. IgD+/− and IgD++ B cells B cells are present as scattered more superficial cells in the perifollicular area. IgD-expressing B cells also accumulate around branching arterioles in the red pulp. (e) Double-staining for CD4 (brown) and CD271 (blue) in a follicle. CD4+ T-cells are located in the CD271− superficial area and in the centre of the follicle, which may correspond to a GC. A periarteriolar region with mixed B and T cells is located at the left side of the follicle (asterisk). (f) Double-staining for IgD (brown) and CD20 (blue) in a PALS (left) and a follicle (right). IgD− CD20+ B cells predominate at the surface of the follicle. The superficial PALS is occupied by a mixture of IgD+ and IgD− CD20+ B cells. IgD+/− B cells cannot be recognized due to co-staining for CD20. (g) Double-staining for IgM (brown) and CD20 (blue) in a secondary follicle with a small part of the GC. The GC and the superficial part of the follicle are primarily composed of IgM− CD20+ B cells. (h) Double-staining for SMA (brown) and CD20 (blue) in a follicle. SMA+ stromal cells and CD20+ B cells accompany an artery (lower right). These cells continue around the follicle, which displaces the SMA+ cells to the periphery. (a) 51-year-old male patient, (b, c, e) 17-year-old male patient, (d, f–h) 22-year-old male patient. All patients had suffered an extraabdominal trauma. (a) Cryosection, (b–h) paraffin sections. (b) Ultravision-AP for CD271 followed by ABC-DAB with tyramide amplification for CD27. (c–h) ABC-DAB detection for first primary antibody followed by Ultravision-AP with Fast Blue chromogen for second primary antibody. Scale bars = 100 μm.
The following additional results were most relevant with respect to perifollicular stromal cell phenotype: double-staining for SMA and CD141 demonstrated a small number of CD141+ SMA− stromal cells located at the inner circumference of the perifollicular SMA+ cells (not shown). Staining for CD141 after MAdCAM-1 did not reveal additional cells, whereas the reversed sequence suggested that the outermost perifollicular MAdCAM-1+ stromal cells were either CD141− or CD141+/− (not shown). There was also a very small inner follicular layer of MAdCAM-1+ stromal cells, which were CD141− or CD141+/− (not shown).
As mentioned above, CD90 was present in the largest population of white pulp stromal cells including FDCs (Fig.1h). However, FDCs in germinal centres as opposed to those in the mantle zones seemed to be CD90−. When stained after CD90, SMA did not reveal additional stromal cells in the white pulp, but only in the red pulp. In the reversed combination many SMA− CD90+ stromal cells were located at the follicular periphery. After staining for CD90, MAdCAM-1+ perifollicular stromal cells were not detected outside the CD90+ cells, but there was an inner area with MAdCAM-1+ CD90− or CD90+/− stromal cells (not shown).
Consecutive staining for CD105 and MAdCAM-1 also revealed that the stromal cells surrounding follicles had an inner layer of cells differing in phenotype. The innermost stromal cells around follicles were MAdCAM-1+ CD105−, whereas the outer cells were CD105+ (Fig.1g). MAdCAM-1+ CD105− stromal cells did not occur in the PALS, because all stromal cells were at least weakly positive for both antigens. Similar to MAdCAM-1, CD141 was also more strongly expressed in the inner perifollicular layer, when stained after CD105. Whether the innermost perifollicular cells were CD105− or CD105+/− could not be determined.
Hence, not only the stromal cells at the superficial PALS but also those surrounding follicles are phenotypically heterogeneous with more heterogeneity occurring in perifollicular stromal cells. CD90 (Fig.1h), SMA, CCL21, CXCL12 (Fig.2a), CXCL13 (Fig.2b,c), CD105, MAdCAM-1 and CD141 appeared to be expressed in or associated with stromal cells of the follicular surface. It can, however, not be excluded that part of the chemokine staining pattern was also due to chemokines adsorbed to fibres close to cells. The vast majority of the positive cells were different from FDCs expressing CNA.42 or CD271. It can therefore be deduced that at least three different non-FDC stromal cell layers are present around follicles, namely an outermost layer expressing CD90 and/or MAdCAM-1, an intermediate layer positive for all antigens investigated except CD271 and an inner layer positive for MAdCAM-1 and/or for CD141, but negative for SMA, CD105, CD271 and most likely also negative for CD90 (Table3).
Table 3.
Phenotype of non-follicular dendritic cell stromal cells in superficial areas of human splenic follicles1
| Area | CD271 | CD90 | MAdCAM-1 | CD141 | CD105 | SMA |
|---|---|---|---|---|---|---|
| Most superficial | +/− (similar to red pulp) | + | + | +/− or − | + | +/− or − (similar to red pulp) |
| Intermediate | − | + | + | + | + | + |
| Innermost | − | +/− or − | + | +/− or − | − | − |
Synopsis of subtractive double-staining experiments.
Follicle-associated non-FDC stromal cells, B cells and CD4+ T cells
In previous publications we showed by double-staining that most CD27+ cells at the follicular periphery are B cells.19,20 We now stained CD271 versus CD27 (Fig.3b), SMA versus CD27 (not shown) as well as MAdCAM-1 versus CD27 (Fig.1f) and demonstrated that the CD271− region and adjacent areas at the follicular surface are associated with CD27+ B cells. The innermost CD27+ B cells were, however, located between the CD271+ FDCs in a region corresponding to the mantle zone (Fig.3b). This was also shown by double-staining for MAdCAM-1 versus CD27, which left an inner rim of CD27+ B cells not accompanied by MAdCAM-1+ stromal cells (Fig.1f). This area most likely corresponds to the iMZ (more precisely: to the outer mantle zone), which was not defined with respect to MAdCAM-1 in previous publications.19,20 The findings clearly demonstrate that CD27+ B cells are located in different stromal compartments and cannot be used to delineate an MZ equivalent in human spleens. In addition, CD27+ B cells were also present in an area superficial to the CD271− ring where CD271+/− stromal cells and sheathed capillaries occurred (Fig.3b). Corresponding to the results obtained for CD27, IgD+/− B cells occurred at the surface of the CD271+ FDC network in the mantle zone (Fig.3c) or inside the superficial shell of SMA+ stromal cells (Fig.3d). Reduced IgD expression has been described for human MZ B cells.
