Abstract
Aldosterone administration in rats results in several cardiac alterations. Previous studies have demonstrated that proanthocyanidins, phenolic bioactive compounds, have cardioprotective effects. We studied the potential beneficial effects of the proanthocyanidin-rich almond skin extract (PASE) on the cardiac alterations induced by aldosterone-salt treatment, their effects in mineralocorticoid receptor activity and we sought to confirm proanthocyanidins as the specific component of the extract involved in the beneficial cardiac effects. Male Wistar rats received aldosterone (1 mg/Kg/day) +1% NaCl for 3 weeks. Half of the animals in each group were simultaneously treated with either PASE (100 mg/Kg/day) or spironolactone (200 mg/Kg/day). The ability of PASE to act as an antagonist of the mineralocorticoid receptor was examined using a transactivation assay. High performance liquid chromatography was used to identify and to isolate proanthocyanidins. Hypertension and diastolic dysfunction induced by aldosterone were abolished by treatment with PASE. Expression of the aldosterone mediator SGK-1, together with fibrotic, inflammatory and oxidative mediators were increased by aldosterone-salt treatment; these were reduced by PASE. Aldosterone-salt induced transcriptional activity of the mineralocorticoid receptor was reduced by PASE. HPLC confirmed proanthocyanidins as the compound responsible for the beneficial effects of PASE. The effects of PASE were comparable to those seen with the mineralocorticoid antagonist, spironolactone. The observed responses in the aldosterone-salt treated rats together with the antagonism of transactivation at the mineralocorticoid receptor by PASE provides evidence that the beneficial effect of this proanthocyanidin-rich almond skin extract is via as a mineralocorticoid receptor antagonist with proanthocyanidins identified as the compounds responsible for the beneficial effects of PASE.
Background
Aldosterone exerts direct cardiac effects that contribute to pathological structural remodeling in the ventricular myocardium [1], [2]. In the clinical setting, elevated serum levels of aldosterone have been shown to be associated with high mortality in patients with severe congestive heart failure [3] and several clinical trials have established the beneficial effects of mineralocorticoid receptor antagonists for the treatment of heart failure [4], [5]. It has been demonstrated that aldosterone promotes myocardial perivascular and interstitial fibrosis [6], [7] and left ventricular hypertrophy [8]. Moreover, the flexibility of myocardial tissue is reduced, increasing the filling pressure of the heart and contributing to diastolic dysfunction [9]. On the other hand, the presence of cardiac hypertrophy is known to be an important risk factor for cardiovascular morbidity and mortality [10]–[12].
Proanthocyanidins are a large family of phenolic bioactive compounds (oligomers or polymers) composed of flavan-3-ol monomer subunits linked together. Based on their composition they are subdivided into three different families: procyanidins -composed exclusively of catechin and epicatechin monomers; prodelphinidins –containing at least one unit of gallocatechin or epigallocatechin together with units of catechin or epicatechin; and properlagonidins- containing at least one unit of afzelechin or epiafzelechin together with units of catechin or epicatechin. Proanthocyanidins have shown antioxidant [13], anti-inflammatory [14], anti-hypertensive [15], anti-platelet [16], anti-thrombotic [17] and hypocholesterolemic [18] activities. Furthermore, epidemiological studies have strongly suggested that regular consumption of proanthocyanidins may decrease the risk of cardiovascular diseases [19]. Polyphenolic constituents including oligomeric proanthocyanidins and resveratrol in red wine have been demonstrated to have cardioprotective effects [20], [21]. Polyphenols’ health properties have been attributed to the direct antioxidant effect of these phytochemicals, which act as free radical scavengers. However, recent data has revealed that polyphenols could interact with cell signaling pathways and modulate the activity of transcription factors with consequent regulation of gene expression [22]–[24]. This suggests that these cellular and molecular targets mediate the most relevant mechanisms of action underlying the biological effects of polyphenols.
We [25], [26], and others [27]–[30] have previously characterized a model in which administration of aldosterone plus 1% salt to rats results in cardiac hypertrophy, fibrosis, hypertension and diastolic dysfunction. This response, which is mediated by the mineralocorticoid receptor (MR), is attenuated by the MR antagonists, spironolactone and eplerenone [30], [31]. Although both antagonists of the mineralocorticoid receptor in current clinical use, spironolactone and eplerenone, are steroidal compounds there are now several reports of non-steroidal compounds with potent mineralocorticoid-specificity-antagonist activity [32]–[34].
None of the previous studies has examined the effect of proanthocyanidins in a cardiac disease model induced by aldosterone where many adverse cardiovascular alterations including inflammation, fibrosis and oxidative stress encounter. Therefore, since previous studies have shown beneficial effects of proanthocyanidins on cardiovascular diseases, the purpose of this study was to evaluate the effects of a proanthocyanidin-rich extract obtained from almond skin on cardiac alterations induced by aldosterone-salt administration and to further investigate the molecular mechanisms involved. Here, we analyzed structural, functional and molecular alterations induced in the rat heart by mineralocorticoid-salt treatment as well as the effects of PASE (Proanthocyanidin-rich Almond Skin Extract) on the serum and glucocorticoid regulated kinase type 1 (SGK-1) gene expression, recognized transcriptional target of MR actions. In order to further study the mechanisms of the effects of PASE, we analyzed the aldosterone-induced transcriptional activity of the mineralocorticoid receptor in the presence of increasing doses of PASE. Furthermore, we sought to confirm that proanthocyanidins were the extract compounds involved in the cardiac effects so; we obtained purified fractions of PASE by High performance liquid chromatography and, analyzed the aldosterone-induced transcriptional activity of the mineralocorticoid receptor in the presence of the purified fractions. The effects of PASE alone were compared with those of spironolactone.
