Introduction
Atherosclerosis is a chronic inflammatory disease characterized by lipid-containing inflammatory lesions of large and medium sized arteries. It is primarily a disease of the inner layer of the arterial wall, the intima. As the disease advances, the adventitia however also participates in the pathogenesis of the atherosclerosis. Hermann et al have proposed that the development of human atherosclerotic lesions can be considered to involve three distinct stages1. In the first stage, early alterations in cellular function result from interaction of environmental risk factors and genetic predisposition. The second stage is characterized by the proliferation of adventitial vasa vasorum with subsequent extension of the neovessels into the inner media, and eventually into the enlarging plaque. In vulnerable plaques, vessel density increases from 2-fold to 4-fold in disrupted plaques, compared with several obstructive stable lesions. Chronic lesions can enter the third stage with further neovascularization especially in the vulnerable shoulder areas of the plaques. At this stage, the intraplaque neovessels may rupture, leading to intraplaque hemorrhage. This may be due to compromised integrity of microvascular endothelium, and plaque weakening secondary to inflammation. The exacerbation of tightly intertwined plaque inflammatory activity and neovascularization results in plaque rupture leading to arterial thrombosis with ensuing clinical syndromes.
Revolution in the field of radiology in the last three decades has enabled imaging of inflammation and neovascularization within atherosclerotic tissue. In this article, we review advances in various clinical and pre-clinical imaging modalities aimed at unravelling the pathobiology of atherosclerosis.
Ultrasound imaging
Use of ultrasound (US) for molecular imaging of cardiovascular system is an extension of contrast echocardiographic principles already in clinical use. Non-targeted US contrast agents (UCAs) purely act as intravascular blood tracers behaving as red blood cells within the microcirculation. Targeted UCAs decorated with ligands, by affinity-based interaction, localize to a site where a specific target (usually receptor) is pathologically up regulated. Typically an UCA is composed of microbubbles (MBs) that generally contain a gas core with a stabilizer shell of protein, lipid or biocompatible polymers. Submicron gas-containing liposomes and acoustically active emulsion-based nanoparticles also exist. On exposure to US waves, MBs vibrate and resonate creating an acoustic signal different from tissue backscatter, which enables US systems to maximize detection of UCAs.
a. Non-targeted ultrasound imaging
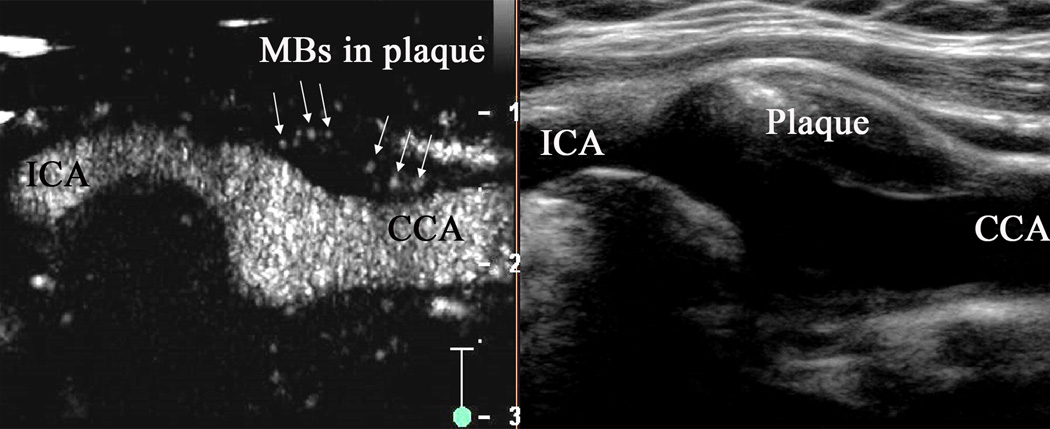
Using non-targeted UCA, contrast-enhanced US (CEUS)-imaging of neovascularization of carotid atherosclerotic plaques is feasible, with CEUS-visualized neovessels (Figure 12) having good histological correlation with CD31-stained neovessels3. Excellent correlation is however unlikely since tunica adventitia and part of media is left behind during carotid endartrectomy (CEA). The degree of plaque enhancement is not related to the degree of carotid stenosis. High plaque enhancement is prevalent among echolucent plaques with plaque echogenicity inversely correlated with grade of intraplaque neovascularization. Since plaque echolucency is considered a marker of high-risk lesions, it highlights the ability of CEUS to differentiate between stable and unstable plaques. CEUS can differentiate between patients with symptomatic and asymptomatic carotid disease. An association between the presence and degree of adventitial vasa vasorum and intraplaque neovascularization as graded on CEUS, with history of cardiovascular disease and previous cardiovascular ischaemic events has also been reported4. CEUS has been used to assess the impact of statins on LDL levels and CEUS-assessed neovascularization5. Plaque neovascularization regressed in 46% plaques in patients over a period of 6 months. It was associated with reduction in LDL levels. A prospective clinical study to assess association of CEUS-quantified neovascularization with future cardiovascular events is awaited.
Figure 1.
Carotid artery with intraplaque neovascularization on CEUS2. Plaque at the origin of the internal carotid artery on B-mode US imaging (right panel). Corresponding artery on contrast-enhanced US (left panel) with visible MBs within the plaque (arrows).
Above studies relied on subjective assessment of extent of neovascularization on CEUS. To improve this, dynamic cine clip-based time-intensity curve for wash-in time of an UCA and intensity of plaque-enhancement has been used6. Besides being acceptably reproducible, this quantitative approach enabled differentiation between plaques of patients with and without ischemic stroke. By developing an algorithm using cine clip-based ‘ratio of neovascularization area to the total plaque area’ and applying motion compensation, excellent correlation between this CEUS- and histology-based parameter has also been reported7. The histological validation of CEUS-assessed neovascularization has however some inherent limitations: data displayed in each US frame is composed of data integrated over a width of a few millimeters rather than from a narrow plane, which is different from the one displayed in the histology slices (in micrometers). Changes in the plaque architecture on histological processing such as on fixation potentially compound this limitation.
In contrast to the use of transcutaneous US for imaging carotid artery, there is severe relative tissue-catheter motion (from cardiac contractility) and weak signal strength from the microcirculation in coronary arteries. This renders coronary artery vasa vasorum imaging challenging. UCAs by enhancing the acoustic signal from coronary vessel wall have the potential to overcome such limitations. Carlier et al reported the initial feasibility study in human coronary arteries using non-harmonic intravascular US (IVUS) in vivo8. Vasa vasorum was not visualized but since plaque perfusion is believed to most likely result from vasa vasorum, enhancement was considered a representation of vasa vasorum density. Unlike availability of CEA specimens, histological validation using coronary atheroma is not possible in clinical studies. Non-harmonic imaging technique assumes linear behavior of the MBs, but it may be difficult to differentiate acoustic signals from the UCA and tissue, reducing the sensitivity and specificity of this technique. By harnessing non-linear properties of the MBs, it can be stimulated to emit energy at higher (harmonic) or lower (subharmonic) lower than the transmitted central frequency (fc). In a coronary phantom experiment, Goertz et al reported the improvement in contrast-to-tissue ratio (CTR) with second harmonic (2fc) and subharmonic imaging (1/2fc)9. Although prototype IVUS imaging systems were used for these feasibility studies, these imaging strategies seem promising and are compatible with commercial platforms10. It is anticipated that by their implementation widespread clinical use may be enabled.
