Abstract
Inflammatory bowel disease (IBD), a chronic intestinal inflammatory condition that affects millions of people worldwide, results in high morbidity and exorbitant health-care costs. The critical features of both innate and adaptive immunity are to control inflammation and dysfunction in this equilibrium is believed to be the reason for the development of IBD. miR-155, a microRNA, is up-regulated in various inflammatory disease states, including IBD, and is a positive regulator of T-cell responses. To date, no reports have defined a function for miR-155 with regard to cellular responses in IBD. Using an acute experimental colitis model, we found that miR-155−/− mice, as compared to wild-type control mice, have decreased clinical scores, a reversal of colitis-associated pathogenesis, and reduced systemic and mucosal inflammatory cytokines. The increased frequency of CD4+ lymphocytes in the spleen and lamina propria with dextran sodium sulphate induction was decreased in miR-155−/− mice. Similarly, miR-155 deficiency abrogated the increased numbers of interferon-γ expressing CD4+ T cells typically observed in wild-type mice in this model. The frequency of systemic and mucosal T helper type 17-, CCR9-expressing CD4+ T cells was also reduced in miR-155−/− mice compared with control mice. These findings strongly support a role for miR-155 in facilitating pro-inflammatory cellular responses in this model of IBD. Loss of miR-155 also results in decreases in T helper type 1/type 17, CD11b+, and CD11c+ cells, which correlated with reduced clinical scores and severity of disease. miR-155 may serve as a potential therapeutic target for the treatment of IBD.
Keywords: Crohn's disease, inflammatory bowel disease, miR-155−/−, T helper type 1/type 17, ulcerative colitis
Introduction
Inflammatory bowel disease (IBD), a relapsing inflammatory condition of unknown aetiology, is characterized as a common yet serious chronic condition of the human bowel. Although the exact cause of IBD remains unknown, epidemiological and experimental studies suggest that both environmental and genetic factors associated with dysregulation of the mucosal immune system are involved in the pathogenesis of this disease.1–3 Mucosal changes in IBD are characterized by prominent infiltration into the colon of various cells, including T lymphocytes, macrophages and neutrophils. Although chronic IBD inflammation is maintained by T helper type 1 (Th1) -driven immune responses, the overall mechanism responsible for IBD is believed to involve a complex interplay between varieties of inflammatory mediators. Recently, Th17 infiltrating cells have received significant attention because increased expression of these cells is evident in patients with Crohn's disease, as well as experimental models of colitis.4–6 However, the function of interleukin-17 (IL-17) in intestinal inflammation remains controversial, as IL-17 has been shown to be important in both inducing and abating chemically induced colitis in mice.7,8
MicroRNAs (miRNAs) are endogenous small RNA molecules of 22 nucleotides in length that post-transcriptionally regulate multiple genes by binding to target mRNAs, thereby inhibiting their translation. To date, more than 100 miRNAs have been identified in cells of the innate and adaptive immune systems. Given its recent association with the regulation of immune function, as well as cancer development,9 miR-155 has received a great deal of interest. For example, miR-155 has been reported to be expressed by both B and T lymphocytes, as well as macrophages, following bacterial and viral activation.10,11 In addition, miR-155−/− mice exhibit an impaired immune response to various pathogens and immunizations, demonstrating an alteration in Th cell subset polarization.12 In monocytes, miR-155 expression increases after Toll-like receptor activation.13 Upon maturation, this expression similarly increases in various subsets of mouse and human dendritic cells (DCs) in response to various Toll-like receptor ligands and interferon-α (IFN-α).14,15 Moreover, DCs lacking miR-155 fail to effectively activate T cells.12 These findings suggest that miR-155 is critically important in T-cell and monocyte activation, as well as in DC maturation.
Increased expression of miR-155 has been reported in many inflammatory diseases, including ulcerative colitis and Crohn's disease.16,17 However, relatively little is known about the capacity of miR-155 to regulate mucosal immunity, particularly in IBD.18 Therefore, during the genesis and effector phases, we examined the role of miR-155 in intestinal inflammation in an experimental model of colitis. Using miR-155-deficient and BL/6 mice, we examined and characterized the cellular populations known to take prominent roles in experimental colitis development, including Th1/Th17, CCR9, macrophages and DCs.
Materials and methods
Animals
Our research team bred female miR-155−/− mice on a C57BL/6 background. Female wild-type C57BL/6 mice, aged 8–12 weeks, were purchased from Jackson Laboratories (Bar Harbor, ME). Animals were housed at the University of South Carolina's School of Medicine Animal Facility. To minimize their pain and distress, mice were maintained in isolator cages under normal light and dark cycles and conventional housing conditions. Experimental groups consisted of six mice; studies were repeated at least three times. The University of South Carolina's Institutional Animal Care and Use Committee approved all animal experimentation used in this study.
Colitis induction by dextran sodium sulphate
Experimental colitis was induced using dextran sodium sulphate (DSS) as previously described.19 Briefly, 8-12-week-old miR-155−/− mice and C57BL/6 mice received either plain water or drinking water containing 1% DSS (MP Biomedical, LLC, Solon OH) ad libitum for 7 days, followed by 7 days of plain water. This routine was repeated for three cycles totalling 42 days. The body weight of mice was monitored every 2 days after the initiation of DSS. Other symptoms of sickness, including diarrhoea, stool consistency and blood in faecal matter were monitored during this time. At the end of the experimental period, blood was collected and colon samples were washed with PBS, cut longitudinally, fixed in formalin, and embedded in paraffin.
