Abstract
Neuronal axons use specific mechanisms to mediate extension, maintain integrity, and induce degeneration. An appropriate balance of these events is required to shape functional neuronal circuits. The protocol described here explains how to use cell culture inserts bearing a porous membrane (filter) to obtain large amounts of pure axonal preparations suitable for examination by conventional biochemical or immunocytochemical techniques. The functionality of these filter inserts will be demonstrated with models of developmental pruning and Wallerian degeneration, using explants of embryonic dorsal root ganglion. Axonal integrity and function is compromised in a wide variety of neurodegenerative pathologies. Indeed, it is now clear that axonal dysfunction appears much earlier in the course of the disease than neuronal soma loss in several neurodegenerative diseases, indicating that axonal-specific processes are primarily targeted in these disorders. By obtaining pure axonal samples for analysis by molecular and biochemical techniques, this technique has the potential to shed new light into mechanisms regulating the physiology and pathophysiology of axons. This in turn will have an impact in our understanding of the processes that drive degenerative diseases of the nervous system.
Keywords: Neuroscience, Issue 89, neuron, axon, filter inserts, culture system, dorsal root ganglion, axonal degeneration
Introduction
The axonal compartment of neurons shows a large degree of functional independence from the somato-dendritic compartment. In projection neurons, axons contain most of the neuronal protein1,2. The study of the physiology and pathophysiology of axons has gained momentum in the neuroscience community because recent studies have shown that early axonal dysfunction appears to be a common feature of neurodegenerative diseases and neuropsychiatric disorders3. It seems likely that a better understanding of axonal-exclusive mechanisms under normal and pathological conditions will shed light on the early events that drive dysfunction.
The present protocol describes how to use culture inserts bearing a porous membrane (filter) to obtain large amounts of pure axonal material that is suitable for biochemical and immunocytochemical analyses. The use of filter inserts to study axonal biology was first implemented by Steward and colleagues4 and further developed by Twiss and colleagues5 to its present configuration. The technique is now in current use by groups studying different aspects of axonal and dendrite biology, with slight modifications in each case to accommodate for the population of neurons studied, the treatments performed and the type of biochemical analyses used6-9. The present protocol does not intend to accommodate all the alternatives for such a variety of applications, but rather provide a simple approach that is suitable for most applications. In particular, the protocol described here uses a triple coating that maximizes axonal yield for biochemical analyses and is most suitable to study axonal degeneration –other coatings described in the literature produced less axons that retract and degenerate faster when deprived from trophic factors9.
In this procedure, neuronal cell bodies remain isolated atop the filter while axons pass through the pores and grow along its bottom surface. Pure axons (free of glia and neuronal cell body contaminants) that grow on the bottom surface of the filter can be collected for biochemical analyses or can be fixed in situ and examined by immunocytochemical techniques9. The procedure relies on the use of embryonic sensory neurons from the dorsal root ganglia (DRG). Embryonic DRGs are widely used to study axonal biology because neurites from these cells undergo fast and robust growth in vitro when maintained in nerve growth factor (NGF), and because they rapidly undergo degeneration when deprived of this factor. Also, DRG neurons lack dendrites, so that all the neurites collected by this method are purely axons.
Filter inserts represent a great advantage compared to other approaches used to study axons. Studies relying on the use of microscopy to differentiate processes occurring in the cell soma from those in axons provide limited biochemical information. As another example, compartmentalized culture systems (e.g., Campenot chambers10 or microfluidic devices11) are useful for imaging approaches and for differential treatment of cell compartments but provide only small amounts of axonal material, precluding the use of these techniques for biochemical analyses which require relatively large amounts of sample. Further, their use requires significant training as well as specialized devices, and they are time consuming. Another approach often used to isolate axons from explants is to manually remove an explant center (containing neuronal cell bodies) before axonal sample collection. Although this can produce large quantities of axon-enriched preparations, axons in this preparation can be enveloped in glia and rapidly removing 20 explant’s bodies consecutively from 6 wells (as an example of a minimal experiment) is time-consuming and at the limit of feasibility.
In contrast, the method presented here produces abundant and pure axonal preparations that can then be analyzed by virtually any biochemical technique that is commonly used to analyze whole-cell lysates, like western blot, immunoprecipitation6, northern blot5 mass spectrometry6, RNA purification7,12,13, among others. Moreover, the protocol can be applied to immunofluorescence (IF) techniques, as it is possible to fix axons growing in the bottom side of the filter to further examine them by IF. This approach greatly facilitates the analysis of axonal-specific processes since there are no cell bodies in the final preparation. This is a significant advantage since a high immunofluorescent signal from cell bodies often undermines examination and analysis of a weaker signal originating from thin structures such as axons.