Methods
Experimental design and animals
Forty male Wistar rats (256±3 g; Harlan Ibérica, Barcelona, Spain) were used in the study according to the guidelines for ethical care of experimental animals of the European Union and granted and approved by the Universidad Complutense Ethics Review Board following the National Guideline 53/2013. The Universidad Complutense Ethics Review Board specifically approved this study. Rats were fed standard rat chow and tap water ad libitum and kept in a quiet room at constant temperature (20 to 22°C) and humidity (50 to 60%). A control group and 4 experimental groups of animals were used in the study, 8 animals each group. Before allocating animals to treatment, blood pressure was measured to group them under the same mean blood pressure by the tail-cuff method. Aldosterone (1 mg/Kg/day) dissolved in corn oil or vehicle alone was subcutaneously injected once daily for 3 weeks; these rats received NaCl 1% as drinking water. Half of the animals in each group were simultaneously treated with either PASE (100 mg/Kg/day in the drinking water) or spironolactone as a positive control (200 mg/Kg/day, subcutaneous). PASE (25.1% proanthocyanidins w/w), used in this study contains the phenolic fraction of almonds (Prunus dulcis) with different degrees of polymerization, and is obtained by a standardized process, which concentrates the procyanidins, properlagonidins and prodelphinidins from almonds, including oligomers and polymers [35]–[37]. At the end of the treatment period, animals were anesthetized (Ketamine, Imalgene 1000, 70 mg/Kg, and Xilacine, Rompun 2%, 6 mg/Kg; intraperitoneal injection) and a catheter (Science FT211B of 1.6F diameter; Ontario, Canada) was inserted and advanced through the right carotid artery getting into the left ventricle as previously described [38]. The catheter was connected to a data acquisition system (PowerLab/800. ADInstruments, Castle Hill, Australia). Signals were monitored and digitally stored for their analysis with the commercial software Chart for Windows v4.2 (London, United Kingdom). Systolic and diastolic blood pressure (SBP and DBP), heart rate (HR), left ventricle end diastolic pressure (LVEDP), left ventricle systolic pressure (LVSP), the first derivative of LV pressure rise over time (+dP/dt) and the first derivative of LV pressure rise over time (−dP/dt) were measured. After measuring hemodynamic parameters, the animals were sacrificed by exsanguinations through the catheter inserted in the carotid artery, the heart removed, weighed and rapidly frozen in liquid nitrogen for molecular studies. The ratio of heart to body weight was used as the index of cardiac hypertrophy.
Cardiac Collagen Quantification
A piece of LV from each rat was fixed in 3, 7% paraformaldehyde for 24 hours, and then stored in 70% alcohol until its use. The tissues were dehydrated, and fixed in paraffin and then cut into 4 µm slices. These slices were stained with Sirius Red F3BA (0.5% in saturated aqueous picric acid; Aldrich Chemical Company, Madrid, Spain). Quantification of collagen content was performed using an image analysis system (Leica Microsystems, Barcelona, Spain). The analyses were performed in four different sections of each slide of the heart and ten photographs from each section were taken. A single investigator, unaware of the experimental groups, performed these analyses.
Real Time RT-PCR to Detect mRNA Expression
RNA isolation
Frozen rat hearts were pulverized in liquid nitrogen. RNA isolation was performed using an RNA extraction kit (Qiagen Sciences, Maryland, USA) and quantified by measurement of optical density at 260 nm (BioPhotometer, Eppendorf, Hamburg, Germany). The RNAs were stored at –80°C until their use.
cDNA synthesis
Genomic DNA was eliminated from the DNA with a mixture of gDNA Wipeout Buffer and RNPASE free water incubated for 2 min at 42°C. Then, 1 µg of total RNA was reverse transcribed using Quantiscript Reverse Transcriptase for 15 min at 42°C and 3 min at 95°C (Qiagen, Sciences, Maryland, USA).
Quantitative RT-PCR analysis
Real-time PCR was performed using a SmartCycler (Cepheid, Sunnyvale, California, USA). Taqman technology was used for qRT-PCR and Taq DNA polymerase (Qiagen Sciences, Maryland, USA) was used. Oligonucleotides, modified with fluorescence label at the 5′-end and with quencher at the 3′-end, were added to a reaction system. The relative quantitation of the gene expression was performed using the comparative CT method [39]. The data was normalized using 18 S ribosomal RNA and expressed as % relative expression vs. control group. Table 1 shows primers sequences.
Table 1. Primers sequences.
| Genes | Sense | Antisense | Probe |
| 18S | 5′CGCAAATTACCCACTCCCGACCC3′ | 5′GGCTACCACATCCAAGGAAG3′ | 5′ CAATTACAGGGCCTCGAAAGA 3′ |
| CTGF | 5′TGGCCCTGACCCAACTATGAT3′ | 5′GCACTTTTTGCCCTTCTTAATGTT3′ | 5′AGGCCAACTGCCTGGTCCAGACCA 3′ |
| TGF-β | 5′GGGCTTTCGCTTCAGTGCT 3′ | 5′TCGGTTCATGTCATGGATGGT3′ | 5′TCAGTCCCAAACGTCGAGGTGACCTG3′ |
| MMP-2 | 5′CGTGGTGAGATCTTCTTCTTCAAGGA3′ | 5′CCTCATACACAGCGTCAATCTTTTC3′ | 5′ACACCACGTGACAAGCCCACAGGTC 3′ |
| TIMP-2 | 5′GGAGGAAAGAAGGAATATCTAATTGCAG3′ | 5′CCAGGGCACAATAAAGTCACAGA3′ | 5′CATCTTGCCATCTCCTTCCGCCTTCC3′ |
| IL-1β | 5′TCTTCGAGGCACAAGGCAC3′ | 5′CAGAGGTCCAGGTCCTGGAA3′ | 5′ACCTGAGCTCGCCAGTGAAATGATGGCTT3′ |
| TNF-α | 5′GGTGATCGGTCCCAACAAGGA 3′ | 5′CACGCTGGCTCAGCCACTC 3′ | 5′TGGCCCAGACCCTCACACTCAGATCA3′ |
| eNOS | 5′AAGACGCTGCTTGGGATCC3′ | 5′AGCCTGGGAACCACTCCTTT3′ | 5′AGGAAGTTACAGAGCCGGCCCACCC3′ |
| p22phox | 5′GGACAGAAGTACCTGACCGCT3′ | 5′CAGGCACGGACAGCAGTAAG3′ | 5′AGGACAGCCCGGACGTAGTAATTTCTGGT3′ |
| SGK-1 | 5′GCACGCCTGAGTATCTCGC3′ | 5′AGGCCATAGAGCATCTCATACAAGAC3′ | 5′CCCGAGGCACCACCAGTCCACT3′ |
GeneBank accession numbers: 18S NR045132.1, CTGF AC127189.4, TGF-β NM021578.2, MMP-2 BC074013.1, TIMP-2 BC084714.1, IL-1β, NG008851.1, TNF-α NM012675.3, eNOS NM021838.2, p22phox NM024160.1 and SGK-1 NM001193569.1.