Cell isolation
At the experimental end point, spleens and mesenteric lymph nodes (MLN) from individual mice in all groups were dissociated; red blood cells were lysed using lysis buffer (Sigma, St Louis, MO). After centrifugation, single-cell suspensions of spleen and MLN were passed through a sterile filter (Sigma) to remove any debris. Subsequently, cell suspensions were washed twice in RPMI-1640 (Sigma) and stored in medium containing 5% fetal bovine serum on ice or at 4° until later use on the same day. Cells from the intestinal lamina propria (LP) were isolated as described previously.20 In brief, the small intestine/colon was cut into 1-cm strips and stirred in PBS containing 1 mm EDTA at 37° for 30 min. The intestinal tissue was digested with collagenase type IV (Sigma) in RPMI-1640 (collagenase solution) for 45 min at 37° with moderate stirring. After each 45-min interval, the released cells were centrifuged and stored in complete medium. Mucosal pieces were again treated at least twice with fresh collagenase solution and cells were then pooled. LP cells were further purified using a discontinuous Percoll gradient (Pharmacia, Uppsala, Sweden) collecting at the 40–75% interface. Lymphocytes were maintained in complete medium as described in detail in our earlier publications.19,21
Flow cytometry staining and analysis
Cells from the spleen, MLN and LP for each experimental group were isolated as described in the preceding section. For three- to four-colour FACS cell-surface antigen staining, cells were pre-blocked with Fc receptors for 15 min at 4°. The cells were washed with FACS staining buffer (PBS) with 1% fetal bovine serum, then stained with the manufacturer's suggested concentration for FITC- or allophycocyanin-conjugated anti-CD4 (GK1.5) (Biolegend, San Diego, CA), CD11b (M1/70) (BD-PharMingen, San Diego, CA), FITC-conjugated IFN-γ (XMG-1·2) (e-Bioscience, San Diego, CA), phycoerythrin (PE) -conjugated anti-IL-17A monoclonal antibody (TC11-18H10.1) (Biolegend), PE-conjugated anti-mouse CCR9 (clone 242503; R & D Systems, Minneapolis, MN) and PE-conjugated anti-mouse CD11c (HL3) (BD-PharMingen) for 30 min at 4° with occasional shaking. The cells were washed twice with FACS staining buffer and thoroughly re-suspended in BD Cytofix/Cytoperm (BD-PharMingen) solution for 20 min. The cells were again washed twice with BD perm/wash solution after storage for 10 min at 4°. Intracellular staining for IFN-γ and analysis was performed according to the BD Bioscience protocol. Cells were then washed thoroughly with FACS staining buffer and analysed by flow cytometry (FC 500 by Beckman Coulter, Fort Collins CO).
Systemic cytokine measurement by Luminex™ analysis
Levels of T helper cell-derived cytokines IL-6, tumour necrosis factor-α (TNF-α), IL12-p40, IL12-p70, IL-17 and IFN-γ in the serum were determined using a luminex ELISA kit (Bio Rad, Hercules, CA). In brief, IL-6, TNF-α, IL-12-p40, IL-12-p70, IL-17 and IFN-γ analyte beads contained in assay buffer were added to pre-wetted vacuum wells followed by 50 μl of assay beads. The buffer was then removed and the wells underwent a wash cycle. Next, 50 μl of standard or serum was added to each well and the plate was incubated for 1 hr and subjected to continuous shaking (at setting #3) using a Lab-Line™ Instrument Titer Plate Shaker (Melrose, IL). The filter bottom plates were then washed and vortexed at 300 g for 30 seconds. Subsequently, 25 μl of anti-mouse detection antibody was added to each well and incubated for 30 min at room temperature. Then, 50 μl of streptavidin-phycoerythrin solution was added and each plate was again incubated for 10 min at room temperature with continuous shaking. We then added 125 μl of assay buffer; we measured BioRad™ readings using a Luminex™ System (Austin, TX) and calculated using BioRad software. The Ab BioRad™ Multiplex Analysis Pathways (MAP) assays are capable of detecting > 10 pg/ml for each analyte.
Systemic IL-23 and latent transforming growth factor-β ELISA
Serum IL-23 and latent transforming growth factor-β (TGF-β) levels were determined by an ELISA kit, according to the manufacturer's protocol (Biolegend). In brief, after washing of pre-coated wells with 300 μl of washing buffer, 50 μl of serum sample (undiluted for IL-23 and 1 : 50 dilution for latent TGF-β), standards, and 50 μl of assay buffer were added to appropriate wells. Micro-plates were covered and incubated for 2 hr at room temperature with constant shaking. After washing with the wash buffer, 100 μl of mouse detection antibody was added to micro-plate wells, which were then incubated with constant shaking for 1 hr at room temperature. After washing with buffer, 100 μl of avidin-HRP A (IL-23) and avidin-HRP D (TGF-β) were added and samples were incubated for 30 min at room temperature. After washing with buffer, 100 μl of substrate solution (E for IL-23) and substrate (D for TGF-β) were added to the wells and incubated at room temperature for 20 min. After that, the reaction was stopped by adding stop solution, and the plates were read at 450 nm.
miR-155 gene expression after DSS induction
Briefly, at the end of experiments spleen cells were isolated from mice in all groups. The cells were stained with CD4 antibody and sorted on the FACS Aria II sorter. After purity was checked, more than 96% pure cells were used for total RNA isolated from CD4+ T cells using a Qiagen miRNeasy Mini Kit (Valencia, CA) according to the manufacturer's instructions. RNA was reverse transcribed into cDNA using a TaqMan® MicroRNA reverse transcription kit (Applied Biosystems, Foster City, CA). Briefly, RNA was reverse transcribed into cDNA in a 15-μl reaction volume containing 5 μl RNA (100 ng) in RNase-free water, 1·5 μl 10 × room temperature buffer, 0·15 μl deoxyNTPs mixture, 0·19 μl RNase inhibitor, 1·0 μl multiscribe reverse transcriptase (50 U/μl), 4·16 μl RNase-free water, and 3 μl of primers [either snoRNA202 (as an internal control) or mmu_miR-155 (Applied Biosystems)]. Reverse transcription was performed at 16° for 30 min, 42° for 30 min and 85° for 5 min, followed by quick chilling on ice and storage at −20° until subsequent amplification. Quantitative real-time RT-PCR analysis was performed according to the manufacturer's instructions (Applied Biosystems). DNA amplification was carried out in 10·00 μl Taqman Universal PCR Master Mix (AmpliTaq Gold DNA Polymerase, Passive Reference 1, buffer, dNTPs, AmpErase UNG), 1·33 μl cDNA, 7·67 μl RNase-free water, and 1·00 μl primer [either snoRNA202 (internal control) or mmu_miR-155 in a final volume of 20 μl/well]. Samples were loaded in a MicroAmp 96-well reaction plate. Plates were run using the Applied Biosystems Sequence Detection System. After 2 min at 50° and 10 min at 95°, samples were co-amplified by 40 repeated cycles, one of which consisted of a 15-second denaturing step at 95° and a 1-min annealing/extending step at 60°. Data were analysed with Applied Biosystems software using the cycle threshold, which is the value calculated and based on the time (measured by PCR cycle number) at which the reporter fluorescent emission increases beyond a threshold level based on the background fluorescence of the system. This reflects the cycle number at which cDNA amplification is first detected. Samples were run in duplicate.
Histology
The colon was preserved using 10% buffer neutral formalin followed by 4% paraformaldehyde, then embedded in paraffin. Fixed tissues were sectioned at 6 μm and stained with haematoxylin & eosin for microscopic examination. Intestinal sections were graded according to the number and severity of lesions.