Two applications of the culture method to study axonal degeneration are described; a model of developmental pruning and a model of injury-induced degeneration (commonly referred to as Wallerian degeneration). The modeling of developmental pruning is based on the fact that sensory neurons in vivo compete for limiting amounts of target-derived NGF and those failing to receive enough neurotrophic support degenerate14. This phenomenon can be mimicked in embryonic DRG cultures by withdrawing NGF from the culture media, which, as happens in vivo, initiates axonal degeneration followed by cell body destruction. To model Wallerian degeneration, the top side of the filter with the cell bodies is scraped away. The axons that had grown onto the bottom side of the filter become physically separated from the cell bodies and thereafter undergo the rapid and stereotypic degenerative process known as Wallerian degeneration15, named after A. Waller who first reported the phenomenon in 185016.
Protocol
NOTE: The following procedures are in accordance with guidelines approved by the Canadian Council of Animal Care. All efforts are made to minimize pain and distress of animals during procedures.
1. Coating of Filters
NOTE: When culture filter inserts are placed in wells of multi-well plates, two compartments are defined: the top compartment is the region above the insert and the bottom compartment is the one below the insert Figure 1A.a-b. Explants are seeded in the top compartment where they attach to the top side of the filter; many axons grow through the filter and extend on the bottom side of the filter, within the bottom compartment Figure 1A.c. The standard working volumes (i.e. for applying coating solution, washes, culture media, etc) are 2 ml for the bottom compartment and 1 ml for the top compartment and are going to be referred to in the protocol as “standard volumes”. Add solutions starting with the bottom compartment by placing the tip of the pipette in the gap between the insert and the side of the well. Also, tilt the insert to one side while dispensing the solution to prevent bubbles from being trapped on the bottom of the filter.
Start coating the filters the day before plating. Under the sterile tissue culture hood, place 24 mm filter inserts in a 6-well receiving plate. Incubate inserts with poly-D-lysine in water (1 mg/ml) for 1 hr at RT using standard volumes: 2 ml in the bottom compartment and 1 ml in the top.
Aspirate poly-D-lysine and rinse once with water. Aspirate water, close the lid and leave plates to dry in the hood O/N. During aspiration of liquids, make sure to remove a remnant of liquid that becomes trapped directly under the filter.
The next morning, dilute laminin in water (10 µg/ml), mix and warm at 37 °C. Incubate inserts with laminin 1 hr in cell incubator at 37 °C, using standard volumes.
Aspirate laminin and add collagen in water (0.1 mg/ml) and incubate for 1 hr at RT. In this case use 2 ml for the bottom compartment and 2 ml for the top compartment.
Aspirate collagen and wash once with sterile water. The filters are now ready to receive culture media and DRG explants, as described in STEP 3.
2. Dissecting Embryonic Dorsal Root Ganglion (DRG) Explants
Anesthetize a 13-day pregnant female mouse by an IP injection of Avertin (250 mg/kg). Wait 10-15 min and check for lack of corneal and pedal withdrawal reflexes. Once reflexes are absent, perform cervical dislocation.
Disinfect all surgical instruments and female abdomen with 70% ethanol. Allow instruments to dry. Hold the skin of the abdomen and cut through the skin and muscle layers to expose the abdominal cavity.
Remove the uterus by detaching from the cervix and ovaries. In a sterile hood, remove the embryos from the uterus with scissors and collect them in a Petri dish filled with ice-cold L15 media. Keep on ice. NOTE: Refer to previous technical reports for further details regarding how to obtain embryos of 13 days of gestation17,18.
Under the microscope, lay the embryo on a side and use small spring scissors to remove the head. Make an incision along the lateral sides to discard the chest, belly, limbs and tail. Keep the back containing the spine, lay ventral side up and remove all visceral organs.
With small spring scissors, expose the entire spinal cord by cutting the spinal column from its ventral side along the midline. Hold the carcass down with one pair of forceps (#3) and use a second pair to hold the spinal cord by its most rostral aspect.
Slowly and gently pull the cord away from the vertebral cavity and pinch off DRGs from the spinal cord using ultra-thin forceps (#5). Group the DRGs to be collected on the bottom of the dish.
Collect grouped DRGs by aspirating with a 200 µl pipette tip that has been cut to enlarge its opening. Place the DRGs in a tube and leave on ice until the DRG collection is finished. NOTE: White/transparent tips are preferred because DRGs do not stick to the walls of this tip (as compared to standard yellow tips). Flushing tips with media before use further prevents DRGs from sticking to its walls.