Tissue culture and transactivation assay
The transactivation assays were performed in CV-1 cells as described previously [40]. The cells were seeded at a density of 8×104 cells/well in 12-well plates in Dulbecco’s modified Eagle’s medium (DMEM; Sigma) +10% FBS and incubated overnight before transfection. Transfections were performed using FuGene6 (Roche Molecular Biochemicals, Indianapolis, IN) as per manufacturer’s protocol, and the medium was changed to DMEM supplemented with 10% charcoal-stripped FBS. The cells were transfected with 500 ng of expression vector containing full-length human mineralocorticoid receptor (hMR) together with 500 ng of the luciferase reporter plasmid MMTV-LUC. The hMR expression construct is pRShMR [41]. Post-transfection, the cells were incubated with spironolactone (100 nM) or PASE (10 nM, 100 nM and 1 µM) in the presence or absence of aldosterone (1 nM) for 24 h. The “n” per experiment was 4 and all in vitro experiments are representative of 3 independent experiments. Luciferase activity was determined using the Dual Luciferase Assay system (Promega, Madison, MI) according to the manufacturer’s instructions. The MMTV-Luc plasmid described previously [42] was used and pRL-tk plasmid containing the Renilla luciferase gene (Promega, Madison, MI) was used as a control. Light units were measured in an EnVision Multilabel Reader (PerkinElmer, Waltham, MA).
High performance liquid chromatography (HPLC)
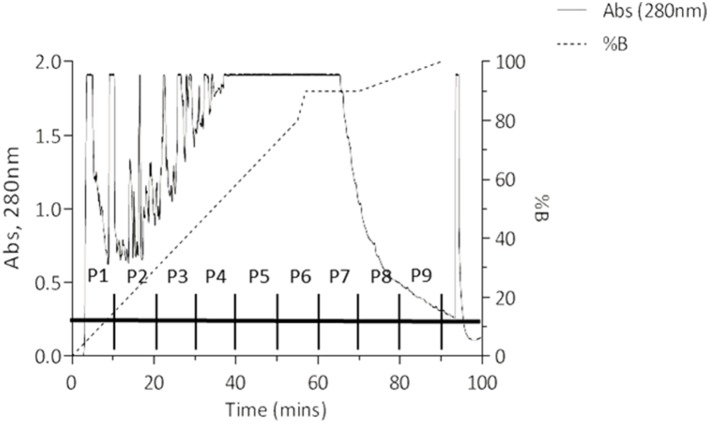
HPLC separation of components within PASE employed a method similar to Monagas et al. [43], with a Waters (Milford, MA) Novapak C18 60Å 4 µm, 30×0.39 cm column, pre-equilibrated in Buffer A (2% CH3COOH in H2O) at a flow rate of 1 ml/min at room temperature. The PASE sample (0.2 g total) was dissolved in buffer A and filtered (0.22 µm) prior to injection, after which a 55 minute linear gradient to 80% buffer B (2% CH3COOH, 25% CH3CN) was started. The column was then washed with increasing amounts of buffer B as follows (2 min, 80–90% buffer B; 13 min hold at 90% buffer B; 20 min, 90–100% buffer B), with a final 10 min wash with 2% CH3COOH, 75% CH3CN (total program time = 100 mins). Fractions were collected at 1 minute intervals and the absorbance of the elute monitored at 280 nm, obtaining 100 fractions. To simplify the transactivation assay, pools of every 10 fractions were prepared and labeled P1 to P10 (see Figure 1). Transactivation assays were then performed as described above. The cells were incubated with each pool (P1–P10) and with each corresponding blank to exclude buffer interferences at a dose of 1 µM. Subsequently, transactivation assays were performed using individual fractions of the positive pool which worked as the reference group (aldosterone 1 nM+spironolactone 100 nM).
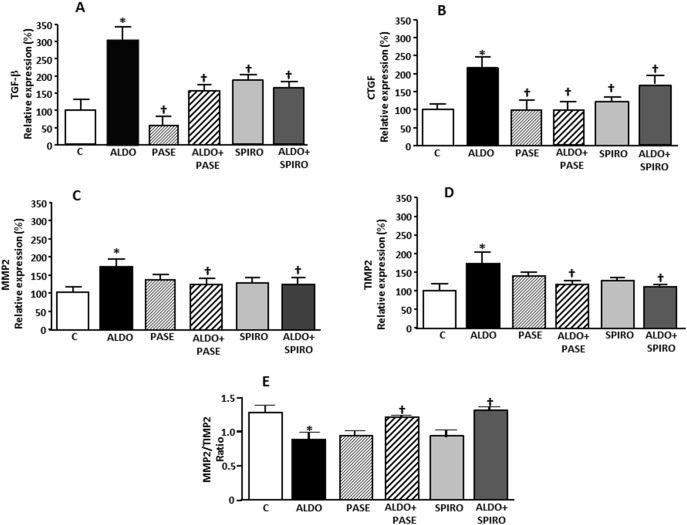
Figure 1. TGF-β (A), CTGF (B), MMP2 (C), TIMP2 (D) mRNA levels, and MMP2/TIMP2 ratio (E) in control (C), aldosterone-salt-treated animals (ALDO), PASE (PASE), aldosterone-salt plus PASE treated animals (ALDO+PASE), spironolactone treated animals (SPIRO) and aldosterone-salt plus spironolactone (ALDO+SPIRO).
Data are expressed as mean ± SEM derived from 8 animals per group. *p<0.05 vs. C; †p<0.05 vs. ALDO.
Statistical analysis
The data was analysed using a one-way analysis of variance, followed by a Bonferroni test if differences were noted (GraphPad Software Inc., USA). A p-value of 0.05 or less was considered significant.
Results
Hemodynamic parameters
Table 2 shows hemodynamic values obtained at end of the study. SBP, DBP, LVSP and LVEDP were significantly higher in aldosterone-salt-treated animals than in controls. –dP/dt was lower in aldosterone-salt-treated rats than in controls and +dP/dt was similar in all groups except in the ALDO+PASE group, which was higher than the aldosterone-salt group. PASE treatment prevented the changes observed with aldosterone-salt treatment.