Apart from IgD+/− and IgD++ B cells (see below) CD3+ T cells and CD4+ T cells were also found in the CD271− region (Fig.3e). Taken together, our findings indicate that all perifollicular MAdCAM-1+ stromal cells are associated with densely arranged CD27+ B cells and fewer scattered IgD++ B cells.
Switched and non-switched B cells at the surface of the PALS and in the outer marginal zone of follicles
Double-staining for IgD and CD20 revealed that a substantial number of IgD+ and of IgD− CD20+ or IgD+/− CD20+ B cells formed a band at the surface of the PALS, which continued into the oMZ of the follicles (Fig.3f). In contrast to the iMZ, where most B cells had a low expression of IgD (Fig.3c,d), the oMZ contained a mixture of two B-cell types, namely a major number of CD20+ B cells with low or absent IgD expression and a minor number of IgD++ B cells, which were most likely CD20+ (Fig.3c,d). Sequential double staining for IgM versus CD20 and IgM versus IgD (not shown) additionally demonstrated that the B cells in the mantle zone of the follicles comprised a high number of IgM+ cells, while at the outermost follicular surface and at the surface of the PALS substantial numbers of IgM− CD20+ B cells were present (Fig.3g). This phenomenon had been previously recognized by detection of co-expression.19 Hence, the surface of the follicles as well as the superficial PALS are not only characterized by special stromal cells, but they also seem to be especially attractive for switched B cells, although larger numbers of IgM+ cells also occur. Scattered B cells appeared to be more strongly stained for IgD than the recirculating B cells in the mantle zone of the follicles. Hence, it cannot be excluded that the superficial IgD++ B cells form a separate cell population.
Stromal cells and lymphocytes associated with the arterial tree
At the surface of red pulp arterioles CD271+ stromal cells formed a dense ring of elongated adventitial cells directly adjacent to the smooth muscle layer. The cells were arranged in a longitudinal as well as a circular orientation. Similar to the white pulp, expression of SMA and MAdCAM-1 occurred in branched CD271− cells surrounding these elongated CD271+ cells (Fig.1b). Interestingly, even larger red pulp arterioles were accompanied by accumulations of B and T cells. Both types of lymphocytes were either completely mixed or B cells were slightly predominant (Fig.3c,e,h). Larger arteries were, however, often covered by a PALS. Trabecular arteries did not possess lymphocyte sheaths. Follicles were either associated with a PALS, or with arterial vessels surrounded by a mixture of B cells and T cells in a meshwork of SMA+ and MAdCAM-1+ stromal cells (Fig.3d,g,h). Strong SMA and MAdCAM-1 expression always occurred in branched periarterial stromal cells at the entrance of such vessels into the follicles and faintly SMA+ and/or MAdCAM-1+ stromal cells further accompanied the arteries for some distance into the follicle interior until the vessel was finally embedded in B cells. The surroundings of the arterial vessels within follicles could, however, not be investigated throughout their entire course. In addition, it was not evident in non-serial sections, which generation of arteries entered the follicles. It is clear that one and the same arterial vessel may run inside a follicle and inside a PALS.52 However, because follicles tended to be located near the branching points of arteries (Figs1f and 3c,d,h), B- and T-cell zones may also be supplied by different generations of arterial vessels. The findings show, that there is a ubiquitous extrafollicular periarteriolar compartment in human spleens that is supported by CD271− stromal cells expressing SMA and MAdCAM-1 containing a mixed population of B and T cells.
Discussion
Our study investigates the microanatomy of adult human spleens with special emphasis on white pulp stromal cells and B lymphocytes. We demonstrate, that the phenotype of human splenic white pulp stromal cells is highly species-specific and differs from that in mice or rats.
The most fundamental differences concern the phenotype of FRCs and stromal cells at the surface of white pulp T-cell zones and follicles. The present publication shows additional phenotypic complexity of stromal cells, especially at the outermost follicular surface previously termed the oMZ.19–21 We clearly demonstrate that there are at least three types of stromal cells in the splenic white pulp, namely CD271+ FRCs, CD271+ FDCs and an additional stromal cell type located at the surface of PALS and follicles that resembles FRCs, but is negative or only minimally positive for CD271.
We apply a subtractive immunohistological double-staining method. This method is based on the fact that cells stained with the first primary antibody are covered with a polymerized colour precipitate, which prevents access of the second primary reagent. Hence, only cells which are left unstained in the first step are revealed in a different colour when the second primary antibody is detected. The method is adjusted to avoid colour mixing as far as possible. Some information about double-positive cells may, however, be gained by reversing the sequence of primary antibodies. If the staining pattern of each antigen is known, it is highly likely that both antigens are co-expressed if no single-positive cells appear in both combinations. However, direct demonstration of co-expression is not possible with the method used. In addition, single-positive cells may be missed in areas of dense staining for the first antigen.