3. Seeding and Maintenance of Dorsal Root Ganglion Explants on Filters
Add complete DRG media at 37 °C to the coated inserts using standard volumes. Supplement the bottom compartment media with 15 ng/ml of NGF, while keeping the top compartment NGF-free. NOTE: Although NGF efficiently diffuses across the filter and its concentration equilibrates within hours, we have observed that applying NGF to the bottom compartment directly before seeding significantly increases axonal yield on the bottom surface of the filter.
Transfer the DRGs from the tube to the filters using a 1,000 µl pipette with a tip trimmed to enlarge its opening.
Draw media into the tip 2-3x before collecting the DRGs. This prevents them from sticking to the inner side of the tip. The same tip can be re-used for all wells. The use of uncolored tips (versus standard blue tips) reduces DRG sticking to the tip surface.
To transfer DRGs, set the pipette at 800 µl. Aspirate approximately 200 µl of media from the top side of the filter, then go to the DRG-containing tube and pipette a small volume up and down – to resuspend DRGs that have sunk to the bottom. Aspirate the whole volume and pipette DRGs submerging the tip in the middle of the insert.
Once all inserts are loaded, close the plate lid and swirl the plate 10x, tapping it gently on the hood bench between each swirl. These movements increase axonal yield by helping DRG explants sink and attach to the filter. This also helps distribute explants evenly so that axons from different explants don’t overlap. NOTE: The explant seeding procedure described in the previous steps significantly increases the overall rate of explant attachment to the substrate (up to 75%). Most significantly, the pipetting procedure readily prevents explants from floating on the media surface instead of sinking to the bottom of the insert.
For biochemical analyses, seed DRGs from one embryo (around 30) per filter. For morphological analyses, use either half or a third of the DRGs from one embryo (10-15). This decision must be made before collecting the DRGs into tubes, so that all DRGs of a tube are seeded into a single well (whether it is 30, 15, or 10 DRGs).
Maintain cultures in a cell incubator at 37 °C, changing media every 2-3 days. To change media, aspirate the top compartment first, followed by the bottom compartment. Add fresh media in standard volumes starting with the bottom compartment. NOTE: To prevent cells/axons from drying, do not change media in more than 3 inserts at the same time. DRGs grown on filters in the presence of NGF will extend long axons, reaching up to 2 mm after 2 days in culture.
4. Trophic Factor Withdrawal Treatment
NGF withdrawal induces axonal degeneration with axonal fragmentation (determined by phase contrast and β-III-tubulin IF) that first appears around 12 hr after withdrawal. Complete fragmentation is achieved by 36 hr. How long the degeneration is left to proceed for has to be chosen by the end user according to the experimental design.
After the 2-day axonal extension phase, change media from top and bottom compartments to media that lacks NGF and contains anti-NGF antibodies (1 µg/ml) to sequester any remaining (or endogenously produced) NGF.
Return plates to cell incubator (37 °C) to allow NGF withdrawal-induced degeneration to proceed for the desired amount of time.
Proceed to protein extraction (step 6) for examination by western blot or directly examine the filters by IF (step 7).
5. Axonal Transection to Induce Wallerian Degeneration
This procedure leaves the axons on the bottom side of the filter detached from their cell body, but otherwise intact. Thereafter, these axons rapidly initiate Wallerian degeneration. Axonal fragmentation (evidenced by phase contrast or β-III-tubulin IF) first appears by 3 hr after transection; by 9 hr, axons are completely fragmented and are detached from the filter. How long the degeneration is left to proceed for has to be chosen by the end user according to the experimental design.
Scrape the top side of the filter, containing grown DRG explants, with a cell-scraper. Ensure complete removal of top material by moving scraper in X-, Y- and circular directions.
Discard the media from the top compartment containing the floating explants that have been scraped from the filter and replace with 1 ml of pre-warmed, complete DRG media containing NGF (10 ng/ml).
Return plate to cell incubator (37 °C) to allow Wallerian degeneration to proceed for the amount of time chosen by the end user.
Proceed to protein extraction (step 6) for examination by western blot or directly examine the filters by IF (step 7).
6. Protein Extraction of Axonal Samples
Fill collection tubes (1.5 ml) with 75 µl of Laemmli sample buffer. Keep tubes on ice while harvesting axons from the entire set of filters.