Table 2. Hemodynamic parameters and collagen content.
| CONTROL | ALDO | PASE | ALDO+PASE | SPIRO | ALDO+SPIRO | |
| SBP (mm Hg) | 119±1.9 | 145±2.4* | 114±4.1† | 119±2.6† | 116±5.4† | 113±2.1† |
| DBP (mm Hg) | 89±1.2 | 108±3.1* | 91±3.4† | 92±4.1† | 83±1.2† | 91±2.2† |
| LVSP (mm Hg) | 121±1.7 | 146±9.3* | 115±2.3† | 123±2.5† | 117±2.3† | 116±1.8† |
| LVEDP (mm Hg) | 3.8±0.2 | 9.7±0.4* | 4±0.5† | 3.1±0.7† | 4.9±1.1† | 3.9±0.6† |
| HR (BPM) | 385±27 | 359±46 | 348±29 | 391±45 | 381±38 | 363±44 |
| +dP/dt (mm Hg/s) | 9390±163 | 8177±236 | 8128±388 | 10065±416† | 8367±325 | 8230±299 |
| –dP/dt (mm Hg/s) | −7106±479 | −4859±398* | −6125±356† | −9789±301† | −5988±196† | −6020±241† |
| HW/BW (mg/g) | 2.8±0.6 | 3.1±0.12* | 2.6±0.06† | 2.7±0.1† | 2.4±0.2† | 2.5±0.1† |
| COLLAGEN (%) | 0.057±0.01 | 0.220±0.02* | 0.065±0.01† | 0.051±0.02† | 0.042±0.02† | 0.065±0.01† |
SBP, systolic blood pressure; DBP, diastolic blood pressure; LVEDP, left ventricular end-diastolic pressure; LVSP, left ventricular systolic pressure, HR, heart rate; +dP/dt the first derivative of LV pressure rise over time; –dP/dt, the first derivative of LV pressure decline over time. HW/BW; heart weight/100 g body weight.
*p<0.05 vs CONTROL;
p<0.05 vs ALDO.
Hypertrophy and fibrosis
Relative heart weight (HW/BW) was significantly higher in aldosterone-salt-treated rats. These animals showed increased myocardial collagen content (Table 2) as well as increased expression of the genes encoding the fibrotic mediators, transforming growth factor beta (TGF-β) and connective tissue growth factor (CTGF), compared to controls (Figures 1A and 1B). PASE treatment also prevented the increase in these parameters induced by aldosterone-salt treatment. Matrix metalloprotease 2 (MMP2) and matrix metalloprotease inhibitor 2 (TIMP2) mRNA levels were higher but the MMP2/TIMP2 ratio was lower in aldosterone-salt-treated rats compared to controls (Figures 1C, 1D and 1E). PASE treatment also prevented the increase of MMP2 and TIMP2 mRNA levels observed with aldosterone-salt treatment and increased the MMP2/TIMP2 ratio. MMP2/TIMP2 ratio was significantly higher in aldosterone-salt-treated rats when PASE was administered as well as in SPIRO group (Figure 1E).
Inflammation, oxidation and eNOS
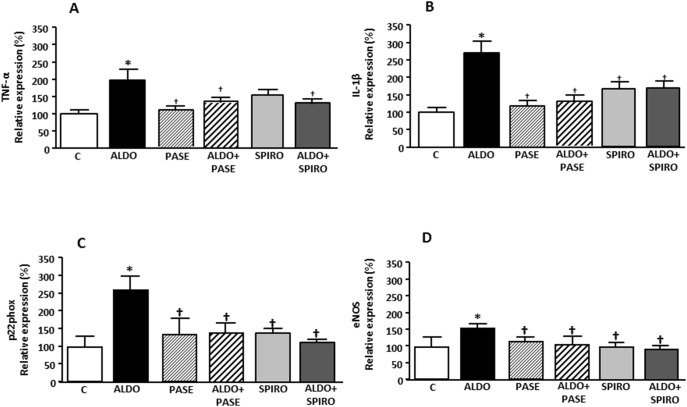
Tumor necrosis factor alpha (TNF-α) and interleukin 1 beta (IL-1β) mRNA levels were higher in aldosterone-salt-treated rats than in controls; these levels were reduced in ALDO+PASE and ALDO+SPIRO groups (Figures 2A and 2B) compared to aldosterone-salt treated rats. Similarly, aldosterone-salt-treated rats showed increased p22phox and endothelial nitric oxide synthase (eNOS) mRNA levels compared to control rats. Treatment with PASE when administered with the aldosterone-salt decreased the levels of both oxidative parameters compared to aldosterone-salt-treated rats (Figures 2C and 2D).
Figure 2. TNF-α (A), IL-1β (B), p22phox (C) and eNOS (D) mRNA levels in control (C), aldosterone-salt-treated animals (ALDO), PASE (PASE), aldosterone-salt plus PASE treated animals (ALDO+PASE), spironolactone treated animals (SPIRO) and aldosterone-salt plus spironolactone (ALDO+SPIRO).
Data are expressed as mean ± SEM derived from 8 animals per group. *p<0.05 vs. C; †p<0.05 vs. ALDO.
SGK-1 expression
All of the aforementioned changes induced by aldosterone-salt were accompanied by increased SGK-1 mRNA levels, which were markedly reduced when aldosterone-salt rats were treated with PASE (Figure 3).
Figure 3. SGK-1 mRNA levels in control (C), aldosterone treated animals (ALDO), PASE (PASE), aldosterone plus PASE treated animals (ALDO+PASE), spironolactone treated animals (SPIRO) and aldosterone-salt plus spironolactone (ALDO+SPIRO).
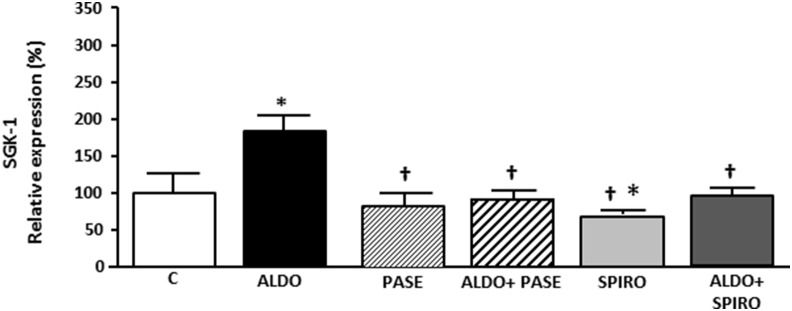
Data are expressed as mean ± SEM derived from 8 animals per group. *p<0.05 vs. CONTROL; †p<0.05 vs. ALDO.