Quantifying inflammatory score
The histological slides from intestinal tissues and livers (quality control) were examined and scored by two individuals in a blinded fashion. A score (0–12) was given based on previously established criteria.20 The summation of scores provided a total colonic disease score per mouse. Inflammation was graded by extent (focal, multifocal, diffuse or extensive areas) and depth or penetration of inflammation (into the lamina propria, submucosa and subserosa), then given a numerical value of 0–12 based on the following criteria: grade 0, no change observed from normal tissue; grade 1, 1 to few multifocal mononuclear cell infiltrates in the lamina propria, minimal hyperplasia; grade 2, intestinal lesions involved with several multifocal, mild inflammatory cell infiltrates in the lamina propria; grade 3, lesions with moderate inflammation and epithelial hyperplasia; grade 4, inflammation involves most of the intestinal sections. The summation of these scores provided a total colonic disease score that ranging from 0 to 12, with grade 4 lesions in proximal, middle and distal colon segments.
Statistics
The data are expressed as the mean ± SEM and compared using a two-tailed Student's t-test, analysis of variance, and/or an unpaired Mann–Whitney U-test. The results were analysed using the Statview II statistical program (Abacus Concepts, Berkeley, CA) for Macintosh computers, and were considered statistically significant if P values were < 0·05.
Results
Changes in body weight during experimental colitis
Previous studies have suggested that a reduction in body weight is the hallmark for monitoring the severity of experimental colitis. Here, we examined the effect of DSS on changes in body weight in miR-155−/−, wild-type, and naive (i.e. with no DSS exposure) C57BL/6 mice. Because we observed no significant changes in body weight in naive C57BL/6 mice throughout the study, statistical comparisons were made only between the miR-155−/− and wild-type mice given DSS exposure. In wild-type mice, the development of chronic colitis was shown by c.14–17% decreases in body weight, which continued to decline throughout the study (Fig.1). These mice also had occasional rectal bleeding. In contrast, miR-155−/− mice had modest decreases in body weight and little or no evidence of rectal bleeding. These results suggest that miR-155 deficiency abrogates colitis-induced weight loss in these mouse cohorts.
Figure 1.
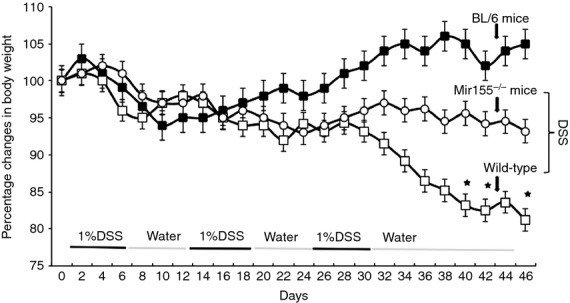
Change in body weight of BL/6, wild-type, and miR-155−/− mice after dextran sodium sulphate (DSS) exposure. Control BL/6, wild-type and miR-155−/− mice were given no treatment (▪ control). Others were given DSS alone (1%) in drinking water for 7 days (□ wild-type mice; ○ miR-155−/− mice). After 7 days, DSS was replaced with a water cycle (ad libitum) for another 7 days. This was repeated for two more cycles of 7 days. The body weight of mice was recorded every 2 days. Changes in body weight were expressed as the percentage of baseline body weight. The statistical significance between values for each group was assessed by analysis of variance. Data represent the mean of three experiments involving six mice per group (n = 18).
Alterations in T-helper cell responses in miR-155−/− mice after DSS induction
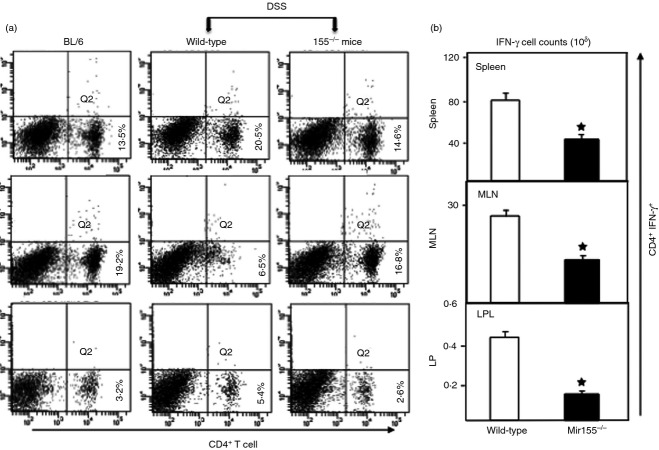
Previous studies have shown that T cells express the miR-155 transcript following bacterial or viral challenge and activation.12 Further, miR-155 deficiency has been linked to impairments in immune pathways related to antigen presentation and T-cell immunity.10,12 Given that miR-155 can influence T-cell responses and that Th1 activity is thought to be a hallmark of colitis progression, we examined these mice for the presence of CD4+ T-cell subpopulations in the spleen, MLN, and LP, using flow cytometric analysis. After DSS induction, the frequency of CD4+ T cells increased in the spleen and LP of wild-type mice compared with naive mice; however, no increases were observed in miR-155−/− mice (Fig.2a). Conversely, upon DSS exposure, the percentage of CD4+ T cells in MLN declined in wild-type mice compared with naive BL/6 mice, while this effect was not evident in miR-155−/− mice.
Figure 2.
Changes in CD4+ T cells and T helper type 1 (Th1) response after dextran sodium sulphate induction. Spleen cells, mesenteric lymph nodes (MLN) and lamina propria (LP) lymphocytes were isolated from three groups of mice as described in Fig.1, stained for CD4+ and interferon-γ (IFN-γ) cell markers, and analysed by flow cytometry. The numbers in the lower right quadrant indicate the total percentage of CD4+ T cells (a). The changes in the number of IFN-γ-expressing CD4+ T cells are shown in (b). Data from a representative of three independent experiments involving six mice per group are shown. Asterisks indicate statistically significant differences between wild-type and miR-155−/− groups; *P < 0·01.
Next, we examined the numbers of Th1 cells in these mice. As shown in Fig.2b, irrespective of the tissue site examined, the number of CD4+ T cells expressing IFN-γ was lower in miR-155−/− mice than in wild-type mice (Fig.2b). These findings clearly indicate that in wild-type mice, DSS-induced colitis leads to a considerable increase in the Th1 response and the percentages of T-helper lymphocytes in the spleen and LP, but decreases the frequency of CD4+ T cells in the MLN. However, using miR-155−/− mice, the DSS-induced increase in CD4+ T lymphocytes in the spleen and LP, as well as the decrease in MLN, was abrogated.