b. Targeted ultrasound imaging
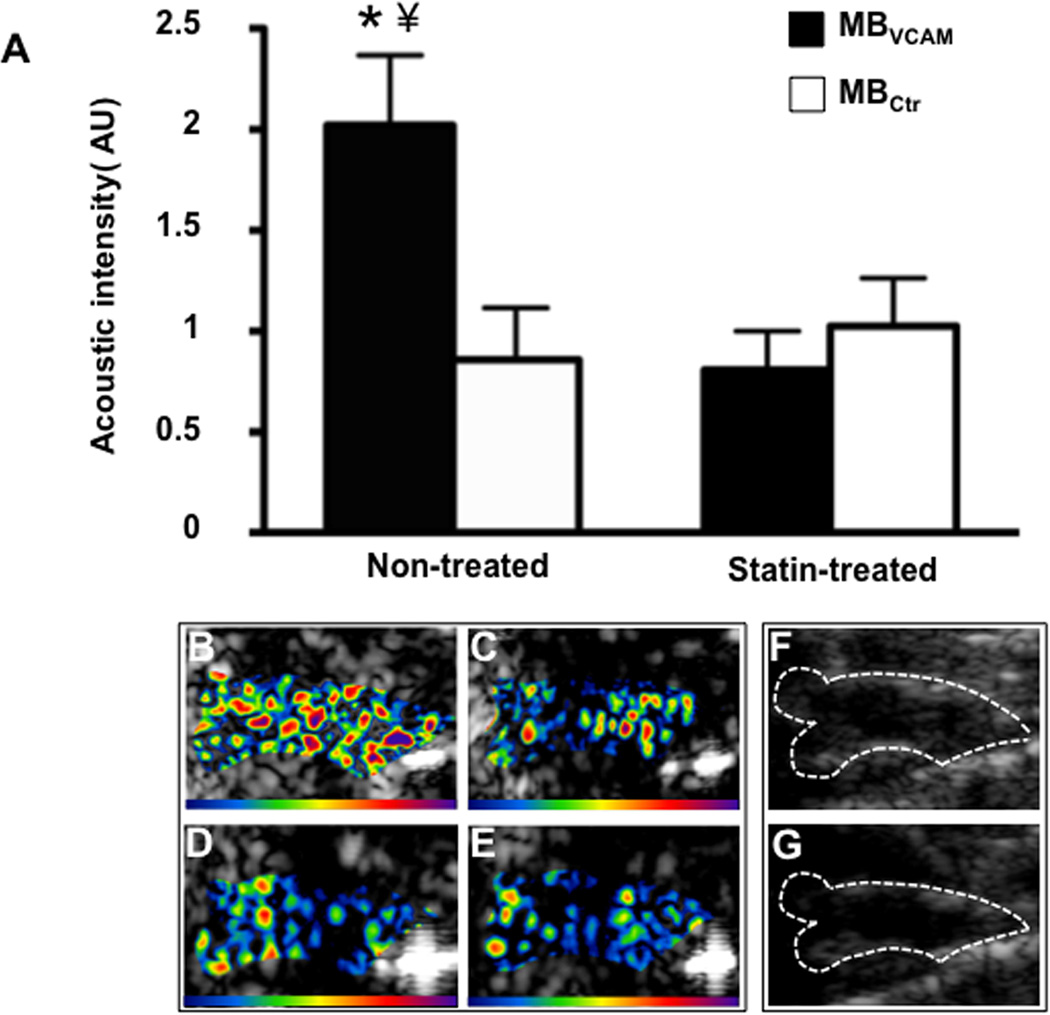
Ultrasound has been used for imaging molecular targets, which are specific to inflammation and associated neovascularization. It has been through designing of bio-functionalized UCAs having affinity for specific targets. To enable imaging of disease-specific targets, receptors that are expressed due to inflammation such as VCAM-1, E-selectin and P-selectin and have no/less constitutive expression, are better targets for receptor targeted-US imaging. A comprehensive IVUS study demonstrated that anti-VCAM, anti-ICAM, anti-tissue factor (TF), anti-fibrin, and anti-fibrinogen conjugated emulsion-based liposomes (ELIPs) could successfully identify different components of atherosclerotic tissue in a single-step process11. The ‘emulsion-based’ nanoparticles however have low acoustic reflectivity in circulation compared to gaseous MBs, which has been a major limitation to their use. Kaufmann et al used VCAM-1 targeted MBs to image inflammation in atherosclerosis12. Since VCAM-1 is expressed on the endothelium of arteries and in vasa-vasorum, it allows imaging of both inflammation and neovascularization simultaneously. The reduction in VCAM-1 expression with use of statins has also been successfully quantified using VCAM-1 targeted MBs (Figure 2)13. Other potential targets for imaging inflammatory neovascularization in atherosclerosis would be ICAM-114; or VEGF and α-integrin as used in disease models such as oncology studies. Inability of MBs to penetrate deeper plaque components such as lipid content limits their use in imaging other clinically relevant imaging targets. Unlike use of non-targeted MB US imaging, the feasibility of targeted molecular US imaging in humans remains unreported.
Figure 2.
Molecular imaging of the ascending aorta13. (A) Mean ± standard error of the mean background-subtracted signal intensity for MBs targeted to VCAM-1 (MBVCAM) and control MBs (MBCtr) in non-treated and statin treated animals. A significant difference in the signal intensity of MBs targeted to VCAM-1 (MBVCAM) was observed compared to control MBs (MBctr), *p<0.01 in non-treated animals, A significant reduced signal intensity of MBVCAM after statin treatment was observed, ¥ p<0.01. Examples of color-coded CEU images from a non-treated animal after injection of MBVCAM (B), and of MBCtr (C). Images from a statin treated animal after injection of MBVCAM (D), and of MBCtr (E). The color scale for the CEU images is shown at the bottom of each frame. A significantly signal difference can be seen in statin treated animal (Figure B versus Figure D). (F) and (G) illustrate the outline of the ascending aorta on B-mode US images which was used as a region of interest for acoustic intensity measurements.
Magnetic resonance imaging
MR imaging utilizes the inherent MR relaxation properties [T1 and T2] of different plaque components and the surrounding tissue to characterize plaque components without contrast media (CM). On T1-weighted images, plaque fibrous tissue may appear isointense to hypointense and lipid may be isointense to hyperintense. However, fibrous tissue has high signal intensity on T2-weighted images while lipid content appears hypointense. Calcium appears hypointense on T1- and T2-weighted images. Use of CM enables signal enhancement of the tissues, overcoming the issue of limited sensitivity associated with multi-contrast MR imaging.
a. Non-targeted MR imaging
1. Contrast-enhanced MR imaging
It involves the acquisition of pre-contrast images of the tissue of interest, followed by intravenous injection of CM and subsequent acquisition of post-contrast images at an appropriate time point after CM administration. Gadolinium based-CM bind to albumin and the formed complex exits the vessel lumen at sites of albumin leakage into the extraluminal space leading to wall enhancement. Upon penetrating the plaque, the gadolinium moiety is no longer restricted to albumin and accumulates in the extracellular matrix of the plaque (which contains hydrophobic material such as collagen or proteoglycans). Being also lipophobic, gadolinium does not significantly enter the lipid core of the plaque, leading to preferential enhancement of the fibrous tissue. A limitation of conventional gadolinium-based CM has been the rapid distribution into extracellular space and a relatively rapid clearance by the kidneys. Using gadolinium-based CM-enhanced in vivo carotid MR imaging in patients undergoing CEA, wall enhancement results in better differentiation between fibrous tissue and lipid rich necrotic core. Wall enhancement is observed near the luminal surface and adventitia. Using a novel CM with prolonged intravascular phase, Cornily et al showed that early plaque enhancement in rabbit aorta had positive correlation with neovessel density and with macrophage density during the late phase15. Recently, in a serial follow up study of 10 patients undergoing gadolinium-enhanced MR imaging, it was shown that the changes in the contrast-to-noise ratio at varying time intervals after acute myocardial infarction parallel changes in the C-reactive protein16. This may be an indicator of the underlying inflammatory activity associated with acute coronary syndromes. Both studies had some limitations. Both studies had small number of patients. There was no comparison with intravascular US, which though invasive, remains the gold standard for coronary plaque assessment. Lastly, the presence of calcified plaques makes the assessment of the wall enhancement difficult particularly when computed tomography is used for co registration. Larger studies are however required to confirm the efficacy of delayed contrast enhancement techniques in the assessment of severity of atherosclerotic activity.