Wash the filter with DRG explants in PBS and scrape the top side of the filter. Remove the insert from the plate, clean the top side of the filter with a cotton-tip applicator and aspirate any extra PBS from the top and bottom sides.
Place insert upside down on bench and cut the filter out of the insert using a sharp scalpel. Hold the filter with forceps, bottom up. Make a cut from the circumference to the center of the filter with scissors.
Place filter on top of the collection tube, and with forceps, press the center of the filter down the tube. While doing this, a funnel should form. Push the funnel-shaped filter to the bottom of the tube so that it is submerged in the Laemmli sample buffer.
Complete protein extraction by boiling the tubes for 4 min. Remove the filter using forceps to place it upside-down into the top of the tube and performing a brief spin (2,000 x g for 15 sec). Discard the dried filters.
Resolve 10 µl of the axonal protein preparation in a SDS-PAGE followed by western blot to detect the proteins of interest. NOTE: As a reference, an axonal preparation from 20 explants grown for 2 days and lysed in 100 µl of RIPA lysis buffer will have a protein concentration of 4-6 µg/µl.
7. Immunofluorescence Examination of Axons on Filters
The following steps outline the general manipulations to perform IF staining on filter inserts, exemplified by the detection of β-III-tubulin. Fixative, incubation times, concentrations and other particulars should be optimized for other antigen-antibody pairs.
Utilize previously used 6-well plates, thoroughly cleaned, for the following washes and incubations of the inserts.
Briefly wash inserts in PBS. Fix them in freshly prepared 4% paraformaldehyde in PBS for 10 min at RT on shaker.
After fixation, scrape and clean the top side of the insert to collect the axons that grew exclusively on the bottom surface of the filter. Proceed with standard incubations for IF.
Incubate inserts in blocking solution (TBS, 0.05% Tween 20, 5% skim milk), using standard volumes, for 1 hr at RT with gentle shaking.
Transfer inserts to primary antibody diluted in blocking buffer (anti-β-III-tubulin, 1:5,000), using standard volumes. Incubate O/N (~18 hr) at 4 °C with gentle shaking.
Wash the first antibody twice in PBS (2 X 10 min at RT).
Transfer inserts to fluorescent-conjugated secondary antibodies diluted in blocking buffer (goat anti-mouse, 1:2,000), using standard volumes. Incubate covered from light for 2 hr at RT with gentle shaking.
Wash the secondary antibody 3x in PBS (3 x 10 min at RT).
After IF is complete, take insert out, pour away PBS from the last wash and clean the top side of the filter with a cotton-tip applicator. Aspirate any liquid from the top and bottom sides.
Place insert upside-down on bench, and cut the filter out of the insert with a sharp scalpel.
Hold the filter with forceps, downside up, and place on microscopy slide. Overlay with a coverslip and fluorescent-compatible mounting media. Let dry flat in cold room, covered from light. Examine and capture images using a fluorescent microscope. NOTE: When stored at 4 °C and protected from light, these preparations can be kept for weeks with minimal loss of fluorescence.
- OPTIONAL: Alternatively, IF can be performed on filters that have been removed from the insert. To do that, scrape and clean the top side of the filters directly after fixation.
- Take filters out of the inserts with a sharp scalpel and place filters axons-up on the bottom of a six-well plate.
- Afterwards, perform incubations for IF as indicated in 7.4-7.8 and 7.11. The advantage of this alternative is that it saves reagents, since, for example, incubation volumes can go down to 500 µl (compared to the 3 ml used when filters are directly stained on the inserts as shown in 7.1-7.11).
Representative Results
Dorsal root ganglion explants from E13.5 mice grow extensively on filters coated sequentially with poly-D-lysine, laminin and collagen (Figure 1B, left), reaching up to 2 mm within 2 days in culture. This substrate combination yields particularly exuberant growth; other combinations yield axons that are considerably shorter (Figure 1B, right). Moreover, DRGs maintained on filters extend more and longer axons than DRGs grown on plastic Figure 1C.
Filters with pores of 1 µm in diameter, used in this protocol, retain cell bodies on the upper side of the filter, while allowing neurites to grow through the pores and extend on the bottom surface. This is clearly shown by epifluorescence microscopy of filters stained for nuclei (DAPI) and β-III-tubulin Figure 1D. In intact filters it is possible to observe cell nuclei, both from neurons and non-neuronal cells, concentrated in the explant center and some irradiating from it. However, when the top side of the filter is scraped, nuclei are gone and only axons can be detected, on the filters bottom side.