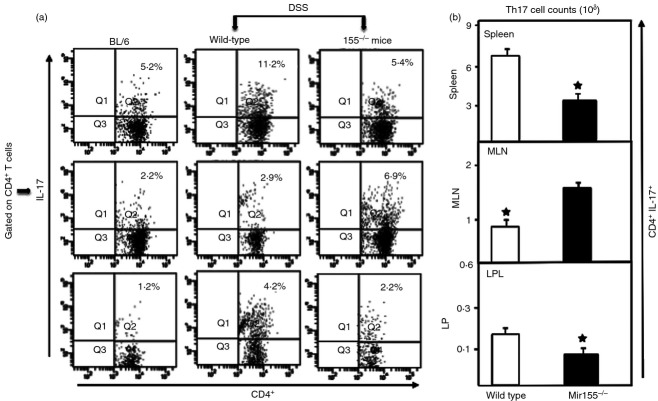
miR-155−/− mice demonstrate impairment in the Th17 response associated with colitis
The expression of both mucosal and systemic Th17 (IL-17A) CD4+ cells decreased in the spleens and LP of miR-155−/− mice compared with wild-type mice (Fig.3a). In contrast, we found an increase in Th17 cells in the MLN of miR-155−/− mice compared with wild-type mice. We found a significant decline in the number of Th17-expressing CD4+ T cells in the spleens and LP of miR-155−/− mice compared with those in wild-type mice (Fig.3b). However, we again found significant increases in the numbers of Th17 cells in MLN of miR-155−/− mice. The altered numbers and frequency of Th17 cells in MLN with miR-155−/− suggests that these cells increase at the inductive stage of colitis in response to DSS, but do not migrate to the LP to carry out their effector functions.
Figure 3.
miR-155−/− mice show diminished expression of interleukin-17A+ (IL-17A+) expressing CD4+ T cells. Spleen, mesenteric lymph nodes (MLN) and lamina propria (LP) lymphocytes were isolated from all groups of mice at the experimental end point, as described in Fig.1. Changes in the frequency and expression of IL-17A+ expressing CD4+ T cells from spleen lymphocyte, MLN and LP are shown in (a). The numbers in the upper right quadrant indicate the total percentage of IL-17A+ cells (a). Changes in the number of CD4+ T cells expressing IL-17A are shown in (b). Experiments involved six mice per group. Asterisks indicate statistically significant differences between wild-type and miR-155−/− groups; i.e. *P < 0·01.
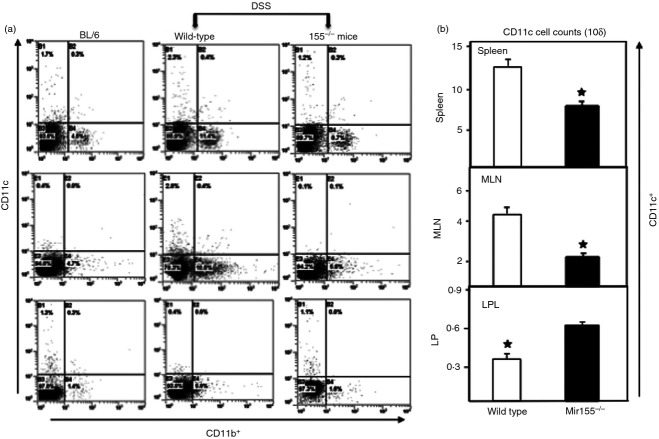
miR-155−/− mice show diminished CD11b+ and CD11c+ responses
It has been shown that miR-155 expression is up-regulated upon activation of macrophages10 and monocytes in response to TLR.13 Moreover, given that DCs lacking miR-155 fail to activate T cells effectively,12 miR-155 up-regulation appears to be a defining feature in DC maturation and function. Based on these findings, we next examined the changes in numbers of CD11b+ and CD11c+ cells in wild-type and miR-155−/− mice in response to DSS-induced colitis. miR-155−/− mice, as compared to wild-type mice, showed decreased frequency of both CD11b+ and CD11c+ cells in the spleen and MLN (Fig.4). However, we observed a slight increase in CD11c+ cells in the LP of miR-155−/− mice as compared to wild-type mice. This finding indicates that miR-155−/− mice experience a decline in their populations of CD11b+ and CD11c+ cells after colitis induction, suggesting a mechanism that possibly contributes to the observed decrease in colitis in these mice.
Figure 4.
Dextran sodium sulphate exposure is ineffective in macrophages and dendrites of miR-155−/− mice. Spleen, mesenteric lymph nodes (MLN), and lamina propria (LP) immune cells were isolated from all groups of mice at the experimental end point as described in Fig.1. Changes in the frequency and expression of CD11b+ and CD11c+ cells from spleens, MLN and LP are shown (a). Changes in the number of CD11c are shown in (b). Asterisks indicate statistically significant differences between wild-type and miR-155−/− groups; i.e. P < 0·01 (*).
Systemic cytokine levels did not change in miR-155−/− mice
It has been shown that IL-12, IL-23 (with a IL-12p40 subunit) and IFN-γ are critical in the induction and progression of colitis.22,23 Increased expression of TNF-α, IL-6 and IL-17 in inflammatory diseases, including IBD and experimental colitis, has been well documented.4,24 Therefore, we next determined whether miR-155 deficiency could affect the increase in systemic cytokine concentrations that is characteristic of IBD. Our results showed that serum IL-6, TNF-α, IFN-γ, IL-12p70 and IL-17 levels increased in wild-type mice as compared to miR-155−/− mice (Fig.5). Surprisingly, we observed an increase in serum levels of IL-12p70 in miR-155−/− mice compared with wild-type mice (Fig.5). These results indicate that the absence of miR-155 leads to a reduction in systemic inflammatory cytokine levels in a model of experimental colitis and may explain, at least in part, the improved clinical outcomes observed in these mice.
Figure 5.