2. Dynamic contrast-enhanced MR imaging
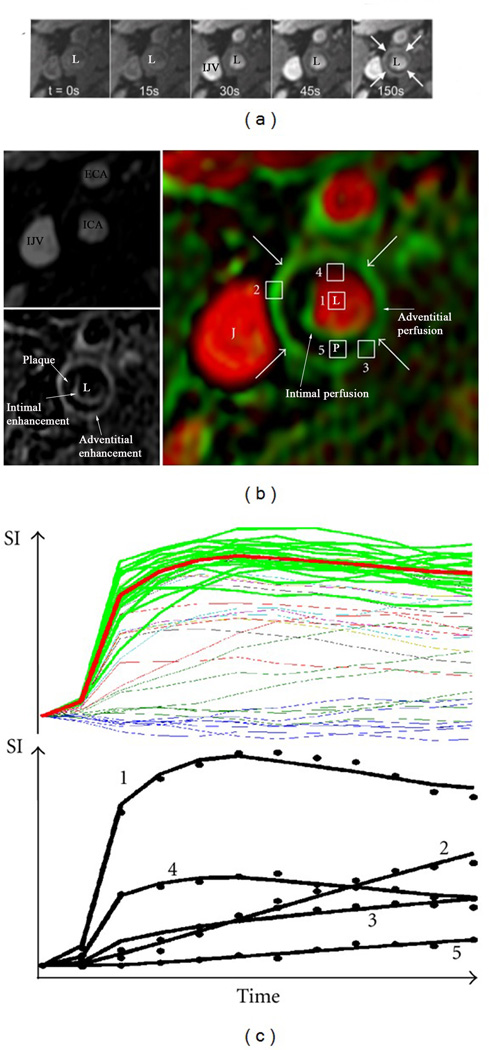
Assessment of the extent and permeability of the plaque neovasculature has become possible by serial acquisition of MR images before and after the administration of gadolinium-based CM and examination of the kinetics of CM uptake in the tissue of interest17 with appropriate data modeling [kinetic modeling (Figure 318)] or with non-model based approaches such as area under curve (AUC)]. This technique is called dynamic contrast enhanced (DCE)-MR imaging. It has high temporal and spatial resolution, which allows detailed assessment of activity of various plaque components. Reproducibility of the AUC measurements19 and kinetic modeling parameters has been reported20, however DCE-MR derived parameters seem to depend on the type of CM used. A strong correlation exists between the transfer constant (Ktrans) [a parameter obtained after kinetic modeling] of the CM into the extracellular space, neovasculature area and plaque inflammation as quantified by macrophage area21. Statins, which have the potential of reducing the inflammatory activity of atherosclerotic plaques, significantly reduce Ktrans22. These findings suggest that Ktrans indirectly represents plaque inflammation. In a rabbit model of atherosclerosis, the relationship of neovessel count in atherosclerotic plaque, neovessel permeability (as determined by AUC from DCE-MR) and plaque inflammation (as determined by 18-fluorine-flurodeoxyglucose (18F-FDG) has been reported23. DCE-MR imaging is therefore a potentially useful technique capable of providing information about the plaque neovascularization and interlinked inflammation.
Figure 3.
Illustration of vasa vasorum imaging via dynamic contrast-enhanced (DCE) MR imaging18. Sequential images after injection of gadolinium contrast agents at different time points (a). L indicates arterial lumen and IJV indicates the internal jugular vein. The sequential images are used in a kinetic model to create parametric images (b) of partial plasma volume (Vp)[top left, ECA: external carotid artery, ICA: internal carotid artery, IJV: internal julgular vein] and transfer constant (Ktrans) (bottom left). These have been fused into the color-coded image at right (arrows indicate adventitia, J: jugular vein, L: lumen, P: plaque). A pixel has been drawn in different sections indicated by numbers i.e. a pixel in the lumen (1), adventitial pixels with high (2) and low (3) Ktrans, a pixel with partial volume of the lumen (4), and an interior plaque pixel (5). Demonstration of intensity vs. time curves for all pixels in the 2cm2 region (all lines) for the set of blood curves extracted by the clustering algorithm (top, green lines) and their average (red line). The bottom curve shows typical fitting results for the kinetic model with corresponding points indicated by numbered pixels shown in (b).
However, this technique has some limitations: Imaging the microvessels, particularly the ones that are at vulnerable sites such as at plaque shoulder requires high in-plane spatial resolution. High temporal resolution, which is important for accurate arterial input function (AIF) estimation, has to be sacrificed to achieve higher spatial resolution. This can prove further challenging when the arterial wall thickness is only 1–2mm such as in early atheromatous lesions. The vessel tortuosity and plaque architecture may cause partial volume effects, which can make measurement of parameters [such as AIF] required for kinetic modeling difficult. AUC not only reflects tissue blood flow and vessel permeability but is also an indirect measure of the interstitial space and therefore has no simple physiological meaning. Clinical studies using DCE-MR imaging rely on signal intensities for calculation of kinetic parameters, it makes intrinsically difficult to compare studies conducted at different times and different centers unless appropriate calibration measures are taken. More prominent susceptibility effects with higher magnetic field strengths can also prove challenging. With above challenges in mind, development and validation of new acquisition methods is required that would allow accurate, repeatable and reproducible quantification of physiological parameters for assessment of inflammatory neovasculature.