Figure 1E shows an example of a filter bottom that originally sustained the growth of approximately 17 explants. In this example, the top side was cleaned before fixation and therefore the immunofluorescent signal represents pure axons on the bottom side of the filter. Protein extraction from axonal preparations (bottom side of the filter) are devoid of nuclear proteins Figure 1F, demonstrating that the preparation is free of cell bodies. Alternatively, when working with RNA extraction, it is possible to confirm the purity of the axonal preparation by comparing the presence of differentially localized mRNAs (i.e. γ-actin) between whole-cell lysates and axonal preparations (as shown by others5).
Developmental pruning of sensory neurons can be modeled in filters subjected to trophic factor deprivation. After 2-3 days of axon extension in the presence of NGF, the media is changed to one lacking NGF and containing an anti-NGF antibody that will sequester any remaining NGF Figure 2A. At the desired time points, filters are processed for biochemistry or immunocytochemistry.
Figure 2B shows typical examples of bottom sides of filters stained for β-III-tubulin before and after deprivation. After 12 hr of deprivation there is little axonal fragmentation, although some axons start to show beading. However, extensive axonal degeneration is achieved after 36 of deprivation.
Wallerian degeneration induced by axon transection of sensory neurons is easily modeled in explants grown on filters by scraping the top side of the filter, leaving behind the distal portion of the axons that grew on the bottom side of the filter Figure 3A. Figure 3B shows typical examples of bottom sides of filters stained for β-III-tubulin before and after in-filter axotomy. At 3 hr after axotomy, there is little fragmentation, although some axons start to show beading. By 7.5 hr, most axons are fragmented or completely gone. Pretreatment of explants with nicotinamide adenine dinucleotide (NAD+, 5 mM, 30 min pretreatment) protects from Wallerian degeneration induced on filters, as it does in other in vitro and in vivo models19.
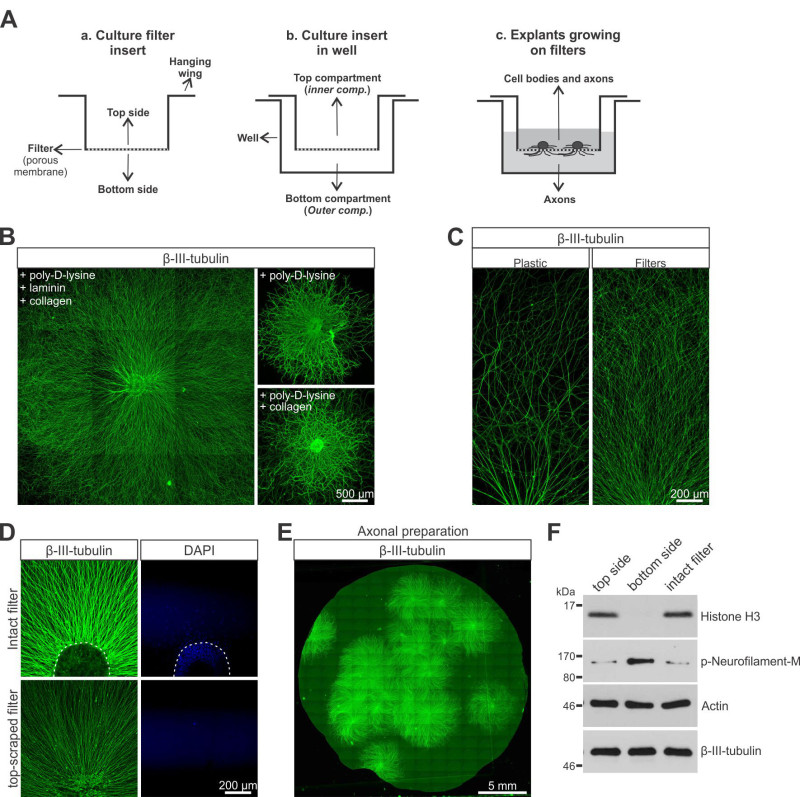 Figure 1. Filter inserts produce large quantities of pure axonal preparations. A) A schema of an insert (a), of an insert in a well (b) and the final configuration with DRG explants (c). B) The sequential coating of filters with poly-D-lysine, laminin and collagen results in explants that grow more and longer axons compared to those grown with poly-D-lysine alone or poly-D-lysine and collagen. The images were produced by tiling overlapping, low-magnification pictures using imaging software. C) Under the same substrate coating conditions, DRG explants maintained on filters show considerably more exuberant axonal growth than explants grown on plastic. D) The axonal preparation obtained by scraping the top side of the filter is devoid of cell nuclei. E) Example of axons that originated from approximately 17 explants and grown on the bottom side of a 24 mm filter. As much as 30 explants can readily grow in these preparations, yielding enough axonal material for common biochemical approaches. F) Immunoblots of lysates collected from filter tops versus bottoms demonstrate that histone H3, a nuclear marker, is confined to the filter tops. The total protein content was normalized across the different samples. Please click here to view a larger version of this figure.