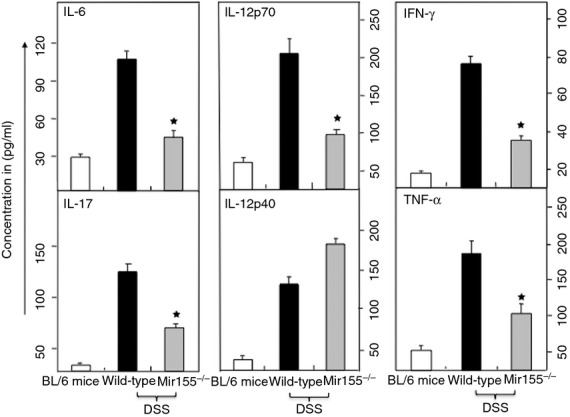
Systemic levels of interleukin-6 (IL-6), tumour necrosis factor-α (TNF-α), IL17, IL-12p70m and interferon-γ (IFN-γ) are reduced in miR-155−/− mice. After sacrifice, serum levels of IL-17, IL-12p70, IL-12p40, IFN-γ, IL-6 and TNF-α were determined by Bio-Rad ELISA multiplex kit, which is capable of detecting > 15 pg/ml of these analytes. The data presented are the mean concentrations of IL-17, IL-12p70, IL-12p40, IFN-γ, IL-6, and TNF-α ± SEM from three separate experiments. Asterisks (*) indicate statistically significant differences (P < 0·01) between wild-type and miR-155−/− mouse groups.
miR-155 expression and systemic IL-23 and latent TGF-β level during colitis
Several lines of evidence indicate that TGF-β is important in inducing tolerance and control of autoimmune inflammation in the gut. TGF-β signalling in T cells blocks the induction of inflammation at gut mucosal sites.25 Increased expression of miR-155 has been reported in many inflammatory diseases, but relatively little is known regarding the capacity of miR-155 to regulate mucosal immunity, particularly in IBD.18 Therefore, we next examined whether DSS induction increases miR-155 expression in CD4+ T cells. Our results clearly show that mice treated with DSS have enhanced expression of miR-155 compared with naive counterparts (Fig.6a).
Figure 6.
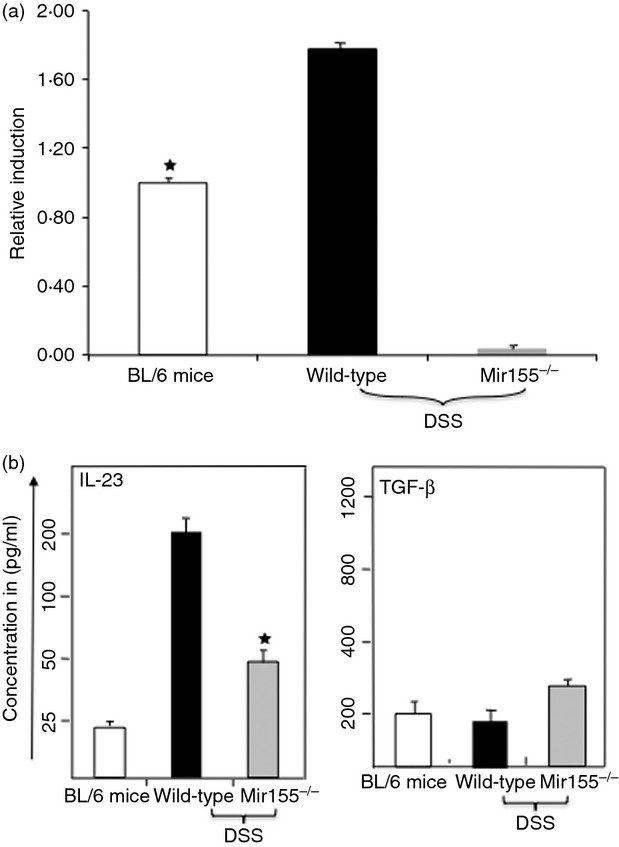
Systemic level of interleukin-23 (IL-23), and transforming growth factor-β (TGF-β), as well as miR-155 expression, during colitis. After sacrifice, serum levels of IL-23 and TGF-β were determined using a Biolegend ELISA assay kit. The data presented are the mean concentrations of IL-23 and TGF-β ± SEM from three separate experiments. Asterisks (*) indicate statistically significant differences (P < 0·01) between wild-type and miR-155−/− mouse groups. The data presented are the mean concentrations of IL-23 and TGF-β involving six mice per group.
The results also show that serum IL-23 level increased in wild-type mice compared with miR-155−/− mice given DSS (Fig.6b). Further, we observed a slight increase in serum levels of latent TGF-β in miR-155−/− mice compared with wild-type mice (Fig.6b). Taken together, all of these findings indicate that DSS induction enhances the expression of miR-155, and that the absence of miR-155 leads to a reduction in IL-23 cytokine levels and a slight increase in the latent TGF-β level in a model of experimental colitis which may explain the beneficial effect in mice.
Severity of colitis is diminished in miR-155−/− mice
At the experimental end point, changes in colon pathology were examined in the three groups of mice. As expected, the colons of naive mice exhibited hypertrophied epithelial layers at multiple sites with only a few inflammatory infiltrates composed mainly of lymphocytes. The colon histology of wild-type mice treated with DSS showed extensive small to multifocal cellular infiltrates composed mainly of lymphocytes and macrophages (Fig.7 middle panel). In contrast, lack of miR-155 reversed the observed increase in cellular infiltrates and severity of disease after DSS treatment (Fig.7 right panel). These results, together with reduced disease severity and inflammatory infiltrates, support a protective effect of miR-155 deficiency in experimental colitis.
Figure 7.
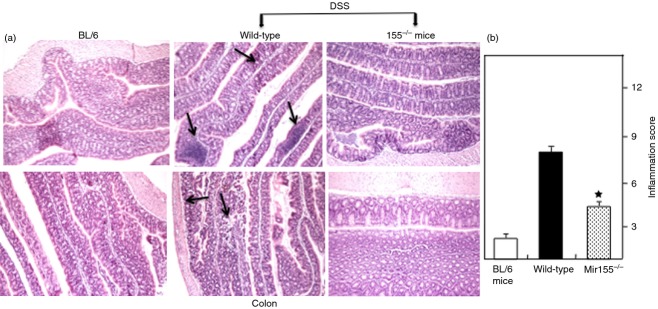
Histological characterization and inflammation score. Histological sections of the colon from the groups of mice described in Fig.1 are presented. Wild-type mice (middle panel) showed significant lymphocyte infiltration and distortion of glands, while miR-155−/− mice (a, left panel) showed markedly decreased lymphocyte infiltration. Naive BL/6 mice showed no cellular infiltration. The pathological changes included diffuse leucocyte infiltrates and thickening of the lamina propria in the area of distorted crypts in the colon. The inflammation score is depicted (b, right panel). Representative sections from three separate experiments (20× magnification) are shown, with groups containing six mice each.