It needs to be considered that adult human splenic follicles normally occur as non-polarized quiescent secondary follicles.21 Our investigation only applies to such follicles. We do not know how the distribution of stromal antigens is altered during immune reactions. Most likely B-cell reactions leading to polarization of splenic follicles will induce more widespread expression of several of the antigens investigated. The inter-individual differences found in our study may mirror the immune state of each patient. In addition, local factors may be involved. Hence, the molecules expressed in splenic stromal cells may be different from that in lymph nodes, especially from that in nodes draining the skin, because surface-draining lymph nodes are more directly exposed to antigens. In addition, the cause for removal is different in both organs, as most of the spleens investigated in our study came from healthy victims of accidents, while lymph nodes are preferentially removed for diagnosis in case of pathological enlargement or cancer. Assessing stromal cell phenotype in pathological organs or in the immunologically more active spleens of children was not within the scope of our investigation, but needs to be performed.
Previous investigations have revealed that the superficial region of human splenic follicles is much more complex than found in mice or rats.1,18–21 The broad MZ that surrounds the PALS and the follicles on all sides in rats1,16 and to some extent also in mice, is a species-specific phenomenon more or less absent in humans.
If the nomenclature of splenic white pulp regions established in mice and rats needs to be retained for humans (which is not very practical) and if the presence of CD27+ B cells is used to locate the MZ, then an iMZ and an oMZ have to be defined at the surface of human splenic follicles.19,20 If, however, staining for FDC antigens is combined with detection of CD27+ B cells, then the iMZ appears as an outer part of the follicular mantle zone occupied by CD27+ B cells.21 Hence, it is impossible to define an outer border of the mantle zone in adult human spleens by staining for any type of B cells. Our present results indicate that the best way of defining regions in adult human splenic follicles is to detect stromal cells. In our hands, MAdCAM-1 and CD271 are presently the most comprehensive antigens to distinguish superficial stromal cells (MAdCAM-1+) from FDCs (CD271+) in follicles. The MAdCAM-1+ region does, however, not correspond to a rat or mouse MZ as mentioned above. Besides absence of a marginal sinus and marginal metallophilic macrophages, the MAdCAM-1+ area also does not contain all follicle-associated CD27+ B cells. Finally, CD27+ B cells are more or less absent in the region superficial to the human PALS.19,20 Our immunohistological findings concerning the special structure of the MZ equivalent in humans have been confirmed by others13,28,55,56 and correspond to previous publications.57–59
We now show, that the oMZ of human splenic follicles contains stromal cells of a special phenotype, which are arranged as concentric shells of cells exhibiting slight phenotypical differences. These cells either lack CD271 or exhibit only low expression. Molecules occurring in these special cells and beyond are SMA, MAdCAM-1, CD141 (thrombomodulin), CD105 (endoglin), CD90 (Thy-1), CCL21, CXCL12 and CXCL13. It has also been described, that cytokeratins 8 and 18 are present in human perifollicular stromal cells.18,60,61 At least three different stromal shells associated with CD27+ B cells are distinguishable around secondary follicles of most adult individuals (Table3). CD90 and MAdCAM-1 are the most widespread antigens in perifollicular stromal shells, while CD105, CD141 and SMA occur in fewer cells. The width of the shells differs among individuals. It is likely that stromal antigen expression depends on the activity of the associated follicle. The phenotypical differences of stromal cells most likely depend on the diffusion range of mediators produced within the follicles. However, stromal cells of the oMZ themselves also produce chemokines such as CXCL13 or/and CCL21 in amounts sufficient for immunohistological detection. This fact may cause the presence of IgD++ B cells in the entire superficial white pulp and of CD4+ T cells in a perifollicular position.
The phenotypical heterogeneity of the superficial stromal cells of T- and B-cell regions in human spleens makes it difficult to find a better comprehensive name for their location than ‘superficial white pulp’. Naming the area is further complicated by the fact that the superficial stromal cells are phenotypically related to, but not identical to, the FRCs of the PALS and that they also occur independent of a PALS or follicle. The following hypothetical model of vessels and stromal cells unifies all observations reported in our present and previous studies. Human splenic arterial vessels are covered by a layer of SMA+ MAdCAM-1+ CD271− stromal cells, which is permanently passed and maintained by T and B lymphocytes. These stromal cells are pushed to the periphery of the white pulp when T-cell zones with CD271+ FRCs and follicles with CD271+ FDCs develop. Hence, a continuous band of stromal cells is present, which either directly covers the vessels or is dislocated by a PALS or a follicle. This band again approaches the arterial vessel, in areas lacking such specializations as nicely visualized by several double-staining combinations (Figs1b and 3h). The stromal cell layer covering arterial vessels might be attractive both for B and T lymphocytes, because it expresses CCL21 and CXCL13. In contrast to rodents, humans do not exhibit a continuous PALS surrounding larger arterial vessels, but the T-cell regions are of limited length. The predominant periarterial ‘sheath’ is a small arrangement consisting of T cells and B cells (B.S. Steiniger, unpublished data), which is displaced from the vessel surface by PALSs and follicles. If the immigration kinetics of recirculating lymphocytes into the human spleen correspond to those of mice and rats,7,62 B and T cells can be assumed to enter the white pulp by ‘retrograde’ migration along small arterial vessels until they finally segregate into PALS and into follicles around larger arteries. The small vessels might correspond to the ‘MZ-bridging channels’ known from rats and mice.63,64 Hence, mixed lymphocyte populations have to be expected around all arterioles in the red pulp. In humans it is highly likely, that immigrating lymphocytes do not need to pass any endothelial cells, because most red pulp capillaries are not connected to sinuses and have open ends.21
The MAdCAM-1+ cells lining the marginal sinus in mice14,49,50 are most likely related to the human superficial MAdCAM-1+ white pulp stromal cells. Hence, in mouse spleens the perisinusoidal MAdCAM-1+ cells do not represent endothelia, but stromal cells lining a special part of the open circulation. However, the amount of MAdCAM-1 expressed is vastly different in humans and mice. Adult rats do not express MAdCAM-1 in the spleen at all.65,66 In addition, podoplanin and RANKL (CD254) are not found in adult human splenic stromal cells by the immunohistological methods used in the present investigation. This is remarkable because podoplanin has been described to occur in FRCs and MRCs in mouse lymph nodes and spleen,4 while RANK-L is only present in mouse MRCs.4,54 We confirm the distribution of podoplanin and RANKL also for human lymph nodes, but not for adult human spleens. Hence, the stromal cells we describe in the present publication are phenotypically different from mouse splenic MRCs. However, in mice and humans at least three different white pulp stromal cell types seem to exist. Our previous investigations in rat spleens1 have demonstrated that SMA is expressed in elongated stromal cells accompanying the marginal sinus. Hence, a special population of superficial white pulp stromal cells, which is MAdCAM-1− SMA+, also exists in rats.