Proanthocyanidins identification
Fractions were collected at 1 minute intervals and the absorbance of the elute monitored at 280 nm, which yielded 100 fractions. Proanthocyanidin were identified from the chromatogram obtained by monitoring the absorbance and based on Monagas et al [43] where they identified proanthocyanidins between 20 and 40 minutes after sample was injected in the HPLC (Figure 4).
Figure 4. HPLC chromatogram and pools representation.
Column: Waters Novapak C18 60Å 4 µm, 30×0.39 cm. Flow rate = 1 ml/min. Buffer A: 2% CH3COOH. Buffer B: 2% CH3COOH, 25% CH3CN. Arrow indicates column strip with 2% CH3COOH, 75% CH3CN. Sample volume loaded = 1 ml. Fractions collected at 1 min intervals. Pools compound of 10 individual fractions; P1∶1–10, P2∶11–20, P3∶21–30. P4∶31–40, P5∶41–50, P6∶51–60, P7∶61–70, P8∶71–80, P9∶81–90 and P10∶91–100.
Transcriptional activity of mineralocorticoid receptor
To assess whether the results obtained with PASE treatment, which paralleled those with spironolactone, represent specific antagonism of the MR, the ability of the total extract to block MR activity in a transcriptional assay was examined. PASE treatment was without activity alone and resulted in an antagonism of the response to aldosterone (Figure 5).
Figure 5. Effect of PASE on aldosterone-induced human mineralocorticoid receptor (hMR) transcriptional activity.
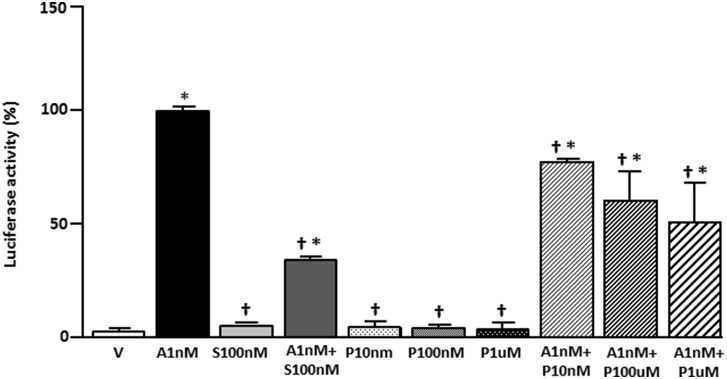
CV-1 cells transfected with hMR expression and MMTV-LUC reporter plasmids were treated with vehicle (V) or aldosterone 1 nM (A1 nM) in presence of spironolactone 100 nM (S100 nM) or PASE 10 nM, 100 nM or 1 µM (P10 nM, P100 nM or P1 µM). Each data point represents the mean ± SEM derived from 3 independent experiments. *p<0.01 vs V; †p<0.05 vs. A1 nM.
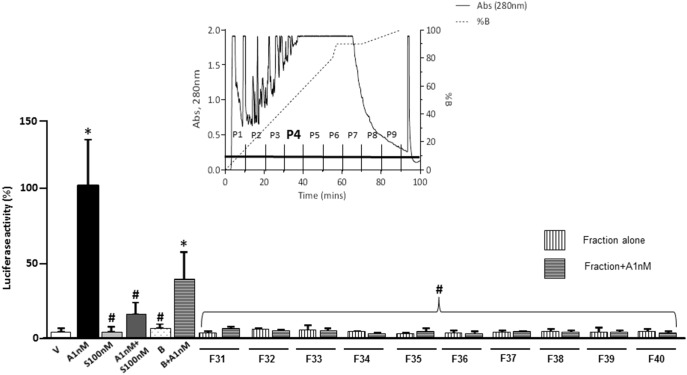
Aldosterone-induced MR transactivation was assessed in the presence of the PASE fractions obtained by HPLC. We observed a decrease of MR activity in pooled fractions from P2, P4, P5, P7 and P10 (data not shown). The ability of each of these fractions alone to inhibit the aldosterone-induced MR transactivation was examined. P4 and P10 were able to block the MR mediated response to aldosterone (Figure 6). Since Fractions in P10 are at the end of the column when high CH3CN was used to strip the column, we did not investigate these further. We therefore assessed the MR activity of the individual fractions (F31 to F40) which formed the P4 pool. Individual fractions from F31 to F40 were able to block MR activity (Figure 6).
Figure 6. Effect of P4 individual fractions on aldosterone-induced human mineralocorticoid receptor (hMR) transcriptional activity.
CV-1 cells transfected with hMR expression and MMTV-LUC reporter plasmids were treated with vehicle (V) or aldosterone 1 nM (A1 nM) in presence of spironolactone 100 nM (S100 nM) or fractions (F) 31 to 40 (1 µM), blank (B) and blank+aldosterone1 nM (B+A1 nM). Each data point represents the mean ± SEM derived from 3 independent experiments. *p<0.01 vs V; †p<0.05 vs. A1 nM.
Discussion
The present study shows for the first time that the treatment with a proanthocyanidins-rich almond skin extract prevents cardiac hypertrophy, fibrosis, inflammation, oxidative stress, hypertension and diastolic dysfunction induced by aldosterone plus salt administration in rats. PASE is able to reduce gene expression of the aldosterone-induced gene SGK-1 too. Furthermore, the study identifies proanthocyanidins as the PASE compounds responsible for the beneficial cardiac effects observed reducing aldosterone-induced transcriptional activity of the mineralocorticoid receptor in vitro. The effects of PASE treatment in aldosterone-salt rats on cardiac parameters were paralleled to those observed with the mineralocorticoid receptor antagonist spironolactone.
Many studies have explored the beneficial effects of proanthocyanidins in a purely descriptive way focusing on putative antioxidant effects. In the present study, we provide additional original, mechanistic insight into the effect of this extract on cardiac pathophysiology.