DSS induction increased homing in MLN and LPL of BL/6 mice
We also examined whether DSS exposure induces T-cell homing or migration to inductive and effector sites. In all groups of mice, expression of CCR9+ CD4+ cells changed little in the spleen (Fig.8). In contrast, there was a significant increase in these populations in MLN and LPL of BL/6 mice given DSS as compared to the frequency of these cells in miR-155−/−-treated mice (Fig.8). The altered frequency of CCR9+ CD4+ cells in the MLN and LPL in mice that received DSS compared with the frequency of these cells in miR-155−/− mice suggested that CCR9+ CD4+ cells increase at both inductive and effector sites for their effector functions.
Figure 8.
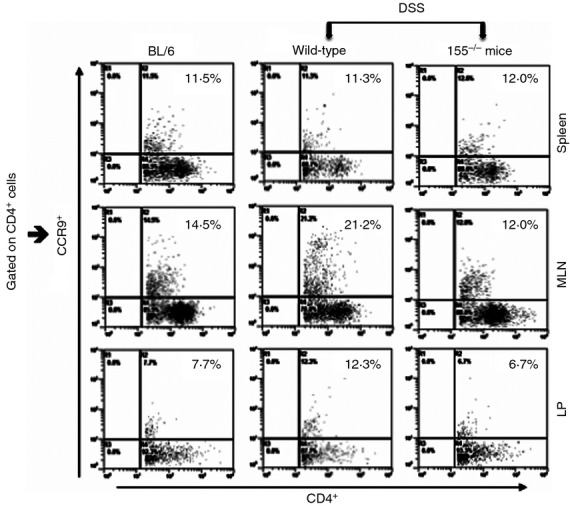
miR-155−/− mice show reduced homing of CCR9+ expressing CD4+ T cells. Spleens, mesenteric lymph nodes (MLN) and lamina propria (LP) lymphocytes were isolated from all groups of mice at the experimental end point as described in Fig.1. Changes in the frequency and expression of CCR9+-expressing CD4+ T cells from spleen, MLN and LPs are shown. The numbers in the upper right quadrant indicate the total percentage of CCR9+ cells gated on CD4 cells.
Discussion
Dysregulation of miR-155 is observed in several autoimmune diseases, including colitis.26,27 In fact, increased expression of miR-155 has been reported in many inflammatory diseases, including IBD.16,17 It has also been demonstrated that miR-155 has a key role in Th1 differentiation and IFN-γ production.28 For instance, a recent study using a non-infectious disease model and miR-155 null mice confirmed the involvement of miR-155 in mediating Th subset differentiation, including the defective development of Th17 cells, which was linked to experimental autoimmune encephalomyelitis resistance.
In the present study, we investigated the role of miR-155 in protecting mucosa-associated immunity in a chemically induced mouse model of colitis. We found that miR-155-deficient mice are resistant to DSS-induced colitis, demonstrating only mild colitis-associated symptoms (i.e. minor change in body weight and no diarrhoea or blood in faeces). On the cellular level, miR-155−/− mice exposed to DSS exhibit diminished frequencies of Th1/Th17 cells, macrophages, and dendritic cells, which correlates with reduced histological disease severity scores.
Several experimental models of IBD indicate that CD4+ T cells play a major part in disease induction. Indeed, the intestinal damage associated with IBD is known to be a consequence of T-cell-mediated injury.29 In support of this, we have shown that adoptive transfer of CD4+ T cells expressing CXCR3, a chemokine for Th1 cells, results in colitis in T-cell recepetor (β × δ)−/− mice.30 Although there are no reports that miR-155 plays a part in T-cell responses in a colitis model, it has recently been shown that miR-155 over-expression can lead to preferential differentiation of CD4+ T cells toward a Th1 phenotype and IFN-γ production.12 Further, it has been shown that miR-155 transcript expression positively correlates with protein levels of IFN-γ.31 In the present study, we observed, following DSS exposure, a significant decrease in CD4+ T cells in the spleens and LP of miR-155−/− mice compared with wild-type mice. Similarly, we found a decrease in IFN-γ-expressing CD4+ T cells in these tissues in miR-155−/− mice. These findings suggest that miR-155 knockout leads to impairments in CD4+ T cells that result in a reduction in the differentiation of these cells at the effector site (colon), as well as to decreased IFN-γ production and, ultimately, protection from advanced development of experimental colitis.
Increased activation of the IL-17 system in the mucosa of IBD patients is not only linked to disease severity4 and inflammatory processes6 but has also been shown to be important for the induction of experimental colitis in mouse models.7 In contrast, one study has shown that IL-17 may offer protection in the DSS-induced acute model of colitis.8 However, our current model is distinct in that we used three cycles of a low dose of DSS, which results in lower antigen exposure and activation of both macrophages and T cells. Using this model, we found that, following DSS exposure both mucosal and systemic Th17 cell frequency is decreased in miR-155−/− mice compared with wild-type mice. Our findings are corroborated by studies that used various disease models. For example, a recent study documented a role of miR-155 in Th17 differentiation and consequently reported that miR-155−/− mice are highly resistant to experimental autoimmune encephalomyelitis.10 Similarly, decreased gastric levels of IL-17 in miR-155−/− mice suggest defective Th17 differentiation and function.18 It is also thought that miR-155 plays a role in Th17 cell responses, the reason being that these cells have been reported to unleash autoimmune inflammatory processes involving miR-155, Ets1, and the clinically relevant IL-23–IL-23R pathway.32 Our data suggest that impairment of Th17 responses in miR-155−/− mice is associated with protection from DSS-induced colitis.
It is well known that mucosal DCs can take up antigens from the gut and present them to CD4+ and CD8+ T cells.33 After the early phase of acute inflammation, DCs undergo maturation and migrate from non-lymphoid to lymphoid tissues, where they stimulate naive T cells.34 DC activation is a critical step in colitis development and reportedly is enhanced in the colons of CD patients.35 In this study, we observed significant decreases in both the frequency and number of CD11c+ cells in the spleen and MLN of miR-155−/− mice compared with wild-type mice. After DSS treatment, a similar pattern was found with respect to CD11b+ cells; there was a decrease in these cells in the spleen, MLN and LP of miR-155−/− mice compared with wild-type mice. These results suggest a dysfunction in both CD11b+ and CD11c+ cells in miR-155−/− mice, which probably offsets the promotion of colitis in this model. It has been shown that miR-155 plays a regulatory role in DC maturation and optimal function of DCs. Further, DCs lacking miR-155 fail to effectively activate T cells.12 MiR-155 deficiency in DCs also leads to reduced SOCS-1 expression and subsequent suppression of IL-12p70 cytokine production in mature DCs.14 This supports our observation of reduced systemic IL-12p70 cytokine levels in miR-155−/− mice. Although our data support a function of miR-155 in reducing CD11b+ cells, which are linked to protection against colitis, in the spleen, MLN, and LPs, a more detailed study on the precise mechanism by which miR-155 regulates these effects on macrophages and DCs is necessary.