Why MAdCAM-1 is so prominently expressed in normal adult human spleens is still open to speculation. In phylogeny, the spleen derives from the gut wall.67,68 It cannot be excluded that CD27+ B cells, which are the most frequent cell type contacting MAdCAM-1+ perifollicular stromal cells in human spleens (or a subpopulation of these cells), recirculate between the spleen and gut-associated lymphatic tissue. These cells might depend on MAdCAM-1+ stromal cells for their survival and for the regulation of their activity. Surface IgA+ B cells occur in the human oMZ,19,69 but it is not clear, whether this feature is unique to the spleen. Especially the expression of IgA2 might be informative, because this isotype is primarily found in gut-associated B cells.70 It has been shown that human transitional B cells home to the gut-associated lymphatic tissue.71 A report on a case of human gut-associated B-cell lymphoma indicates that idiotypically detectable lymphoma B cells can migrate to the splenic MZ.72
The ontogeny of lymph nodes and gut-associated lymphatic tissues has been clarified to a large extent in mice,73–75 although the most primordial signal starting lymph node development is still unknown. Similar mechanisms also work in humans.76 A central role is played by so-termed lymphoid tissue inducer cells, which represent an innate lymphoid cell population derived from the bone marrow. Lymphoid tissue inducer cells stimulate stromal organizer cells to provide an immature stromal network in the respective lymphatic organ. In addition, mouse lymph node and splenic FDCs have been shown to arise from ubiquitous periarterial or periarteriolar stromal cells.6,77 It is likely that this is also true for FRCs, because mesenchymal stem cells occur in almost all organs.78 How splenic follicles arise, is not entirely clear, because in mouse spleens B lymphocytes instead of lymphoid tissue inducer cells appear to be important for the development of the white pulp.79–82 In humans an association of B cells and arterial vessels is already present in fetal spleens.83 The first invading lymphocytes in human fetal spleens are B cells associated with arterioles in vascular lobules. This phenomenon may be due to expression of CXCL13 in smooth muscle cells of the small arterial vessels. In human spleens B lymphocytes are associated with periarteriolar and periarterial SMA+ stromal cells long before birth.83
Double-staining for IgM and CD20 revealed that CD20+ IgM− switched B cells primarily occupied the outermost surface of the follicles corresponding to the oMZ. This finding corresponds to the detection of IgM− CD27+ B cells in the same location by immunofluorescence.19 Hence, our results demonstrate an interesting heterogeneity in the follicular distribution of non-switched and switched B cells, although both cell types were not totally separated. At present, two explanations for this phenomenon come to mind, namely that most IgM+ CD27+ B cells have an affinity for the CD271− type of superficial stromal cells and/or their diffusible products or that IgM− CD27+ B cells have an affinity for non-stromal cells outside the oMZ, for example for neutrophils accumulating in the perifollicular zone18,23,41,69 or vice versa. However, IgM− CD20+ switched B cells also occurred around capillary sheaths22 and around the PALS. Further analysis of switched B cells in the superficial white pulp is prevented by the problem that B-cell surface immunoglobulins other than IgD or IgM cannot be reliably detected by immunohistology because of interference by the ubiquitously distributed high amounts of interstitial immunoglobulins. Reports in mice also indicate that IgM+ and IgG1+ B cells tend to localize differently in the vicinity of contracted germinal centres in the spleen.84 As shown previously, the superficial PALS and the oMZ are not totally identical regions, because CD27+ B cells can be demonstrated to accumulate in the iMZ and oMZ by subtractive double staining, although this is not the case at the surface of the PALS.19,20 It cannot be excluded that CD27+ B cells are located in a different region, i.e. among the FRCs deeper inside the PALS, which prevents their recognition among the CD27+ T cells. Alternatively, switched B cells at the surface of the PALS may be CD27−, while CD27+ switched B cells localize to the follicular surface. The phenotype and distribution of switched B cells needs to be further investigated in human spleens using immunofluorescence methods, which permit a more detailed analysis inside the PALS.
Another peculiarity of the superficial white pulp is the fact that IgD++ B cells occur at the surface of the PALS and of the follicles as already published.19 The special phenotype of the superficial stromal cells may be influenced by mediators secreted by these IgD++ B cells. In contrast, migratory B cells may be attracted by the CXCL13+ stromal cells in this location. The superficial IgD++ B cells also need to be analysed in more detail. In several individuals these B cells seemed to be more intensely stained for IgD than mantle zone B cells.