Figure 1. Filter inserts produce large quantities of pure axonal preparations. A) A schema of an insert (a), of an insert in a well (b) and the final configuration with DRG explants (c). B) The sequential coating of filters with poly-D-lysine, laminin and collagen results in explants that grow more and longer axons compared to those grown with poly-D-lysine alone or poly-D-lysine and collagen. The images were produced by tiling overlapping, low-magnification pictures using imaging software. C) Under the same substrate coating conditions, DRG explants maintained on filters show considerably more exuberant axonal growth than explants grown on plastic. D) The axonal preparation obtained by scraping the top side of the filter is devoid of cell nuclei. E) Example of axons that originated from approximately 17 explants and grown on the bottom side of a 24 mm filter. As much as 30 explants can readily grow in these preparations, yielding enough axonal material for common biochemical approaches. F) Immunoblots of lysates collected from filter tops versus bottoms demonstrate that histone H3, a nuclear marker, is confined to the filter tops. The total protein content was normalized across the different samples. Please click here to view a larger version of this figure.
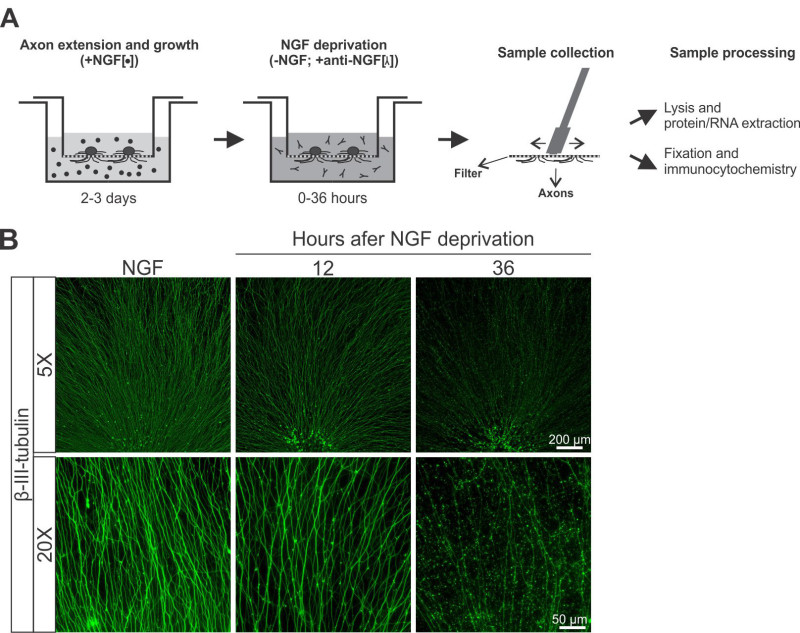 Figure 2. The study of axonal degeneration induced by trophic factor withdrawal in filter inserts. A) Schema outlining a typical NGF-deprivation experiment. DRG explants grow extensive axons in the presence of NGF, and degenerate upon NGF deprivation. B) Representative images at different magnifications (5X and 20X objectives) of axonal preparations from explants maintained in NGF or deprived of NGF for 12 or 36 hr. Please click here to view a larger version of this figure.
Figure 2. The study of axonal degeneration induced by trophic factor withdrawal in filter inserts. A) Schema outlining a typical NGF-deprivation experiment. DRG explants grow extensive axons in the presence of NGF, and degenerate upon NGF deprivation. B) Representative images at different magnifications (5X and 20X objectives) of axonal preparations from explants maintained in NGF or deprived of NGF for 12 or 36 hr. Please click here to view a larger version of this figure.