A large number of studies have described, in both in vitro and in vivo models, the numerous effects of proanthocyanidins-rich extracts on cell signaling. Although these studies are of significant value as a starting point to define the health benefits of proanthocyanidins, they have very limited value when mechanisms of action are discussed, given the difficulty in identifying the molecule(s) responsible for the observed effects. In the present study we have been able to describe not only the mechanism through which PASE is able to induce beneficial cardiac effects but also the molecules responsible for the observed effects. PASE is able to decrease the transcriptional activity of the mineralocorticoid receptor as was seen with the mineralocorticoid receptor antagonist, spironolactone. This result is particularly interesting since we are unaware of previous studies of a rich proanthocyanidins extract acting as a steroid receptor antagonist. Moreover, the HPLC results corroborated that proanthocyanidins are the phenolic compounds of PASE responsible of cardiac beneficial effects observed in aldosterone-salt-treated rats. Previous studies have shown that phenolic compounds could interact with cell signaling pathways. In animal models, it has been shown that dietary polyphenols can modulate expression of numerous genes and proteins in different organs, such as the aorta of apolipoprotein E-deficient mice [44]. This capacity of polyphenols to modulate the expression of genes through modulation of cell signaling pathways has also been described in vitro, e.g. epigallocatechin gallate in hepatocytes [45], flavonoids in vascular endothelial cells [46], [47], or neuronal cells [48]. Recent studies have also shown that polyphenols can modulate other regulators involved in the posttranscriptional regulation of expression of genes, particularly microRNA [49]. Nevertheless, none of these studies have revealed before an improvement of cardiac function related to mineralocorticoid receptors and pointed out proanthocyanidins as the main phenolic compound responsible of the beneficial effects observed in the present study. The study shows also for the first time that PASE was able to reduce the elevated mRNA levels of SGK-1 in aldosterone-treated rats. We have shown that SGK-1 is overexpressed in the heart of aldosterone+salt treated rats were structural, functional and molecular cardiac alterations occur [25]. This allows us to postulate that this mechanism has a central role in the amelioration and normalization of the complex intracellular signaling, involving fibrotic, inflammatory and oxidative pathways, which led to the cardiac hypertrophy and fibrosis induced by aldosterone.
Anti-inflammatory, antioxidant and antifibrotic effects shown in the present study, contribute to the beneficial cardioprotective effects of PASE in aldosterone-salt-treated rats. PASE reduced elevated TNF-α and IL-1β mRNA levels in hearts from aldosterone-salt-treated rats. Previous studies have reported that regular almond proanthocyanidins intake reduce inflammatory biomarkers [50]–[52]. Cell culture experiments, animal studies and human intervention trials have already shown that flavonols and procyanidins exhibit anti-inflammatory and antioxidant properties acting via several molecular targets, involving NF-κB and iNOS regulation [53]. Consumption of grape seed proanthocyanidins reduced levels of hs-C-reactive protein, interleukin-6 (IL-6) and TNF-α in Zucker Fa/fa rats fed a hyperlipidemic diet [54]. In addition to its anti-inflammatory effect, PASE reduced elevated p22phox mRNA levels in aldosterone-salt-treated rats, indicating a potential reduction of oxidative stress. Chronic ingestion of anthocyanins was associated with increased cardiac glutathione concentrations in rats [55]. Moreover, green tea epigallocatechin gallate reduced oxidative stress and bcl-2 levels in hypertrophied rat hearts [56].
Left ventricular remodeling is regulated in an important extent by eNOS and NO signaling [57]. Mice lacking eNOS display significantly aggravated left ventricular remodeling after myocardial infarction compared to wildtypes [58]. Furthermore, left ventricular function was improved, and hypertrophy was reduced in animals with selective overexpression of eNOS in cardiomyocytes [59]. Elevated eNOS expression in aldosterone-salt-treated rats could be considered as a defense mechanism against cardiac hypertrophy and elevated blood pressure, leading to enhance NO release which would be offering cardiac protection [60]–[62]. This concept is further supported by the fact that the reduction of p22phox expression by PASE, and potential reduction of oxidative stress, was accompanied by reduction of eNOS expression.
Aldosterone-salt treatment increased relative heart weight and collagen content reflecting an increase in collagen synthesis by fibroblasts as a reparative response to inflammation and cell death, and to hypertrophy of myocytes [63]–[65]. Our results have shown a decrease in the relative heart weight and collagen content with PASE treatment. Several studies have shown that polyphenols are cardioprotective by reducing cardiac hypertrophy. Administration of an alcohol-free red wine in rats with postinfarction remodeling showed a protective effect on hearts by repressing hypertrophy-associated phosphorylation of protein kinase C (PKC) α/β II and by activating Akt/protein kinase B [66]. PASE treatment reduced overexpression of fibrotic mediators suggesting a novel beneficial effect of proanthocyanidins on cardiac remodeling. Decreased production of MMPs or stimulation of TIMPs will also contribute to fibrosis. Both MMP2 and TIMP2 were elevated in aldosterone-salt-treated rats compared to controls; elevation of TIMP2 was more marked than that of MMP2, and as a consequence, the MMP2/TIMP2 ratio was reduced. These parameters were normalized by PASE, consistent with the ability of PASE to decrease the increased cardiac collagen content and fibrosis induced by aldosterone-salt-treatment.
The beneficial effects of PASE on cardiac hypertrophy, as well as on inflammatory, oxidant and fibrotic mediators, could contribute to the amelioration of cardiac hemodynamic parameters in aldosterone-treated rats. In fact, treatment with PASE reduced SBP and DBP, normalized LVSP and LVEDP, increased +dP/dt and –dP/dt, indicating an improvement of diastolic dysfunction and a stimulation of contractile capacity. PASE also showed an antihypertensive action in aldosterone-treated rats. Previous studies have already reported beneficial effects of flavonoids on blood pressure. Ingestion of green tea containing catechins for 24 weeks, reduced systolic blood pressure, in obese or pre-obese Japanese children [67], and epigallocatechin gallate reduced diastolic blood pressure in overweight or obese adults [68].
Taken together, the beneficial cardiac effects of PASE on the structural, functional and molecular alterations induced by the administration of aldosterone, our results suggest that one of the mechanism through which PASE proanthocyanidins could be acting would be as a mineralocorticoid receptor antagonist. The hypothesis is supported by the reduced expression of the principal mediator of cellular actions of aldosterone, SGK-1, to a similar extent to that observed with MR antagonist together with the decreased aldosterone-induced transactivation of the MR induced by PASE treatment. Moreover, we identified proanthocyanidins as the PASE compounds able to interact with MR and decrease aldosterone-induced transactivation of the MR. Our study provides a novel insight into mechanisms, other than an antioxidant action of proanthocyanidin-rich compounds that mediate their beneficial effects in a model of cardiovascular disease. However, further studies are needed to confirm this hypothesis.