Our investigation shows that certain basic traits of splenic white pulp microanatomy are invariant in humans, mice and rats. Nevertheless, it needs to be kept in mind that the species mentioned do not only differ in several morphological and phenotypical features of splenic microanatomy. There are also fundamental functional discordances among humans and mice, for example in genomic responses during inflammation.85 Adult human spleens even contain entire B-cell compartments not present in mice or rats, namely capillary sheaths composed of specialized stromal sheath cells, macrophages and B cells.22 Regarding these sheaths it is clear that mice and rats are exceptional vertebrate species. Hence, the stromal cells of the white pulp may also function in a species-specific way.
Acknowledgments
We thank Dr A. Hellinger, formerly Clinics of Visceral, Thoracic and Vascular Surgery and Dr P.J. Barth, formerly Institute of Pathology of Marburg University Hospital for their generous cooperation and the contribution of specimens. Dr. M. Briskin generously shared mAb 10A6 detecting MAdCAM-1.
Glossary
- ABC
avidin–biotin complex
- AP
alkaline phosphatase
- DAB
diaminobenzidine
- FRC
fibroblastic reticulum cell
- FDC
follicular dendritic cell
- GC
germinal centre
- iMZ
inner marginal zone
- mAb
monoclonal antibody
- MAdCAM-1
mucosal addressin cell adhesion molecule-1
- MRC
marginal reticulum cell
- MZ
marginal zone
- NBT/BCIP
nitro blue tetrazolium/bromo-chloro indolylphosphate
- NGFR
nerve growth factor receptor
- oMZ
outer marginal zone
- PALS
periarterial lymphatic sheath
- RANKL
receptor activator of NF-κB ligand
- SMA
smooth muscle α-actin
Disclosures
None of the authors has any conflict of interests.
References
- 1.Steiniger B, Barth P. Microanatomy and function of the spleen. Adv Anat Embryol. 2000;151:1–100. doi: 10.1007/978-3-642-57088-9. [DOI] [PubMed] [Google Scholar]
- 2.Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606–16. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 3.Bronte V, Pittet MJ. The spleen in local and systemic regulation of immunity. Immunity. 2013;39:806–18. doi: 10.1016/j.immuni.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katakai T. Marginal reticular cells: a stromal subset directly descended from the lymphoid tissue organizer. Front Immunol. 2012;3 doi: 10.3389/fimmu.2012.00200. article No. 200. doi: 10.3389/fimmu.2012.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Den Haan JM, Mebius RE, Kraal G. Stromal cells of the mouse spleen. Front Immunol. 2012;3 doi: 10.3389/fimmu.2012.00201. article No. 201. doi: 10.3389/fimmu.2012.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aguzzi A, Kranich J, Krautler NJ. Follicular dendritic cells: origin, phenotype, and function in health and disease. Trends Immunol. 2014;35:105–13. doi: 10.1016/j.it.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Nieuwenhuis P, Ford WL. Comparative migration of B- and T-lymphocytes in the rat spleen and lymph nodes. Cell Immunol. 1976;23:254–67. doi: 10.1016/0008-8749(76)90191-x. [DOI] [PubMed] [Google Scholar]
- 8.Liu YJ. Sites of B lymphocyte selection, activation, and tolerance in spleen. J Exp Med. 1997;186:625–9. doi: 10.1084/jem.186.5.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hendricks J, Visser A, Dammers PM, Burgerhof JG, Bos NA, Kroese FG. Class-switched marginal zone B cells in spleen have relatively low numbers of somatic mutations. Mol Immunol. 2011;48:874–82. doi: 10.1016/j.molimm.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 10.Dammers PM, Visser A, Popa ER, Nieuwenhuis P, Kroese FG. Most marginal zone B cells in rat express germline encoded Ig VH genes and are ligand selected. J Immunol. 2000;165:6156–69. doi: 10.4049/jimmunol.165.11.6156. [DOI] [PubMed] [Google Scholar]
- 11.Dammers PM, Kroese FG. Recruitment and selection of marginal zone B cells is independent of exogenous antigen. Eur J Immunol. 2005;35:2089–99. doi: 10.1002/eji.200526118. [DOI] [PubMed] [Google Scholar]
- 12.Weill JC, Weller S, Reynaud CA. Human marginal zone B cells. Annu Rev Immunol. 2009;27:267–85. doi: 10.1146/annurev.immunol.021908.132607. [DOI] [PubMed] [Google Scholar]
- 13.Garraud O, Borhis G, Badr G, Degrelle S, Pozzetto B, Cognasse F, Richard Y. Revisiting the B-cell compartment in mouse and humans: more than one B-cell subset exists in the marginal zone and beyond. BMC Immunol. 2012;13:63. doi: 10.1186/1471-2172-13-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Den Haan JM, Kraal G. Innate immune functions of macrophage subpopulations in the spleen. J Innate Immun. 2012;4:437–45. doi: 10.1159/000335216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kraal G, Mebius R. New insight into the cell biology of the marginal zone of the spleen. Int Rev Cytol. 2006;250:175–215. doi: 10.1016/S0074-7696(06)50005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dijkstra CD, Döpp EA, Joling P, Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985;54:589–99. [PMC free article] [PubMed] [Google Scholar]