b. Targeted MR imaging
1. Iron oxide-based MR imaging
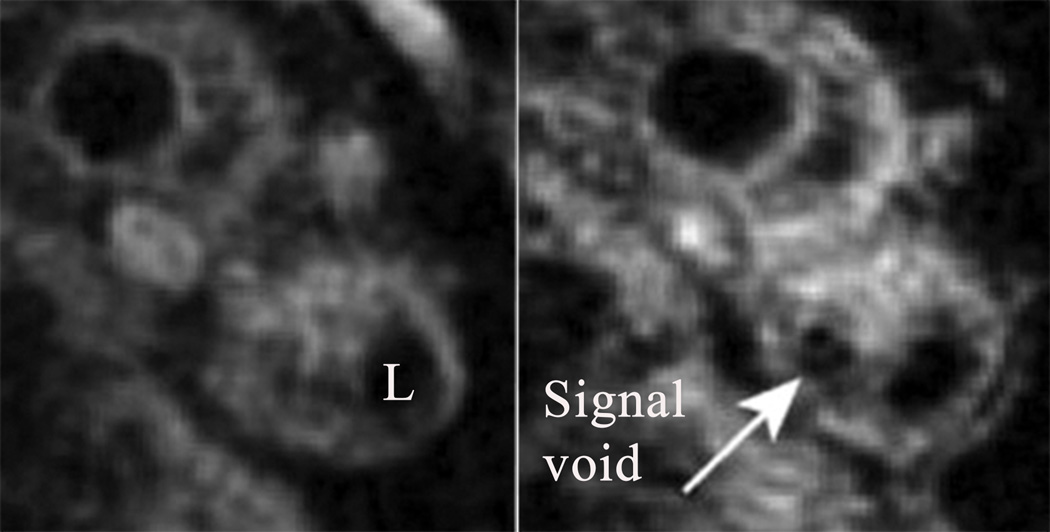
MR imaging also allows imaging of the cellular mediators of inflammation in atherosclerosis by use of targeted CM. Although initially devised for imaging the reticulo-endothelial system, Kresse et al first reported that superparamagnetic iron oxide (SPIO) particles also get incorporated into cells of aortic atherosclerotic plaques in hyperlipidemic rabbits24. Compared to SPIOs, the smaller particle size of dextran-coated ultrasmall superparamagnetic particles of iron oxide (USPIO) and their ability to extravasate via tight capillary pores makes them attractive option for cellular MR imaging. More importantly, USPIO particles are not immediately recognized by the hepatic and splenic reticulo-endothelial system, resulting in prolongation of plasma half-life making them suitable for atheroma imaging. Although the accumulation of USPIO in macrophages is well established, the mechanism of its uptake is not yet well defined. At higher concentrations of USPIO, T2/T2* relaxation effects predominate, with such areas in the tissue appearing hypointense on MR imaging25. The areas of signal loss appear initially at 24hrs (Figure 426), becoming obvious at 36hrs until 48hrs after USPIO administration. Relative change in signal intensity in regions of interest between matched post- and pre-USPIO enhanced MR images was used to quantify the USPIO-induced signal loss. Macrophage staining with CD68 and iron staining with Perls’ stain was used for histological assessment of USPIO localization within carotid plaques. Correlation between MR and histology was however not reported. Trivedi et al observed that although there was good agreement between location of Perls’ stain on histology and location of MR signal void, its agreement with the nature of USPIO signal effect was only moderate27. Strong correlation existed between magnitudes of USPIO effect, Perls’ staining and macrophage count in plaques, which exhibited focal areas of USPIO uptake on MR imaging, compared to plaques with diffuse distribution of USPIO. Plaques with such focal areas were histologically observed to have characteristics of vulnerable plaques. Poor correlation was observed between Perls staining and macrophage localization. Although various possible explanations were given for this observation such as heterogeneity in macrophage population in the plaque, authors attributed it to result most likely from the lack of sensitivity of Perls stain for USPIO. At low USPIO concentrations however T1 effects predominate, causing signal enhancement. This was observed to be prevalent in asymptomatic carotid plaques with thick fibrous cap.
Figure 4.
Pre- (A) and post-USPIO (B) MR spiral T2*-weighted axial imaging showing atheromatous plaque in the internal carotid artery of a symptomatic patient26. L indicates arterial lumen. Focal USPIO uptake can be seen at 24 hours after intravenous injection as an area of signal drop/void (white arrow).
Using serial USPIO-enhanced MR imaging over 3 month period in asymptomatic patients, significant reduction in carotid plaque inflammation with high dose statin lowering therapy compared to low dose therapy had also been reported28. Post-hoc long term follow up (median: 4 yrs) of patients from this trial failed to show any significant association between USPIO signal intensity loss and any subsequent cardio- and cerebrovascular events29. Limitation of this post-hoc study was that it was significantly underpowered to assess the long-term association. The traditional technique of quantifying relative signal loss on MR images as a measure of USPIO-induced changes also has limitations. The differences in patient positioning, magnetic field inhomogeneities and other artifacts may all induce signal loss and may not be indicative of USPIO uptake. In contrast to such semi-quantitative methods, quantitative T2* and T2 MR pulse sequences have been shown to more robust, particularly quantitative T2 sequences due to its inherent insensitivity to magnetic field inhomogeneities. Sadat et al have recently used these quantitative sequences to assess inflammation in human abdominal aortic aneurysms30. Since UPSIO and other negative contrast approaches can be difficult to interpret due to a low signal-to-noise ratio, positive contrast sequences, which can be acquired during same imaging session, may improve image interpretation and analysis31. Despite great potential, so far none of the USPIO products has been approved for atheroma imaging.
Receptor-targeted iron oxide MR imaging
Like receptor targeted UCAs, iron oxide particles tagged with ligands targeted to specific receptors in atheromatous tissue can offer an enhanced non-radiating method of imaging allowing ‘in vivo microscopy’. Using VCAM-1 targeted cross-lined iron oxide (CLIO) nanoparticles; Nahrendorf et al initially demonstrated targeting of all cell types within atheroma expressing VCAM-1 in vitro32. In vivo MR imaging revealed signal enhancement in the aortic root. VCAM-1 CLIO particles co-localized with endothelial cells and other VCAM-1 expressing cells such as macrophages in atheroma on fluorescent microscopy. By conjugating target-specific peptides with UPSIO (cyclic heptapeptide: R832 targeted to VCAM-1 and linear hexapeptide: R826 targeting phosphatidylserine), contrast enhancement of aortic atherosclerotic plaque in mice (20–30 min after CM injection) was observed, which was reproducible33. The plaques enhanced by USPIO-R832 contained macrophages concentrated in the cap and a large necrotic core, whereas USPIO-R826 produced a negative enhancement of plaques rich in macrophages and neutral fats concentrated inside the plaque and with lower global collagen content.
Compared to the non-specific dextran coated iron oxide particles, significantly shorter optimum imaging time for bio-functionalized UPSIO is due to their specific nature. They can be efficiently rendered stealthy by the covalent conjugation of USPIO derivatives with polyethylene glycol (PEG), escaping macrophage uptake. Most recently, other functionalized USPIOs such as those targeting P-selectin, oxidized LDL (OxLDL) and scavenger receptor A1 have been designed for targeted atheroma imaging. Microparticles of iron oxide (MPIOs) dually targeting VCAM-1 and P-selectin in aortic plaques have been reported to have significantly increased binding compared to P-selectin or VCAM-1 only targeted MPIOs, with significantly increased MR signal ex vivo compared to control agent34. By further reducing the size of this MPIO to micron size, efficacy of this CM to image activated endothelium and different stages of development of atheroma has been demonstrated35.
2. Gadolinium-based CM
Gadolinium loaded nanoparticles, which act as T1-CM, incorporate phospholipids, surfactants and lipophilic gadolinium complexes. They were initially used to overcome the limitations of iron-oxide enhanced MR imaging of atheroma in the early days of development of iron-oxide based-CM, such as difficulty in differentiating between iron oxide induced-signal void and artifacts, non-specificity of its uptake and delays in imaging due to delays in CM to penetrate the arterial wall. Use of target-specific lipid-based nanoparticles allows delivery of large number of gadolinium moieties incorporated in the lipid bilayer to the atheromatous tissue, acting as T1-reducing agents that appear bright in T1-weighted MR images. They generally have fast uptake at the target site, rapid clearance, and renal excretion leading to high target to background signal ratio.
Lipinski et al first reported the use of gadolinium-based immunomicelles specifically targeting macrophage scavenger receptor-A (MSR-A) on macrophages in plaques36. Development of annexin-A5-conjugated micellar nanoparticle carrying multiple Gd-labelled lipids for MR imaging and fluorescent lipids for fluorescence microscopy and imaging has also been reported37. It was shown to target phosphatidylserine exposed at the surface of apoptotic cells and macrophages in murine model of aortic atherosclerotic plaque. High-density lipoprotein (HDL) is another native nanoparticle, which due to its natural interaction with atherosclerotic plaques, has been used as an imaging agent. Using HDL-based gadolinium MR imaging CM, Frias et al observed increased signal intensity in areas of aortic plaques rich in macrophages38. However, due to the use of human plasma for manufacturing of such CM, the resulting safety precautions would complicate its clinical translation. This, alongside the pressing interest in HDL and in the treatment of HDL levels led to the development of HDL-mimicking peptides, with absent immunogenicity and ease of synthesis. Cormode reported the first in vivo use of HDL mimicking (with ApoA-I peptide) gadolinium and rhodamine loaded nanoparticle (rHDL) for dual modality imaging (MR and florescence respectively) to enhance macrophage rich areas of plaque in a mouse model39. Further modification of the rHDL, by incorporation of cationic and membrane penetrating lipopeptide (P2A2), showed a more pronounced signal enhancement of atherosclerotic wall on MR imaging. Confocal laser scanning microscopy revealed rHDL-P2A2 nanoparticles co-localized with intraplaque macrophages.