In conclusion, the effects of PASE on cardiac hypertrophy, fibrosis, hypertension and diastolic dysfunction, were associated with a reduction of inflammatory, oxidative and fibrotic mediators. The observed reduction of SGK-1 expression together with the ability of PASE proanthocyanidins to antagonize MR-mediated transactivation in vitro argues for a MR-antagonist effect of this proanthocyanidins-rich extract.
Acknowledgments
We thank Dr. José Carlos Quintela and Natac Biotech S.L. for the technical support.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This work was supported by grants from VI Programa Nacional de Investigación Científica, Desarrollo e Innovación Tecnológica de España (SAF2011-30396) and the National Health and Medical Research Council of Australia through a Fellowship (#1002559) and Project Grant (#1002575) support to PJF. Prince Henry’s Institute is supported by the Victorian Government’s Operational Infrastructure Program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Struthers AD, MacDonald TM (2004) Review of aldosterone- and angiotensin II-induced target organ damage and prevention. Cardiovasc Res 61: 663–670 10.1016/j.cardiores.2003.11.037 [doi];S0008636303007594 [pii]. [DOI] [PubMed] [Google Scholar]
- 2. Rossi G, Boscaro M, Ronconi V, Funder JW (2005) Aldosterone as a cardiovascular risk factor. Trends Endocrinol Metab 16: 104–107 S1043-2760(05)00039-1 [pii];10.1016/j.tem.2005.02.010 [doi]. [DOI] [PubMed] [Google Scholar]
- 3. Swedberg K, Eneroth P, Kjekshus J, Wilhelmsen L (1990) Hormones regulating cardiovascular function in patients with severe congestive heart failure and their relation to mortality. CONSENSUS Trial Study Group. Circulation 82: 1730–1736. [DOI] [PubMed] [Google Scholar]
- 4. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, et al. (1999) The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 341: 709–717 10.1056/NEJM199909023411001 [doi]. [DOI] [PubMed] [Google Scholar]
- 5. Pitt B, White H, Nicolau J, Martinez F, Gheorghiade M, et al. (2005) Eplerenone reduces mortality 30 days after randomization following acute myocardial infarction in patients with left ventricular systolic dysfunction and heart failure. J Am Coll Cardiol 46: 425–431. [DOI] [PubMed] [Google Scholar]
- 6.Sun Y, Ramires FJ, Weber KT (1997) Fibrosis of atria and great vessels in response to angiotensin II or aldosterone infusion. Cardiovasc Res 35: 138–147. S0008636397000977 [pii]. [DOI] [PubMed]
- 7. Satoh M, Nakamura M, Saitoh H, Satoh H, Akatsu T, et al. (2002) Aldosterone synthase (CYP11B2) expression and myocardial fibrosis in the failing human heart. Clin Sci (Lond) 102: 381–386. [PubMed] [Google Scholar]
- 8. Takeda Y, Yoneda T, Demura M, Miyamori I, Mabuchi H (2000) Sodium-induced cardiac aldosterone synthesis causes cardiac hypertrophy. Endocrinology 141: 1901–1904 10.1210/endo.141.5.7529 [doi]. [DOI] [PubMed] [Google Scholar]
- 9. Struthers AD (2004) The clinical implications of aldosterone escape in congestive heart failure. Eur J Heart Fail 6: 539–545 10.1016/j.ejheart.2004.04.013 [doi];S1388984204001278 [pii]. [DOI] [PubMed] [Google Scholar]
- 10. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP (1990) Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 322: 1561–1566 10.1056/NEJM199005313222203 [doi]. [DOI] [PubMed] [Google Scholar]
- 11.Funder JW (2007) The role of aldosterone and mineralocorticoid receptors in cardiovascular disease. Am J Cardiovasc Drugs 7: 151–157. 731 [pii]. [DOI] [PubMed]
- 12.Brilla CG, Weber KT (1992) Mineralocorticoid excess, dietary sodium, and myocardial fibrosis. J Lab Clin Med 120: 893–901. 0022-2143(92)90267-O [pii]. [PubMed]
- 13. Scott BC, Butler J, Halliwell B, Aruoma OI (1993) Evaluation of the antioxidant actions of ferulic acid and catechins. Free Radic Res Commun 19: 241–253. [DOI] [PubMed] [Google Scholar]
- 14. Schafer A, Chovanova Z, Muchova J, Sumegova K, Liptakova A, et al. (2006) Inhibition of COX-1 and COX-2 activity by plasma of human volunteers after ingestion of French maritime pine bark extract (Pycnogenol). Biomed Pharmacother 60: 5–9 S0753-3322(05)00206-4 [pii];10.1016/j.biopha.2005.08.006 [doi]. [DOI] [PubMed] [Google Scholar]
- 15. Kwak CJ, Kubo E, Fujii K, Nishimura Y, Kobuchi S, et al. (2009) Antihypertensive effect of French maritime pine bark extract (Flavangenol): possible involvement of endothelial nitric oxide-dependent vasorelaxation. J Hypertens 27: 92–101. [DOI] [PubMed] [Google Scholar]
- 16. Murphy KJ, Chronopoulos AK, Singh I, Francis MA, Moriarty H, Pet al (2003) Dietary flavanols and procyanidin oligomers from cocoa (Theobroma cacao) inhibit platelet function. Am J Clin Nutr 77: 1466–1473. [DOI] [PubMed] [Google Scholar]
- 17. Sano T, Oda E, Yamashita T, Naemura A, Ijiri Y, et al. (2005) Anti-thrombotic effect of proanthocyanidin, a purified ingredient of grape seed. Thromb Res 115: 115–121 S0049-3848(04)00417-7 [pii];10.1016/j.thromres.2004.07.015 [doi]. [DOI] [PubMed] [Google Scholar]
- 18. Nocun M, Ulicna O, Muchova J, Durackova Z, Watala C (2008) French maritime pine bark extract Pycnogenol reduces thromboxane generation in blood from diabetic male rats. Biomed Pharmacother 62: 168–172 S0753-3322(07)00132-1 [pii];10.1016/j.biopha.2007.07.002 [doi]. [DOI] [PubMed] [Google Scholar]