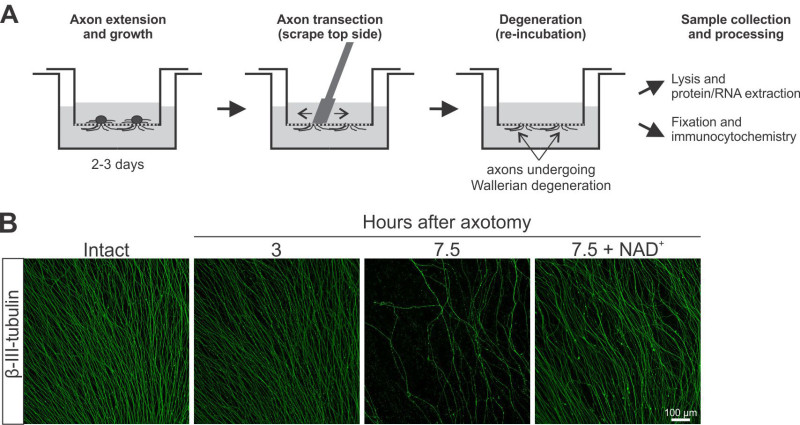 Figure 3. The study of Wallerian axonal degeneration induced by axonal transection in filter inserts. A) Schema outlining a typical axotomy experiment. Axon severing is achieved by scraping the top side of the filter, leaving severed axons (devoid of cell bodies) on the bottom side. B) Representative images of axonal preparations from intact explants or after 3 or 7.5 hr after transection in the presence or absence of 5 mM of NAD+. Please click here to view a larger version of this figure.
Figure 3. The study of Wallerian axonal degeneration induced by axonal transection in filter inserts. A) Schema outlining a typical axotomy experiment. Axon severing is achieved by scraping the top side of the filter, leaving severed axons (devoid of cell bodies) on the bottom side. B) Representative images of axonal preparations from intact explants or after 3 or 7.5 hr after transection in the presence or absence of 5 mM of NAD+. Please click here to view a larger version of this figure.
Discussion
Important parameters to take into account when adapting this technique include the neuronal population that will be studied, the substrate, pore size of the filter and surface area. All these parameters will impact the specificity, quality and quantity of the neurite preparation obtained, and must be considered carefully by the end user. In the examples presented in this protocol, the use of DRG neurons has the advantage of extending only axons, thus giving a pure (specific) axonal preparation.
Axonal growth of embryonic DRGs is very fast compared to neurons from the central nervous system, allowing axonal samples to be prepared after only 2 days in culture. The pore size of 1 µm has proven sufficiently small to prevent cell bodies from migrating into the bottom surface while allowing efficient passage of growing axons. Compared to smaller sizes available (i.e. 0.4 µm) it does not impose selectivity for thin axons. For the study of DRG explants, the 24 mm filter insert (for use in 6-wells plates) produces sufficient quantities of axonal protein sample for most techniques, including immunoprecipitation, which often requires a large amount of protein input. However, smaller sizes (i.e. filter inserts for 12-well plates) are suitable for some applications, especially if using dissociated cells and/or immunocytochemistry.
Although the filter insert efficiently isolates neurites from cell bodies for examination, it is important to note that both compartments share the same culture media, limiting the experimental manipulations to whole-cell treatments. Other isolation approaches, including compartmentalized Campenot chambers or microfluidic chambers, allow for differential treatment of the different compartments. The experimenter must determine which of these cell-compartment isolation techniques is most suitable for their own specific experimental design.
Alternative methods to obtain pure axonal samples include compartmentalized culture systems like Campenot chambers or microfluidic devices or manually removing an explant center (containing neuronal cell bodies). The compartmentalized approaches provide only small amounts of axonal material. Their use requires significant training as well as specialized devices, and they are time consuming. On the other hand, removing the explant center to obtain enriched axonal samples is impractical, requires expertise and is limited to neuronal populations that can be grown as explants. In contrast, the method presented here produces abundant and pure axonal preparations, is fast and does not require extensive training. Foremost, it can also be performed with dissociated neurons.
Of course, the isolation capability of filter inserts can be advantageous beyond axonal degeneration. For example, the biochemistry of regenerating growth cones can be easily studied by scraping the bottom side of filters with grown DRG explants. Within hours, axotomized DRG neurons will regenerate growth cones in the bottom side of the filters that, as described in this protocol, can then be isolated for the purification of regenerating growth cone proteins. Moreover, the axonal samples obtained by this method can be analyzed by biochemical methods other than western blotting. For example, axons can be lysed in RIPA or CHAPS lysis buffers to obtain a protein sample that can be further subjected to immunoprecipitation or pulldown experiments9. Alternatively, axonal samples can be processed for RNA extraction, as previously described5.
The advantage of the culture system presented is not limited to sensory neurons. For example, cortical neurons will extend dendrites and axons that will form synapses on the bottom side of the filter7. Thus, pure neurite samples (enriched with synapses) can be isolated from cell bodies for detailed biochemical analyses of processes that take place exclusively in synapses. The use of this technique is simple and has no outstanding critical steps. However, axonal length and morphology is quite sensitive to the quality of the coating of the filters. Hence, care must be taken to use fresh and homogenous coating solutions for the indicated amounts of time to obtain robust and consistent axonal samples. The widespread use of this technique will help advance our knowledge of the physiology of axons, under normal circumstances and in disease.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This project was supported by grant MOP62827 from the Canadian Institutes of Health Research.