Increases in systemic levels and tissue expression of TNF-α and IL-6 have also been reported in several models of colitis and in patients with Crohn's disease.20,36–38 Interleukin-23 is essential to T-cell-mediated colitis and promotes inflammation via IL-17 and IL-6.6 Further, it is well established that IL-12 drives Th1 differentiation and IFN-γ production. Evidence indicates that these factors play a central role in the progression of colitis.22,39,40 Here, we demonstrate decreased secretion of TNF-α, IL-6, IL-12, IL-17 and IFN-γ levels in the serum of miR-155−/− mice, indicating the involvement of miR-155 in decreasing Th1 responses and reducing IFN-γ. We also noted a significant increase in IL-23 in the group of mice given DSS compared with miR-155−/− mice. This suggests that IL-17 and IL-6 pathways activated by IL-23 may be responsible for intestinal inflammation.
Both Th1 and Th17-type effector cells have been implicated in the progression of intestinal inflammation.6,24 It has been shown that γδ T-cell subsets are distinct and express the receptor for IL-23, a cytokine known to stimulate these cells to produce IL-17.41,42 CCR9 is one of the important chemokines involved in gut-specific migration of leucocytes. The up-regulation of CCR9 regulates trafficking of gut-specific memory or effector T cells to the gut.43 In the present study, we found that mice given DSS, compared with miR-155−/− mice, showed increased expression of CD4+ CCR9+ cells. This clearly suggests that the subsets homing at MLN and LP enhance the IL-17 production mediated by IL-23 to increase chronic inflammation in BL/6 mice compared with miR-155−/− mice.
In summary, we have demonstrated that miR-155−/− mice are protected from advanced DSS-induced colitis development. It is likely that miR-155 mediates this protection through multiple pathways, including impaired IL-17 production by CD4+ T cells, a Th1-biased phenotype, diminished activation of T cells by DCs, and reduced systemic cytokine production, presumably as a consequence of the down-regulation of CD11b+ (both frequency and number). These combined effects are probably responsible for the reduced severity of colitis in miR-155−/− mice. In conclusion, intrinsic cellular expression of miR-155 is required for Th1/Th17 and DC differentiation and function in colitis. However, a more detailed study is needed before drawing any firm conclusions regarding the clinical efficacy of miR-155 inhibition in colitis and IBD.
Acknowledgments
This work was supported in part by National Institutes of Health (NIH) grants R56 DK087836 (UPS), R21CA167058, K01AT007824 (AEM), and P01 AT003961 (MN and PN); Research and Development Funds from the University of South Carolina School of Medicine; and the Intramural Program of the National Institute on Aging, NIH.
Disclosures
All authors assure that there is no competing conflict of interest.
References
- 1.Podolsky DK. Inflammatory bowel disease (1) N Engl J Med. 1991;325:928–37. doi: 10.1056/NEJM199109263251306. [DOI] [PubMed] [Google Scholar]
- 2.Dohi T, Fujihashi K, Kiyono H, Elson CO, McGhee JR. Mice deficient in Th1- and Th2-type cytokines develop distinct forms of hapten-induced colitis. Gastroenterology. 2000;119:724–33. doi: 10.1053/gast.2000.16500. [DOI] [PubMed] [Google Scholar]
- 3.Braegger CP, MacDonald TT. Immune mechanisms in chronic inflammatory bowel disease. Ann Allergy. 1994;72:135–41. [PubMed] [Google Scholar]
- 4.Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pene J, Chevalier S, Preisser L, et al. Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. J Immunol. 2008;180:7423–30. doi: 10.4049/jimmunol.180.11.7423. [DOI] [PubMed] [Google Scholar]
- 6.Yen D, Cheung J, Scheerens H, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–6. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Z, Zheng M, Bindas J, Schwarzenberger P, Kolls JK. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm Bowel Dis. 2006;12:382–8. doi: 10.1097/01.MIB.0000218764.06959.91. [DOI] [PubMed] [Google Scholar]
- 8.Ogawa A, Andoh A, Araki Y, Bamba T, Fujiyama Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin Immunol. 2004;110:55–62. doi: 10.1016/j.clim.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 9.O'Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol. 2010;10:111–22. doi: 10.1038/nri2708. [DOI] [PubMed] [Google Scholar]
- 10.O'Connell RM, Kahn D, Gibson WS, et al. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity. 2010;33:607–19. doi: 10.1016/j.immuni.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tili E, Michaille JJ, Cimino A, et al. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-α stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol. 2007;179:5082–9. doi: 10.4049/jimmunol.179.8.5082. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez A, Vigorito E, Clare S, et al. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316:608–11. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103:12481–6. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu C, Huang X, Zhang X, et al. miR-221 and miR-155 regulate human dendritic cell development, apoptosis, and IL-12 production through targeting of p27kip1, KPC1, and SOCS-1. Blood. 2011;117:4293–303. doi: 10.1182/blood-2010-12-322503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou H, Huang X, Cui H, Luo X, Tang Y, Chen S, Wu L, Shen N. miR-155 and its star-form partner miR-155* cooperatively regulate type I interferon production by human plasmacytoid dendritic cells. Blood. 2010;116:5885–94. doi: 10.1182/blood-2010-04-280156. [DOI] [PubMed] [Google Scholar]
- 16.Takagi T, Naito Y, Mizushima K, et al. Increased expression of microRNA in the inflamed colonic mucosa of patients with active ulcerative colitis. J Gastroenterol Hepatol. 2010;25(Suppl. 1):S129–33. doi: 10.1111/j.1440-1746.2009.06216.x. [DOI] [PubMed] [Google Scholar]
- 17.Fasseu M, Treton X, Guichard C, et al. Identification of restricted subsets of mature microRNA abnormally expressed in inactive colonic mucosa of patients with inflammatory bowel disease. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0013160. doi: 10.1371/journal.pone.0013160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oertli M, Engler DB, Kohler E, Koch M, Meyer TF, Muller A. MicroRNA-155 is essential for the T cell-mediated control of Helicobacter pylori infection and for the induction of chronic gastritis and colitis. J Immunol. 2011;187:3578–86. doi: 10.4049/jimmunol.1101772. [DOI] [PubMed] [Google Scholar]