- 17.Steiniger B, Barth P, Herbst B, Hartnell A, Crocker PR. The species-specific structure of microanatomical compartments in the human spleen: strongly sialoadhesin-positive macrophages occur in the perifollicular zone, but not in the marginal zone. Immunology. 1997;92:307–16. doi: 10.1046/j.1365-2567.1997.00328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steiniger B, Hellinger A, Barth P. The perifollicular and marginal zones of the human splenic white pulp: do fibroblasts guide lymphocyte immigration? Am J Pathol. 2001;159:501–12. doi: 10.1016/S0002-9440(10)61722-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steiniger B, Timphus EM, Jacob R, Barth PJ. CD27+ B cells in human lymphatic organs: re-evaluating the splenic marginal zone. Immunology. 2005;116:429–42. doi: 10.1111/j.1365-2567.2005.02242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steiniger B, Timphus EM, Barth PJ. The splenic marginal zone in humans and rodents – an enigmatic compartment and its inhabitants. Histochem Cell Biol. 2006;126:641–8. doi: 10.1007/s00418-006-0210-5. [DOI] [PubMed] [Google Scholar]
- 21.Steiniger B, Trabandt M, Barth PJ. The follicular dendritic cell network in secondary follicles of human palatine tonsils and spleens. Histochem Cell Biol. 2011;135:327–36. doi: 10.1007/s00418-011-0799-x. [DOI] [PubMed] [Google Scholar]
- 22.Steiniger BS, Seiler A, Lampp K, Wilhelmi V, Stachniss V. B lymphocyte compartments in the human splenic red pulp: capillary sheaths and periarteriolar regions. Histochem Cell Biol. 2014;141:507–18. doi: 10.1007/s00418-013-1172-z. [DOI] [PubMed] [Google Scholar]
- 23.Cerutti A, Cols M, Puga I. Marginal zone B cells: virtues of innatelike antibody-producing lymphocytes. Nat Rev Immunol. 2013;13:118–32. doi: 10.1038/nri3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weller S, Braun MC, Tan BK, et al. Blood IgM “memory” B cells are circulating splenic marginal zone B cells in humans. Blood. 2004;104:3647–54. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tierens A, Delabie J, Michiels L, Vandenberghe P, De Wolf-Peeters C. Marginal-zone B cells in the human lymph node and spleen show somatic hypermutations and display clonal expansion. Blood. 1999;93:226–34. [PubMed] [Google Scholar]
- 26.Weller S, Faili A, Garcia C, et al. CD40-CD40L independent Ig gene hypermutation suggests a second B cell diversification pathway in humans. Proc Natl Acad Sci USA. 2001;98:1166–70. doi: 10.1073/pnas.98.3.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weller S, Reynaud CA, Weill JC. Splenic marginal zone B cells in humans: where do they mutate their Ig receptor? Eur J Immunol. 2005;35:2789–92. doi: 10.1002/eji.200535446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weller S, Mamani-Matsuda M, Picard C, Cordier C, Lecoeuche D, Gauthier F, Weill JC. Somatic diversification in the absence of antigen-driven responses is the hallmark of the IgM+ IgD+ CD27+ B cell repertoire in infants. J Exp Med. 2008;205:1331–42. doi: 10.1084/jem.20071555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheeren FA, Nagasawa M, Weijer K, Cupedo T, Kirberg J, Legrand N, Spits H. T cell-independent development and induction of somatic hypermutation in human IgM+IgD+CD27+ B cells. J Exp Med. 2008;205:2033–42. doi: 10.1084/jem.20070447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seifert M, Küppers R. Molecular footprints of a germinal center derivation of human IgM+(IgD+)CD27+ B cells and the dynamics of memory B cell generation. J Exp Med. 2009;206:2659–69. doi: 10.1084/jem.20091087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein U, Rajewski K, Küppers R. Human immunoglobulin (Ig) M+IgD+peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188:1679–89. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gray D, MacLennan IC, Bazin H, Khan M. Migrant my+delta+ and static my+delta– B lymphocyte subsets. Eur J Immunol. 1982;12:564–9. doi: 10.1002/eji.1830120707. [DOI] [PubMed] [Google Scholar]
- 33.Arnon TI, Horton RM, Grigorova IL, Cyster JG. Visualization of splenic marginal zone B-cell shuttling and follicular B-cell egress. Nature. 2013;493:684–8. doi: 10.1038/nature11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu YJ, Zhang J, Lane PJ, Chan EY, MacLennan IC. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur J Immunol. 1991;21:2951–62. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- 35.Cinamon G, Zachariah MA, Lam OM, Foss FW, Jr, Cyster JG. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat Immunol. 2008;9:54–62. doi: 10.1038/ni1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Capolunghi F, Rosado MM, Sinibaldi M, Aranburu A, Carsetti R. Why do we need IgM memory B cells? Immunol Lett. 2013;152:114–20. doi: 10.1016/j.imlet.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 37.Rosado MM, Gesualdo F, Marcellini V, et al. Preserved antibody levels and loss of memory B cells against pneumococcus and tetanus after splenectomy: tailoring better vaccination strategies. Eur J Immunol. 2013;43:2659–70. doi: 10.1002/eji.201343577. [DOI] [PubMed] [Google Scholar]
- 38.Kruetzmann S, Rosado MM, Weber H, et al. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J Exp Med. 2003;197:939–45. doi: 10.1084/jem.20022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet. 2011;378:86–97. doi: 10.1016/S0140-6736(10)61493-6. [DOI] [PubMed] [Google Scholar]