Another exciting development has been the production of chemically engineered substrates, which undergo a physicochemical change after interacting with their intended target (due to enzymatic cleavage, pH change etc). This physiochemical change would result in a product, with higher relaxivity and which has high target to background signal ratio, facilitating easy detection with an imaging modality. Myeloperoxidase enzyme (MPO) is one such potential target for molecular imaging, which is expressed by neutrophils and macrophages in advanced atherosclerotic lesions. Using such Gd-based probe has been reported to enhance diseased atherosclerotic thoracic aorta, with enhancement areas correlating with MPO-rich areas infiltrated by macrophages on histological examination40.
Targeted imaging of angiogenesis in atherosclerotic tissue with αvβ3-integrin targeted Gd-based nanoparticle has been reported41. αvβ3-integrin is a well-established biomarker of neovascular proliferation. Incorporation of an anti-angiogenic agent, Fumagillin into this imaging probe, was used for its localized delivery to the atheroma in a rabbit model. Seven days after treatment, αvβ3-integrin targeted nanoparticle enhanced imaging revealed reduced MR signal enhancement compared to untreated animals. Reduction in the microvessel count was evident in the treatment group42. Co-administration of αvβ3-integrin targeted fumagillin nanoparticle and atorvastatin was later shown to prolong the anti-angiogenic effect fumagillin.
Nuclear Imaging
Nuclear imaging relies on its ability to provide quantitative information on a functional level of plaque such as metabolic activity or expression levels of functional molecules. It is based on the use of radiolabelled biomarkers (usually called radiotracers), with their signal (‘hot spots’) detectable at the target site by means of imaging techniques such as gamma cameras, positron emission tomography (PET) or single photon emission computerized tomography (SPECT). To obtain a good quality image, radiotracer should have a rapid clearance from the blood stream and a good target-to-background signal ratio. This is particularly important when imaging a small size target such as atheromatous plaque, where a high background signal can impair the image quality. Use of radiotracers with high target specificity is therefore important. Nuclear imaging techniques though have high sensitivity but generally lack adequate spatial resolution. Use and further development of multimodality imaging systems such as PET/CT or PET/MR imaging may help to overcome this limitation due to better spatial resolution.
The feasibility of nuclear imaging in assessing functional activity of human atherosclerotic tissue was reported as early as 1980s. 125I (iodine)-labeled LDL was used and images were acquired by a gamma camera. Due to relatively poor imaging qualities of 125I, Technetium-99m (99mTc) was soon found to be a better alternative due to its short half-life and better gamma emission with a low absorbed radioactive dose for the patient. To improve the kinetics of these biomarkers, the discovery that oxidized LDL was readily taken up by macrophages via scavenger receptors, led to formulation of radiolabelled-oxidized LDL. 99mTc-oxidized LDL was observed to have rapid blood clearance and higher sensitivity in detecting symptomatic carotid plaques, localizing at scavenger receptor sites of macrophages. To differentiate between activated and quiescent macrophages, use of 99mTc-oxidized LDL targeted to folate receptors, which are only expressed on activated macrophages, has been reported43, thereby potentially enabling precise imaging of unstable atherosclerotic sites. In comparison with lipoproteins, peptides clear from the circulation quickly and theoretically could improve identification of atherosclerotic tissue easier. Their utility in humans remains largely unreported. For quantification of macrophage content, radiolabelled monoclonal antibody against amino malonic acid (AMA), a molecule vital to monocyte recruitment and foam cell production within atherosclerotic lesions, had significantly higher uptake in atheromatous aorta compared to normal aortas44. Slow radiotracer clearance from circulation however made in vivo imaging of aortic plaque unsuccessful.
Compared to SPECT imaging which has a resolution of 1–1.5cm, PET-Fluorine-18–labeled deoxyglucose (FDG) imaging can provide 4 to 5 mm resolution. Using18F-FDG PET imaging, Rudd et al reported efficacy of this non-invasive technique in imaging inflammation within atherosclerotic plaques45 (Figure 546). 18F-FDG PET imaging is in fact readout of vascular glucose metabolism, which is believed to be a surrogate of atherosclerotic plaque inflammation. Pre-clinical studies in animal models of atherosclerosis (without diabetes) have largely confirmed that the basis of the signal is inflammation, but not consistently so. Since glucose uptake is higher in macrophages than in other cells within the plaque, it is not surprising when we consider that all cells metabolizing glucose accumulate 18F-FDG. Efficacy of various anti atherosclerotic agents has also been successfully assessed using 18F-FDG PET imaging, by measuring the changes that they cause to the 18F-FDG signals47. The non-invasive read out of inflammation in patients with diabetes using FDG PET is an attractive option, but its uptake by cells is competitively reduced by the presence of elevated blood glucose levels. This remains a limitation for the use of 18F-FDG PET in clinical studies on diabetic patients. Correlation between 18F-FDG PET quantified arterial inflammation and DCE-MR imaging assess neovascularization has also been investigated. Taqueti et al also observed that with increasing macrophage count the FDG PET signal increased and Ktrans value was higher in macrophage-rich plaque areas48. Weak inverse relationship between inflammation measured as 18F-FDG uptake by PET and plaque perfusion by DCE-MR imaging has also been reported49. The likely explanation for the latter observation is there may be a complex relationship between plaque inflammation and neovascularization during the different stages of plaque development.
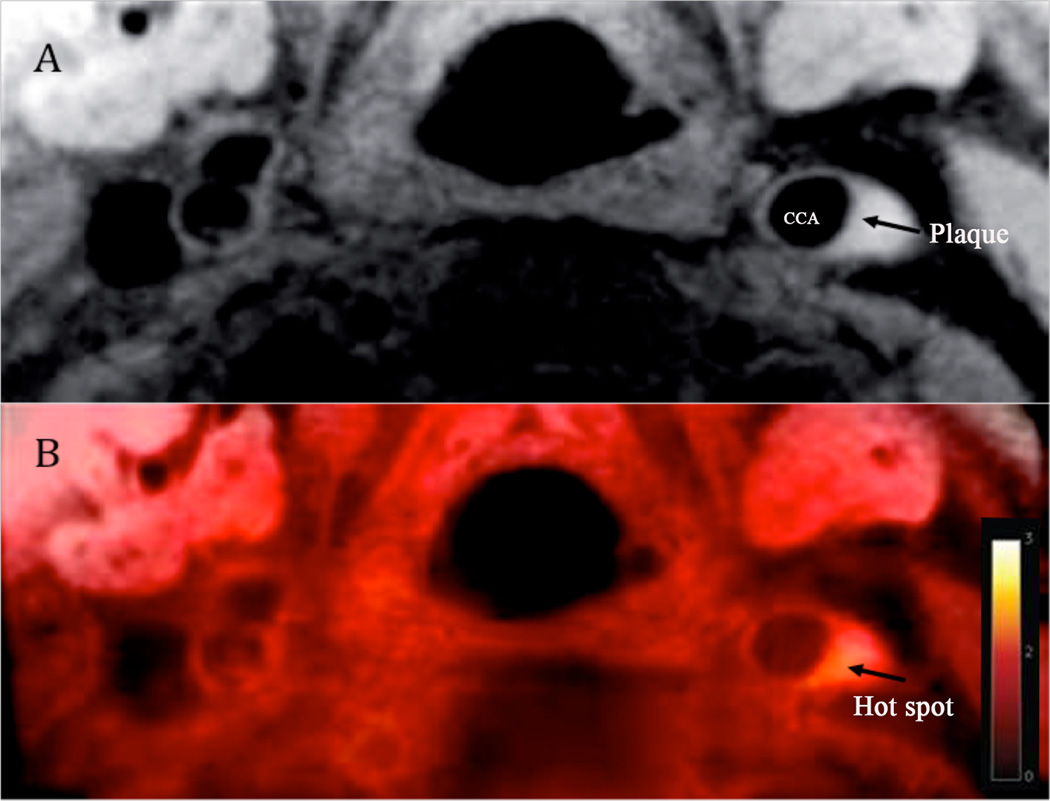
Figure 5.