- 19.Singh UP, Singh NP, Singh B, Hofseth LJ, Price RL, Nagarkatti M, Nagarkatti PS. Resveratrol (trans-3,5,4’-trihydroxystilbene) induces silent mating type information regulation-1 and down-regulates nuclear transcription factor-κB activation to abrogate dextran sulfate sodium-induced colitis. J Pharmacol Exp Ther. 2010;332:829–39. doi: 10.1124/jpet.109.160838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh UP, Singh S, Taub DD, Lillard JW., Jr Inhibition of IFN-γ-inducible protein-10 abrogates colitis in IL-10–/– mice. J Immunol. 2003;171:1401–6. doi: 10.4049/jimmunol.171.3.1401. [DOI] [PubMed] [Google Scholar]
- 21.Singh UP, Singh NP, Singh B, Hofseth LJ, Taub DD, Price RL, Nagarkatti M, Nagarkatti PS. Role of resveratrol-induced CD11b+ Gr-1+ myeloid derived suppressor cells (MDSCs) in the reduction of CXCR3+ T cells and amelioration of chronic colitis in IL-10–/– mice. Brain Behav Immun. 2012;26:72–82. doi: 10.1016/j.bbi.2011.07.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neurath MF, Fuss I, Kelsall BL, Stuber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281–90. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parronchi P, Romagnani P, Annunziato F, et al. Type 1 T-helper cell predominance and interleukin-12 expression in the gut of patients with Crohn's disease. Am J Pathol. 1997;150:823–32. [PMC free article] [PubMed] [Google Scholar]
- 24.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–62. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 25.Gorelik L, Flavell RA. Abrogation of TGFβ signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–81. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 26.Tang Y, Luo X, Cui H, et al. MicroRNA-146A contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis Rheum. 2009;60:1065–75. doi: 10.1002/art.24436. [DOI] [PubMed] [Google Scholar]
- 27.Wu F, Zikusoka M, Trindade A, et al. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2α. Gastroenterology. 2008;135:1624–35. doi: 10.1053/j.gastro.2008.07.068. e24. [DOI] [PubMed] [Google Scholar]
- 28.Banerjee A, Schambach F, DeJong CS, Hammond SM, Reiner SL. Micro-RNA-155 inhibits IFN-γ signaling in CD4+ T cells. Eur J Immunol. 2010;40:225–31. doi: 10.1002/eji.200939381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elson CO, Beagley KW, Sharmanov AT, et al. Hapten-induced model of murine inflammatory bowel disease: mucosa immune responses and protection by tolerance. J Immunol. 1996;157:2174–85. [PubMed] [Google Scholar]
- 30.Singh UP, Singh S, Weaver CT, Iqbal N, McGhee JR, Lillard JW., Jr IFN-γ-inducible chemokines enhance adaptive immunity and colitis. J Interferon Cytokine Res. 2003;23:2000. doi: 10.1089/107999003322485099. [DOI] [PubMed] [Google Scholar]
- 31.Yu Q, Zhu S, Zhou R, et al. Effects of sinomenine on the expression of microRNA-155 in 2,4,6-trinitrobenzenesulfonic acid-induced colitis in mice. PLoS ONE. 2013;8:e73757. doi: 10.1371/journal.pone.0073757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu R, Huffaker TB, Kagele DA, Runtsch MC, Bake E, Chaudhuri AA, Round JL, O'Connell RM. MicroRNA-155 confers encephalogenic potential to Th17 cells by promoting effector gene expression. J Immunol. 2013;190:5972–80. doi: 10.4049/jimmunol.1300351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blanas E, Davey GM, Carbone FR, Heath WR. A bone marrow-derived APC in the gut-associated lymphoid tissue captures oral antigens and presents them to both CD4+ and CD8+ T cells. J Immunol. 2000;164:2890–6. doi: 10.4049/jimmunol.164.6.2890. [DOI] [PubMed] [Google Scholar]
- 34.Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9:10–6. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 35.te Velde AA, van Kooyk Y, Braat H, Hommes DW, Dellemijn TA, Slors JF, van Deventer SJ, Vyth-Dreese FA. Increased expression of DC-SIGN+IL-12+IL-18+ and CD83+IL-12–IL-18– dendritic cell populations in the colonic mucosa of patients with Crohn's disease. Eur J Immunol. 2003;33:143–51. doi: 10.1002/immu.200390017. [DOI] [PubMed] [Google Scholar]
- 36.Autenrieth IB, Bucheler N, Bohn E, Heinze G, Horak I. Cytokine mRNA expression in intestinal tissue of interleukin-2 deficient mice with bowel inflammation. Gut. 1997;41:793–800. doi: 10.1136/gut.41.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reimund JM, Wittersheim C, Dumont S, Muller CD, Kenney JS, Baumann R, Poindron P, Duclos B. Increased production of tumour necrosis factor-α interleukin-1β, and interleukin-6 by morphologically normal intestinal biopsies from patients with Crohn's disease. Gut. 1996;39:684–9. doi: 10.1136/gut.39.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaetke LM, Oz HS, de Villiers WJ, Varilek GW, Frederich RC. The leptin defense against wasting is abolished in the IL-2-deficient mouse model of inflammatory bowel disease. J Nutr. 2002;132:893–6. doi: 10.1093/jn/132.5.893. [DOI] [PubMed] [Google Scholar]
- 39.Trinchieri G. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994;84:4008–27. [PubMed] [Google Scholar]
- 40.Ehrhardt RO, Ludviksson BR, Gray B, Neurath M, Strober W. Induction and prevention of colonic inflammation in IL-2-deficient mice. J Immunol. 1997;158:566–73. [PubMed] [Google Scholar]
- 41.Visperas A, Do JS, Bulek K, Li X, Min B. IL-27, targeting antigen-presenting cells, promotes Th17 differentiation and colitis in mice. Mucosal Immunol. 2014;7:625–33. doi: 10.1038/mi.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sutton CE, Mielke LA, Mills KH. IL-17-producing γδ T cells and innate lymphoid cells. Eur J Immunol. 2012;42:2221–31. doi: 10.1002/eji.201242569. [DOI] [PubMed] [Google Scholar]
- 43.Stenstad H, Ericsson A, Johansson-Lindbom B, et al. Gut-associated lymphoid tissue-primed CD4+ T cells display CCR9-dependent and -independent homing to the small intestine. Blood. 2006;107:3447–54. doi: 10.1182/blood-2005-07-2860. [DOI] [PubMed] [Google Scholar]