References
- Conde C, Caceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat. Rev. Neurosci. 2009;10:319–332. doi: 10.1038/nrn2631. [DOI] [PubMed] [Google Scholar]
- Rasband MN. The axon initial segment and the maintenance of neuronal polarity. Nat. Rev. Neurosci. 2010;11:552–562. doi: 10.1038/nrn2852. [DOI] [PubMed] [Google Scholar]
- Lingor P, Koch JC, Tonges L, Bahr M. Axonal degeneration as a therapeutic target in the CNS. Cell Tissue Res. 2012;349:289–311. doi: 10.1007/s00441-012-1362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre ER, Steward O. Demonstration of local protein synthesis within dendrites using a new cell culture system that permits the isolation of living axons and dendrites from their cell bodies. J. Neurosci. 1992;12:762–772. doi: 10.1523/JNEUROSCI.12-03-00762.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JQ, et al. A functional role for intra-axonal protein synthesis during axonal regeneration from adult sensory neurons. J. Neurosci. 2001;21:9291–9303. doi: 10.1523/JNEUROSCI.21-23-09291.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis D, et al. Differential transport and local translation of cytoskeletal, injury-response, and neurodegeneration protein mRNAs in axons. J. Neurosci. 2005;25:778–791. doi: 10.1523/JNEUROSCI.4235-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon MM, Choi SH, Jamieson CA, Geschwind DH, Martin KC. Identification of process-localized mRNAs from cultured rodent hippocampal neurons. J. Neurosci. 2006;26:13390–13399. doi: 10.1523/JNEUROSCI.3432-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenmann Z, et al. Axonal degeneration is regulated by the apoptotic machinery or a NAD+-sensitive pathway in insects and mammals. J. Neurosci. 2010;30:6375–6386. doi: 10.1523/JNEUROSCI.0922-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsain N, Higgins JM, Parker KN, Johnstone AD, Barker PA. XIAP regulates caspase activity in degenerating axons. Cell Rep. 2013;4:751–763. doi: 10.1016/j.celrep.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Ure DR, Campenot RB. Retrograde transport and steady-state distribution of 125I-nerve growth factor in rat sympathetic neurons in compartmented cultures. J. Neurosci. 1997;17:1282–1290. doi: 10.1523/JNEUROSCI.17-04-01282.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, et al. Axonal mRNA in uninjured and regenerating cortical mammalian axons. J. Neurosci. 2009;29:4697–4707. doi: 10.1523/JNEUROSCI.6130-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minis A, et al. Subcellular transcriptomics-Dissection of the mRNA composition in the axonal compartment of sensory neurons. Dev. Neurobiol. 2013. [DOI] [PubMed]
- Niekerk EA, et al. Sumoylation in axons triggers retrograde transport of the RNA-binding protein La. Proc. Natl. Acad. Sci. U. S. A. 2007;104:12913–12918. doi: 10.1073/pnas.0611562104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas ME, Barres BA. Why is Wallerian degeneration in the CNS so slow. Annu. Rev. Neurosci. 2007;30:153–179. doi: 10.1146/annurev.neuro.30.051606.094354. [DOI] [PubMed] [Google Scholar]
- Waller A. Experiments on the Section of the Glossopharyngeal and Hypoglossal Nerves of the Frog, and Observations of the Alterations Produced Thereby in the Structure of Their Primitive Fibres. Philosophical Transactions of the Royal Society of London. 140:423–429. [Google Scholar]
- Albuquerque C, Joseph DJ, Choudhury P, MacDermott AB. Dissection, Plating, and Maintenance of Dorsal Root Ganglion Neurons for Monoculture and for Coculture with Dorsal Horn Neurons. Cold Spring Harbor Protocols. 2009. [DOI] [PubMed]
- Leach MK, et al. The culture of primary motor and sensory neurons in defined media on electrospun poly-L-lactide nanofiber scaffolds. J Vis Exp. 2011. [DOI] [PMC free article] [PubMed]
- Sasaki Y, Araki T, Milbrandt J. Stimulation of nicotinamide adenine dinucleotide biosynthetic pathways delays axonal degeneration after axotomy. J. Neurosci. 2006;26:8484–8491. doi: 10.1523/JNEUROSCI.2320-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