FDG-MR imaging of carotid atheroma in the left common carotid artery46. (A) Black-blood MRI, an arrow indicating carotid plaque. (B) Superimposed FDG-MRI showing a hot spot (arrow) due to increased FDG uptake in the area of the carotid plaque.
PET-CT imaging has also been recently used to investigate calcification within atheromatous tissue as there is strengthening belief that unstable and metabolically active atheromata have active calcification, which differs from long standing dormant calcification. Hydroxyapatite is the central structural component of vascular calcification and is laid down during the earliest and most active stages of mineralization, believed to be associated with plaque inflammation and necrosis. Since fluoride ions are incorporated into the hydroxyapatite by ion exchange with hydroxyl groups at the crystal surface, using this property to advantage 18F-NaF PET imaging has been used to image atheromatous calcification. Patients with increased coronary 18F-NaF activity have been observed to have higher rates of previous cardiovascular events and higher overall calcium scores. Quantification of coronary 18F-FDG uptake is hampered by myocardial activity. This limitation with 18F-FDG was observed in a most recent prospective clinical trial50. 18F-NaF take up was however observed at all sites of carotid plaque ruptures in patients with previous myocardial infarction and was associated with histological evidence of active calcification, macrophage infiltration, apoptosis, and necrosis. Patients with stable angina had plaques with focal 18F-NaF uptakes, which were associated with more high-risk features on IVUS than those without uptake such as positive remodeling, micro-calcification and necrotic core. These findings highlight the potential benefit of 18F-NaF in differentiating between stable and unstable atheroma particularly in coronary arteries where 18F-FDG PET-CT imaging is limited by myocardial uptake. Further assessment of this imaging technique is required if it were to be used for improving risk stratification, monitoring disease progression, guiding therapeutic interventions, and assessing novel anti-atherosclerotic therapies.
Conclusions
Atherosclerosis remains a leading cause of mortality and morbidity in the developed countries despite significant advances in medical diagnostics and therapeutics. A paradigm shift has been witnessed in the past three decades from looking at atherosclerotic tissue as mere lipid-laden obstructive lesions to looking beyond the arterial lumen. Novel imaging techniques are enabling us to perform functional imaging and in vivo microscopy of plaque inflammation and neovascularization in much greater detail than ever before. Not only are they unravelling the pathobiology of atherosclerosis but also allowing investigation of the efficacy of new anti-atherosclerotic and anti-inflammatory agents. Each technique has its strengths and drawbacks. Identification of the atherosclerotic disease process in the earlier stages of development, well before clinical symptoms ensue, with delivery of therapeutic agents to disease-specific targets with least constitutive expression, to impede or cease the disease process, is the holy grail of functional, cellular and molecular imaging. This will require careful selection of validated imaging endpoints in future studies rather than relying on ‘clinical outcome’ studies, which require large sample size.
Acknowledgments
Sources of Funding: Dr Farouc Jaffer’s research is supported by National Institute of Health Grant [NIH HL R01 108229]. Dr Domenico Ribatti’s research is supported by the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement n.278570.
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Herrmann J, Lerman LO, Mukhopadhyay D, Napoli C, Lerman A. Angiogenesis in atherogenesis. Arterioscler Thromb Vasc Biol. 2006;26:1948–1957. doi: 10.1161/01.ATV.0000233387.90257.9b. [DOI] [PubMed] [Google Scholar]
- 2.Staub D, Schinkel AF, Coll B, Coli S, van der Steen AF, Reed JD, Krueger C, Thomenius KE, Adam D, Sijbrands EJ, ten Cate FJ, Feinstein SB. Contrast-enhanced ultrasound imaging of the vasa vasorum: from early atherosclerosis to the identification of unstable plaques. JACC Cardiovasc Imaging. 2010;3:761–771. doi: 10.1016/j.jcmg.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Shah F, Balan P, Weinberg M, Reddy V, Neems R, Feinstein M, Dainauskas J, Meyer P, Goldin M, Feinstein SB. Contrast-enhanced ultrasound imaging of atherosclerotic carotid plaque neovascularization: a new surrogate marker of atherosclerosis? Vasc Med. 2007;12:291–297. doi: 10.1177/1358863X07083363. [DOI] [PubMed] [Google Scholar]
- 4.Staub D, Patel MB, Tibrewala A, Ludden D, Johnson M, Espinosa P, Coll B, Jaeger KA, Feinstein SB. Vasa vasorum and plaque neovascularization on contrast-enhanced carotid ultrasound imaging correlates with cardiovascular disease and past cardiovascular events. Stroke. 2010;41:41–47. doi: 10.1161/STROKEAHA.109.560342. [DOI] [PubMed] [Google Scholar]
- 5.Deyama J, Nakamura T, Takishima I, Fujioka D, Kawabata K, Obata JE, Watanabe K, Watanabe Y, Saito Y, Mishina H, Kugiyama K. Contrast-enhanced ultrasound imaging of carotid plaque neovascularization is useful for identifying high-risk patients with coronary artery disease. Circ J. 2013;77:1499–1507. doi: 10.1253/circj.cj-12-1529. [DOI] [PubMed] [Google Scholar]
- 6.Huang PT, Chen CC, Aronow WS, Wang XT, Nair CK, Xue NY, Shen X, Li SY, Huang FG, Cosgrove D. Assessment of neovascularization within carotid plaques in patients with ischemic stroke. World J Cardiol. 2010;2:89–97. doi: 10.4330/wjc.v2.i4.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoogi A, Adam D, Hoffman A, Kerner H, Reisner S, Gaitini D. Carotid plaque vulnerability: quantification of neovascularization on contrast-enhanced ultrasound with histopathologic correlation. AJR Am J Roentgenol. 2011;196:431–436. doi: 10.2214/AJR.10.4522. [DOI] [PubMed] [Google Scholar]
- 8.Carlier S, Kakadiaris IA, Dib N, Vavuranakis M, O'Malley SM, Gul K, Hartley CJ, Metcalfe R, Mehran R, Stefanadis C, Falk E, Stone G, Leon M, Naghavi M. Vasa vasorum imaging: a new window to the clinical detection of vulnerable atherosclerotic plaques. Curr Atheroscler Rep. 2005;7:164–169. doi: 10.1007/s11883-005-0040-2. [DOI] [PubMed] [Google Scholar]