- 40.Steiniger B, Bette M, Schwarzbach H. The open microcirculation in human spleens: a three-dimensional approach. J Histochem Cytochem. 2011;59:639–48. doi: 10.1369/0022155411408315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steiniger B. Spleen. In: Delves P, editor. Encyclopedia of Life Sciences. Immunology: John Wiley & Sons Ltd; 2012. doi: 10.1002/9780470015902.a0000900.pub3. [Google Scholar]
- 42.Chen LT, Weiss L. The role of the sinus wall in the passage of erythrocytes through the spleen. Blood. 1973;41:529–37. [PubMed] [Google Scholar]
- 43.Drenckhahn D, Wagner J. Stress fibres in the splenic sinus endothelium in situ: molecular structure, relationship to the extracellular matrix, and contractility. J Cell Biol. 1986;102:1738–47. doi: 10.1083/jcb.102.5.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nombela-Arrieta C, Ritz J, Silberstein LE. The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol. 2011;12:126–31. doi: 10.1038/nrm3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mikami Y, Ishii Y, Watanabe N, Shirokawa T, Suzuki S, Irie S, Isokawa K, Honda MJ. CD271/p75(NTR) inhibits the differentiation of mesenchymal stem cells into osteogenic, adipogenic, chondrogenic, and myogenic lineages. Stem Cells Dev. 2011;20:901–13. doi: 10.1089/scd.2010.0299. [DOI] [PubMed] [Google Scholar]
- 46.Maeda K, Matsuda M, Suzuki H, Saitoh HA. Immunohistochemical recognition of human follicular dendritic cells (FDCs) in routinely processed paraffin sections. J Histochem Cytochem. 2002;50:1475–86. doi: 10.1177/002215540205001107. [DOI] [PubMed] [Google Scholar]
- 47.Pezzati P, Stanisz AM, Marshall JS, Bienenstock J, Stead RH. Expression of nerve growth factor receptor immunoreactivity on follicular dendritic cells from human mucosa associated lymphoid tissues. Immunology. 1992;76:485–90. [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson SJ, Schattemann GC, Gown AM, Bothwell M. A monoclonal antibody against nerve growth factor receptor. Immunohistochemical analysis of normal and neoplastic human tissue. Am J Clin Pathol. 1989;92:415–23. doi: 10.1093/ajcp/92.4.415. [DOI] [PubMed] [Google Scholar]
- 49.Kraal G, Schornagel K, Streeter PR, Holzmann B, Butcher EC. Expression of the mucosal vascular addressin, MAdCAM-1, on sinus-lining cells in the spleen. Am J Pathol. 1995;147:763–71. [PMC free article] [PubMed] [Google Scholar]
- 50.Berlin C, Berg EL, Briskin MJ. α4 β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–95. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 51.Briskin M, Winsor-Hines D, Shyjan A, et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol. 1997;151:97–110. [PMC free article] [PubMed] [Google Scholar]
- 52.Steiniger B, Rüttinger L, Barth PJ. The three-dimensional structure of human splenic white pulp compartments. J Histochem Cytochem. 2003;51:655–63. doi: 10.1177/002215540305100511. [DOI] [PubMed] [Google Scholar]
- 53.Link A, Hardie DL, Favre S, et al. Association of T-zone reticular networks and conduits with ectopic lymphoid tissues in mice and humans. Am J Pathol. 2011;178:662–75. doi: 10.1016/j.ajpath.2010.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Katakai T, Suto H, Sugai M, et al. Organizer-like reticular stromal cell layer common to adult secondary lymphoid organs. J Immunol. 2008;181:6189–200. doi: 10.4049/jimmunol.181.9.6189. [DOI] [PubMed] [Google Scholar]
- 55.Guisado Vasco P, Villar Rodriguez JL, Ibanez Martinez J, Gonzalez Campora R, Galera Davidson H. Immunohistochemical organization patterns of the follicular dendritic cells, myofibroblasts and macrophages in the human spleen – new considerations on the pathological diagnosis of splenectomy pieces. Int J Clin Exp Pathol. 2010;3:189–202. [PMC free article] [PubMed] [Google Scholar]
- 56.Pack M, Trumpfheller C, Thomas D, Park CG, Granelli-Piperno A, Münz C, Steinman R. DEC-205/CD205+ dendritic cells are abundant in the white pulp of the human spleen, including the border region between the red and white pulp. Immunology. 2008;123:438–46. doi: 10.1111/j.1365-2567.2007.02710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Satoh T, Takeda R, Oikawa H, Satodate R. Immunohistochemical and structural characteristics of the reticular framework of the white pulp and marginal zone in the human spleen. Anat Rec. 1997;249:486–94. doi: 10.1002/(SICI)1097-0185(199712)249:4<486::AID-AR8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 58.Tanaka H, Takasaki S, Sakata A, Muroya T, Suzuki T, Ishikawa E. Lymphocyte subsets in the white pulp of human spleen in normal and diseased cases. Acta Pathol Jpn. 1984;34:251–70. doi: 10.1111/j.1440-1827.1984.tb07554.x. [DOI] [PubMed] [Google Scholar]
- 59.Timens W, Poppema S. Lymphocyte compartments in human spleen. An immunohistologic study in normal spleens and noninvolved spleens in Hodgkin's disease. Am J Pathol. 1985;120:443–54. [PMC free article] [PubMed] [Google Scholar]
- 60.Franke WW, Moll R. Cytoskeletal components of lymphoid organs. I. Synthesis of cytokeratins 8 and 18 and desmin in subpopulations of extrafollicular reticulum cells of human lymph nodes, tonsils, and spleen. Differentiation. 1987;36:145–63. doi: 10.1111/j.1432-0436.1987.tb00189.x. [DOI] [PubMed] [Google Scholar]