- 9.Goertz DE, Frijlink ME, de Jong N, van der Steen AF. Nonlinear intravascular ultrasound contrast imaging. Ultrasound Med Biol. 2006;32:491–502. doi: 10.1016/j.ultrasmedbio.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Maresca D, Renaud G, van Soest G, Li X, Zhou Q, Shung KK, de Jong N, van der Steen AF. Contrast-enhanced intravascular ultrasound pulse sequences for bandwidth-limited transducers. Ultrasound Med Biol. 2013;39:706–713. doi: 10.1016/j.ultrasmedbio.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamilton AJ, Huang SL, Warnick D, Rabbat M, Kane B, Nagaraj A, Klegerman M, McPherson DD. Intravascular ultrasound molecular imaging of atheroma components in vivo. J Am Coll Cardiol. 2004;43:453–460. doi: 10.1016/j.jacc.2003.07.048. [DOI] [PubMed] [Google Scholar]
- 12.Kaufmann BA, Sanders JM, Davis C, Xie A, Aldred P, Sarembock IJ, Lindner JR. Molecular imaging of inflammation in atherosclerosis with targeted ultrasound detection of vascular cell adhesion molecule-1. Circulation. 2007;116:276–284. doi: 10.1161/CIRCULATIONAHA.106.684738. [DOI] [PubMed] [Google Scholar]
- 13.Khanicheh E, Mitterhuber M, Xu L, Haeuselmann SP, Kuster GM, Kaufmann BA. Noninvasive ultrasound molecular imaging of the effect of statins on endothelial inflammatory phenotype in early atherosclerosis. PLoS ONE. 2013;8:e58761. doi: 10.1371/journal.pone.0058761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Z, Curaj A, Fokong S, Liehn EA, Weber C, Lammers T, Kiessling F, Zandvoort van M. Rhodamine-loaded intercellular adhesion molecule-1-targeted microbubbles for dual-modality imaging under controlled shear stresses. Circ Cardiovasc Imaging. 2013;6:974–981. doi: 10.1161/CIRCIMAGING.113.000805. [DOI] [PubMed] [Google Scholar]
- 15.Cornily JC, Hyafil F, Calcagno C, Briley-Saebo KC, Tunstead J, Aguinaldo JG, Mani V, Lorusso V, Cavagna FM, Fayad ZA. Evaluation of neovessels in atherosclerotic plaques of rabbits using an albumin-binding intravascular contrast agent and MRI. J Magn Reson Imaging. 2008;27:1406–1411. doi: 10.1002/jmri.21369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibrahim T, Makowski MR, Jankauskas A, Maintz D, Karch M, Schachoff S, Manning WJ, Schomig A, Schwaiger M, Botnar RM. Serial contrast-enhanced cardiac magnetic resonance imaging demonstrates regression of hyperenhancement within the coronary artery wall in patients after acute myocardial infarction. JACC Cardiovasc Imaging. 2009;2:580–588. doi: 10.1016/j.jcmg.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 17.Kerwin W, Hooker A, Spilker M, Vicini P, Ferguson M, Hatsukami T, Yuan C. Quantitative magnetic resonance imaging analysis of neovasculature volume in carotid atherosclerotic plaque. Circulation. 2003;107:851–856. doi: 10.1161/01.cir.0000048145.52309.31. [DOI] [PubMed] [Google Scholar]
- 18.Kerwin WS. Carotid artery disease and stroke: assessing risk with vessel wall MRI. ISRN Cardiol. 2012;2012:180710. doi: 10.5402/2012/180710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calcagno C, Vucic E, Mani V, Goldschlager G, Fayad ZA. Reproducibility of black blood dynamic contrast-enhanced magnetic resonance imaging in aortic plaques of atherosclerotic rabbits. J Magn Reson Imaging. 2010;32:191–198. doi: 10.1002/jmri.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerwin WS, Zhao X, Yuan C, Hatsukami TS, Maravilla KR, Underhill HR. Contrast-enhanced MRI of carotid atherosclerosis: dependence on contrast agent. J Magn Reson Imaging. 2009;30:35–40. doi: 10.1002/jmri.21826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerwin WS, O'Brien KD, Ferguson MS, Polissar N, Hatsukami TS, Yuan C. Inflammation in carotid atherosclerotic plaque: a dynamic contrast-enhanced MR imaging study. Radiology. 2006;241:459–468. doi: 10.1148/radiol.2412051336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong L, Kerwin WS, Chen H, Chu B, Underhill HR, Neradilek MB, Hatsukami TS, Yuan C, Zhao XQ. Carotid artery atherosclerosis: effect of intensive lipid therapy on the vasa vasorum--evaluation by using dynamic contrast-enhanced MR imaging. Radiology. 2011;260:224–231. doi: 10.1148/radiol.11101264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calcagno C, Cornily JC, Hyafil F, Rudd JH, Briley-Saebo KC, Mani V, Goldschlager G, Machac J, Fuster V, Fayad ZA. Detection of neovessels in atherosclerotic plaques of rabbits using dynamic contrast enhanced MRI and 18F-FDG PET. Arterioscler Thromb Vasc Biol. 2008;28:1311–1317. doi: 10.1161/ATVBAHA.108.166173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kresse M, Wagner S, Thode K, Dinkelborg L, Semmler W. MR plaque imaging using superparamagnetic iron oxide particles. Paper presented at: International Society for Magnetic Resonance in Medicine; 1998; Sydney, Australia. [Google Scholar]
- 25.Kooi ME, Cappendijk VC, Cleutjens KB, Kessels AG, Kitslaar PJ, Borgers M, Frederik PM, Daemen MJ, van Engelshoven JM. Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation. 2003;107:2453–2458. doi: 10.1161/01.CIR.0000068315.98705.CC. [DOI] [PubMed] [Google Scholar]
- 26.Tang TY, Muller KH, Graves MJ, Li ZY, Walsh SR, Young V, Sadat U, Howarth SP, Gillard JH. Iron oxide particles for atheroma imaging. Arterioscler Thromb Vasc Biol. 2009;29:1001–1008. doi: 10.1161/ATVBAHA.108.165514. [DOI] [PubMed] [Google Scholar]
- 27.Trivedi RA, Mallawarachi C, JM UK-I, Graves MJ, Horsley J, Goddard MJ, Brown A, Wang L, Kirkpatrick PJ, Brown J, Gillard JH. Identifying inflamed carotid plaques using in vivo USPIO-enhanced MR imaging to label plaque macrophages. Arterioscler Thromb Vasc Biol. 2006;26:1601–1606. doi: 10.1161/01.ATV.0000222920.59760.df. [DOI] [PubMed] [Google Scholar]
- 28.Tang TY, Howarth SP, Miller SR, Graves MJ, Patterson AJ, JM UK-I, Li ZY, Walsh SR, Brown AP, Kirkpatrick PJ, Warburton EA, Hayes PD, Varty K, Boyle JR, Gaunt ME, Zalewski A, Gillard JH. The ATHEROMA (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) Study. Evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. J Am Coll Cardiol. 2009;53:2039–2050. doi: 10.1016/j.jacc.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 29.Degnan AJ, Patterson AJ, Tang TY, Howarth SP, Gillard JH. Evaluation of ultrasmall superparamagnetic iron oxide-enhanced MRI of carotid atherosclerosis to assess risk of cerebrovascular and cardiovascular events: follow-up of the ATHEROMA trial. Cerebrovasc Dis. 2012;34:169–173. doi: 10.1159/000339984. [DOI] [PubMed] [Google Scholar]
- 30.Sadat U, Taviani V, Patterson AJ, Young VE, Graves MJ, Teng Z, Tang TY, Gillard JH. Ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging of abdominal aortic aneurysms--a feasibility study. Eur J Vasc Endovasc Surg. 2011;41:167–174. doi: 10.1016/j.ejvs.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 31.Korosoglou G, Weiss RG, Kedziorek DA, Walczak P, Gilson WD, Schar M, Sosnovik DE, Kraitchman DL, Boston RC, Bulte JW, Weissleder R, Stuber M. Noninvasive detection of macrophage-rich atherosclerotic plaque in hyperlipidemic rabbits using "positive contrast" magnetic resonance imaging. J Am Coll Cardiol. 2008;52:483–491. doi: 10.1016/j.jacc.2008.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nahrendorf M, Jaffer FA, Kelly KA, Sosnovik DE, Aikawa E, Libby P, Weissleder R. Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation. 2006;114:1504–1511. doi: 10.1161/CIRCULATIONAHA.106.646380. [DOI] [PubMed] [Google Scholar]
- 33.Burtea C, Ballet S, Laurent S, Rousseaux O, Dencausse A, Gonzalez W, Port M, Corot C, Vander Elst L, Muller RN. Development of a magnetic resonance imaging protocol for the characterization of atherosclerotic plaque by using vascular cell adhesion molecule-1 and apoptosis-targeted ultrasmall superparamagnetic iron oxide derivatives. Arterioscler Thromb Vasc Biol. 2012;32:e36–e48. doi: 10.1161/ATVBAHA.112.245415. [DOI] [PubMed] [Google Scholar]
- 34.McAteer MA, Schneider JE, Ali ZA, Warrick N, Bursill CA, von zur Muhlen C, Greaves DR, Neubauer S, Channon KM, Choudhury RP. Magnetic resonance imaging of endothelial adhesion molecules in mouse atherosclerosis using dual-targeted microparticles of iron oxide. Arterioscler Thromb Vasc Biol. 2008;28:77–83. doi: 10.1161/ATVBAHA.107.145466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McAteer MA, Mankia K, Ruparelia N, Jefferson A, Nugent HB, Stork LA, Channon KM, Schneider JE, Choudhury RP. A leukocyte-mimetic magnetic resonance imaging contrast agent homes rapidly to activated endothelium and tracks with atherosclerotic lesion macrophage content. Arterioscler Thromb Vasc Biol. 2012;32:1427–1435. doi: 10.1161/ATVBAHA.111.241844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lipinski MJ, Amirbekian V, Frias JC, Aguinaldo JG, Mani V, Briley-Saebo KC, Fuster V, Fallon JT, Fisher EA, Fayad ZA. MRI to detect atherosclerosis with gadolinium-containing immunomicelles targeting the macrophage scavenger receptor. Magn Reson Med. 2006;56:601–610. doi: 10.1002/mrm.20995. [DOI] [PubMed] [Google Scholar]
- 37.van Tilborg GA, Vucic E, Strijkers GJ, Cormode DP, Mani V, Skajaa T, Reutelingsperger CP, Fayad ZA, Mulder WJ, Nicolay K. Annexin A5-functionalized bimodal nanoparticles for MRI and fluorescence imaging of atherosclerotic plaques. Bioconjug Chem. 2010;21:1794–1803. doi: 10.1021/bc100091q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frias JC, Williams KJ, Fisher EA, Fayad ZA. Recombinant HDL-like nanoparticles: a specific contrast agent for MRI of atherosclerotic plaques. J Am Chem Soc. 2004;126:16316–16317. doi: 10.1021/ja044911a. [DOI] [PubMed] [Google Scholar]
- 39.Cormode DP, Briley-Saebo KC, Mulder WJ, Aguinaldo JG, Barazza A, Ma Y, Fisher EA, Fayad ZA. An ApoA-I mimetic peptide high-density-lipoprotein-based MRI contrast agent for atherosclerotic plaque composition detection. Small. 2008;4:1437–1444. doi: 10.1002/smll.200701285. [DOI] [PubMed] [Google Scholar]
- 40.Ronald JA, Chen Y, Belisle AJ, Hamilton AM, Rogers KA, Hegele RA, Misselwitz B, Rutt BK. Comparison of gadofluorine-M and Gd-DTPA for noninvasive staging of atherosclerotic plaque stability using MRI. Circ Cardiovasc Imaging. 2009;2:226–234. doi: 10.1161/CIRCIMAGING.108.826826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winter PM, Morawski AM, Caruthers SD, Fuhrhop RW, Zhang H, Williams TA, Allen JS, Lacy EK, Robertson JD, Lanza GM, Wickline SA. Molecular imaging of angiogenesis in early-stage atherosclerosis with alpha(v)beta3-integrin-targeted nanoparticles. Circulation. 2003;108:2270–2274. doi: 10.1161/01.CIR.0000093185.16083.95. [DOI] [PubMed] [Google Scholar]
- 42.Winter PM, Neubauer AM, Caruthers SD, Harris TD, Robertson JD, Williams TA, Schmieder AH, Hu G, Allen JS, Lacy EK, Zhang H, Wickline SA, Lanza GM. Endothelial alpha(v)beta3 integrin-targeted fumagillin nanoparticles inhibit angiogenesis in atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:2103–2109. doi: 10.1161/01.ATV.0000235724.11299.76. [DOI] [PubMed] [Google Scholar]
- 43.Ayala-Lopez W, Xia W, Varghese B, Low PS. Imaging of atherosclerosis in apoliprotein e knockout mice: targeting of a folate-conjugated radiopharmaceutical to activated macrophages. J Nucl Med. 2010;51:768–774. doi: 10.2967/jnumed.109.071324. [DOI] [PubMed] [Google Scholar]
- 44.Chakrabarti M, Cheng KT, Spicer KM, Kirsch WM, Fowler SD, Kelln W, Griende S, Nehlsen-Cannarella S, Willerson R, Spicer SS, Koch T. Biodistribution and radioimmunopharmacokinetics of 131I-Ama monoclonal antibody in atherosclerotic rabbits. Nucl Med Biol. 1995;22:693–697. doi: 10.1016/0969-8051(95)00008-l. [DOI] [PubMed] [Google Scholar]
- 45.Rudd JH, Warburton EA, Fryer TD, Jones HA, Clark JC, Antoun N, Johnstrom P, Davenport AP, Kirkpatrick PJ, Arch BN, Pickard JD, Weissberg PL. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105:2708–2711. doi: 10.1161/01.cir.0000020548.60110.76. [DOI] [PubMed] [Google Scholar]
- 46.Tang TY, Moustafa RR, Howarth SP, Walsh SR, Boyle JR, Li ZY, Baron JC, Gillard JH, Warburton EA. Combined PET-FDG and USPIO-enhanced MR imaging in patients with symptomatic moderate carotid artery stenosis. Eur J Vasc Endovasc Surg. 2008;36:53–55. doi: 10.1016/j.ejvs.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Tawakol A, Fayad ZA, Mogg R, Alon A, Klimas MT, Dansky H, Subramanian SS, Abdelbaky A, Rudd JH, Farkouh ME, Nunes IO, Beals CR, Shankar SS. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: results of a multicenter fluorodeoxyglucose-positron emission tomography/computed tomography feasibility study. J Am Coll Cardiol. 2013;62:909–917. doi: 10.1016/j.jacc.2013.04.066. [DOI] [PubMed] [Google Scholar]
- 48.Taqueti V, Carli MD, Jerosch-Herold M, Sukhova G, Murthy V, Folco E, Kwong R, Nahrendorf M, Weissleder R, Libby P. Increased microvascular blood flow and permeability associates with FDG signal in human atheroma. J Am Coll Cardiol. 2012;59:E1309-E1309. [Google Scholar]
- 49.Calcagno C, Ramachandran S, Izquierdo-Garcia D, Mani V, Millon A, Rosenbaum D, Tawakol A, Woodward M, Bucerius J, Moshier E, Godbold J, Kallend D, Farkouh ME, Fuster V, Rudd JH, Fayad ZA. The complementary roles of dynamic contrast-enhanced MRI and 18F-fluorodeoxyglucose PET/CT for imaging of carotid atherosclerosis. Eur J Nucl Med Mol Imaging. 2013;40:1884–1893. doi: 10.1007/s00259-013-2518-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joshi NV, Vesey AT, Williams MC, Shah AS, Calvert PA, Craighead FH, Yeoh SE, Wallace W, Salter D, Fletcher AM, van Beek EJ, Flapan AD, Uren NG, Behan MW, Cruden NL, Mills NL, Fox KA, Rudd JH, Dweck MR, Newby DE. F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet. 2013;383:705–713. doi: 10.1016/S0140-6736(13)61754-7. [DOI] [PubMed] [Google Scholar]