- 61.Doglioni C, Dell´Orto P, Zanetti G, Iuzzolino P, Coggi G, Viale G. Cytokeratin-immunoreactive cells of human lymph nodes and spleen in normal and pathological conditions. Virchows Arch A Pathol Anat. 1990;416:479–90. doi: 10.1007/BF01600298. [DOI] [PubMed] [Google Scholar]
- 62.Pellas TC, Weiss L. Migration pathways of recirculating murine B cells and CD4+ and CD8+ T lymphocytes. Am J Anat. 1990;187:355–73. doi: 10.1002/aja.1001870405. [DOI] [PubMed] [Google Scholar]
- 63.Mitchell J. Lymphocyte circulation in the spleen. Marginal zone bridging channels and their possible role in cell traffic. Immunology. 1973;24:93–107. [PMC free article] [PubMed] [Google Scholar]
- 64.Bajenoff M, Glaichenhaus N, Germain RN. Fibroblastic reticular cells guide T lymphocyte entry into and migration within the splenic T cell zone. J Immunol. 2008;181:3947–54. doi: 10.4049/jimmunol.181.6.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iizuka T, Koike R, Miyasaka N, Miyasaka M, Watanabe T. Cloning and characterization of the rat MAdCAM-1 cDNA and gene. Biochim Biophys Acta. 1998;1395:266–70. doi: 10.1016/s0167-4781(97)00142-5. [DOI] [PubMed] [Google Scholar]
- 66.Iizuka T, Tanaka T, Suematsu M, et al. Stage-specific expression of mucosal addressin cell adhesion molecule-1 during embryogenesis in rats. J Immunol. 2000;164:2463–71. doi: 10.4049/jimmunol.164.5.2463. [DOI] [PubMed] [Google Scholar]
- 67.Jönsson V. Comparison and definition of spleen and lymph node: a phylogenetic analysis. J Theor Biol. 1985;117:691–9. doi: 10.1016/s0022-5193(85)80247-2. [DOI] [PubMed] [Google Scholar]
- 68.Glomski CA, Tamburlin J, Chainani M. The phylogenetic odyssey of the erythrocyte. III. Fish, the lower vertebrate experience. Histol Histopathol. 1992;7:501–28. [PubMed] [Google Scholar]
- 69.Puga I, Cols M, Barra CM, et al. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol. 2012;13:170–80. doi: 10.1038/ni.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.He B, Xu W, Santini PA, et al. Intestinal bacteria trigger T-cell independent immunoglobulin A2 class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812–26. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 71.Vossenkämper A, Blair PA, Niloufar S, et al. A role for gut-assocoated lymphoid tissue in shaping the human B cell repertoire. J Exp Med. 2013;210:1665–74. doi: 10.1084/jem.20122465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spencer J, Dogan A. A common migratory highway between human spleen and mucosa-associated lymphoid tissues; data from nature's own experiments. Mucosal Immunol. 2009;2:380–2. doi: 10.1038/mi.2009.91. [DOI] [PubMed] [Google Scholar]
- 73.Van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nat Rev Immunol. 2010;10:664–74. doi: 10.1038/nri2832. [DOI] [PubMed] [Google Scholar]
- 74.Koning JJ, Mebius RE. Interdependence of stromal and immune cells for lymph node function. Trends Immunol. 2012;33:264–70. doi: 10.1016/j.it.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 75.Mebius RE. Organogenesis of lymphoid tissues. Nat Rev Immunol. 2003;3:292–303. doi: 10.1038/nri1054. [DOI] [PubMed] [Google Scholar]
- 76.Cupedo T, Crellin NK, Papazian N, et al. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+CD127+ natural killer-like cells. Nat Immunol. 2009;10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- 77.Krautler NJ, Kana V, Kranich J, et al. Follicular dendritic cells emerge from ubiquitous perivascular precursors. Cell. 2012;150:194–206. doi: 10.1016/j.cell.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Da Silva Meirelles L, Chagastelles PC, Beyer Nardi N. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–13. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 79.Ngo VN, Cornall RJ, Cyster JG. Splenic T zone development is B cell dependent. J Exp Med. 2001;194:1649–60. doi: 10.1084/jem.194.11.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vondenhoff MF, Desanti GE, Cupedo T, Bertrand JY, Cumano A, Kraal G, Mebius RE, Golub R. Separation of splenic red and white pulp occurs before birth in an LTαβ-independent manner. J Leukoc Biol. 2008;84:152–61. doi: 10.1189/jlb.0907659. [DOI] [PubMed] [Google Scholar]
- 81.Nolte MA, Arens R, Kraus M, van Oers MH, Kraal G, van Lier RA, Mebius RE. B cells are crucial for both development and maintenance of the splenic marginal zone. J Immunol. 2004;172:3620–7. doi: 10.4049/jimmunol.172.6.3620. [DOI] [PubMed] [Google Scholar]
- 82.Mebius RE, Nolte MA, Kraal G. Development and function of the splenic marginal zone. Crit Rev Immunol. 2004;24:449–64. doi: 10.1615/critrevimmunol.v24.i6.40. [DOI] [PubMed] [Google Scholar]
- 83.Steiniger B, Ulfig N, Riße M, et al. Fetal and early post-natal development of the human spleen: from primordial arterial B cell lobules to a non-segmented organ. Histochem Cell Biol. 2007;128:205–15. doi: 10.1007/s00418-007-0296-4. [DOI] [PubMed] [Google Scholar]
- 84.Aiba Y, Kometani K, Hamadate M, et al. Preferential localization of IgG memory B cells adjacent to contracted germinal centers. Proc Natl Acad Sci USA. 2010;107:12192–7. doi: 10.1073/pnas.1005443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seok J, Warren HS, Cuenca AG, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA. 2013;110:307–12. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]