- 19. Blade C, Arola L, Salvado MJ (2010) Hypolipidemic effects of proanthocyanidins and their underlying biochemical and molecular mechanisms. Mol Nutr Food Res 54: 37–59 10.1002/mnfr.200900476 [doi]. [DOI] [PubMed] [Google Scholar]
- 20.Belleville J (2002) The French paradox: possible involvement of ethanol in the protective effect against cardiovascular diseases. Nutrition 18: 173–177. S0899900701007213 [pii]. [DOI] [PubMed]
- 21.Guler A, Sahin MA, Yucel O, Yokusoglu M, Gamsizkan M, et al.. (2011) Proanthocyanidin prevents myocardial ischemic injury in adult rats. Med Sci Monit 17: BR326-BR331.coul 882042 [pii]. [DOI] [PMC free article] [PubMed]
- 22. Fraga CG, Oteiza PI (2011) Dietary flavonoids: Role of (−)-epicatechin and related procyanidins in cell signaling. Free Radic Biol Med 51: 813–823 S0891-5849(11)00360-1 [pii];10.1016/j.freeradbiomed.2011.06.002 [doi]. [DOI] [PubMed] [Google Scholar]
- 23. Spencer JP (2010) The impact of fruit flavonoids on memory and cognition. Br J Nutr 104 Suppl 3S40–S47 S0007114510003934 [pii];10.1017/S0007114510003934 [doi]. [DOI] [PubMed] [Google Scholar]
- 24.Afman L, Milenkovic D, Roche HM (2014) Nutritional aspects of metabolic inflammation in relation to health-insights from transcriptomic biomarkers in PBMC of fatty acids and polyphenols. Mol Nutr Food Res. 10.1002/mnfr.201300559 [doi]. [DOI] [PubMed]
- 25. Martin-Fernandez B, De las HN, Miana M, Ballesteros S, Delgado C, et al. (2011) Structural, functional, and molecular alterations produced by aldosterone plus salt in rat heart: association with enhanced serum and glucocorticoid-regulated kinase-1 expression. J Cardiovasc Pharmacol 57: 114–121 10.1097/FJC.0b013e31820088ca [doi]. [DOI] [PubMed] [Google Scholar]
- 26. Martin-Fernandez B, Miana M, De las HN, Ruiz-Hurtado G, Fernandez-Velasco M, et al. (2009) Cardiac L-type calcium current is increased in a model of hyperaldosteronism in the rat. Exp Physiol 94: 675–683 expphysiol.2009.047688 [pii];10.1113/expphysiol.2009.047688 [doi]. [DOI] [PubMed] [Google Scholar]
- 27. Brilla CG, Pick R, Tan LB, Janicki JS, Weber KT (1990) Remodeling of the rat right and left ventricles in experimental hypertension. Circ Res 67: 1355–1364. [DOI] [PubMed] [Google Scholar]
- 28. Wilke A, Funck R, Rupp H, Brilla CG (1996) Effect of the renin-angiotensin-aldosterone system on the cardiac interstitium in heart failure. Basic Res Cardiol 91 Suppl 279–84. [DOI] [PubMed] [Google Scholar]
- 29. Rocha R, Martin-Berger CL, Yang P, Scherrer R, Delyani J, et al. (2002) Selective aldosterone blockade prevents angiotensin II/salt-induced vascular inflammation in the rat heart. Endocrinology 143: 4828–4836. [DOI] [PubMed] [Google Scholar]
- 30.Young MJ, Funder JW (1996) Mineralocorticoids, salt, hypertension: effects on the heart. Steroids 61: 233–235. 0039-128X(96)00020-7 [pii]. [DOI] [PubMed]
- 31. Young M, Funder JW (2004) Eplerenone, but not steroid withdrawal, reverses cardiac fibrosis in deoxycorticosterone/salt-treated rats. Endocrinology 145: 3153–3157 10.1210/en.2004–0005 [doi];en.2004-0005 [pii]. [DOI] [PubMed] [Google Scholar]
- 32. Dietz JD, Du S, Bolten CW, Payne MA, Xia C, et al. (2008) A number of marketed dihydropyridine calcium channel blockers have mineralocorticoid receptor antagonist activity. Hypertension 51: 742–748 HYPERTENSIONAHA.107.103580 [pii];10.1161/HYPERTENSIONAHA.107.103580 [doi]. [DOI] [PubMed] [Google Scholar]
- 33. Fagart J, Hillisch A, Huyet J, Barfacker L, Fay M, et al. (2010) A new mode of mineralocorticoid receptor antagonism by a potent and selective nonsteroidal molecule. J Biol Chem 285: 29932–29940 M110.131342 [pii];10.1074/jbc.M110.131342 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pitt B, Kober L, Ponikowski P, Gheorghiade M, Filippatos G, et al. (2013) Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J 34: 2453–2463 eht187 [pii];10.1093/eurheartj/eht187 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Monagas M, Garrido I, Lebron-Aguilar R, Bartolome B, Gomez-Cordoves C (2007) Almond (Prunus dulcis (Mill.) D.A. Webb) skins as a potential source of bioactive polyphenols. J Agric Food Chem 55: 8498–8507 10.1021/jf071780z [doi]. [DOI] [PubMed] [Google Scholar]
- 36.Barreiros AL, David JP, de Queiroz LP, David JM (2000) A-type proanthocyanidin antioxidant from Dioclea lasiophylla. Phytochemistry 55: 805–808. S0031-9422(00)00297-1 [pii]. [DOI] [PubMed]
- 37. Anderson RA, Broadhurst CL, Polansky MM, Schmidt WF, Khan A, et al. (2004) Isolation and characterization of polyphenol type-A polymers from cinnamon with insulin-like biological activity. J Agric Food Chem 52: 65–70 10.1021/jf034916b [doi]. [DOI] [PubMed] [Google Scholar]
- 38. Zhao X, Wu N, Deng M, Yin Y, Zhou J, et al. (2006) An improved method of left ventricular catheterization in rats. Physiol Meas 27: N27–N33 S0967-3334(06)13913-1 [pii];10.1088/0967-3334/27/6/N01 [doi]. [DOI] [PubMed] [Google Scholar]
- 39. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408 10.1006/meth.2001.1262 [doi];S1046-2023(01)91262-9 [pii]. [DOI] [PubMed] [Google Scholar]
- 40.Rogerson FM, Yao YZ, Elsass RE, Dimopoulos N, Smith BJ, et al.. (2007) A critical region in the mineralocorticoid receptor for aldosterone binding and activation by cortisol: evidence for a common mechanism governing ligand binding specificity in steroid hormone receptors. Mol Endocrinol 21: 817–828. me. 2006-0246 [pii];10.1210/me.2006-0246 [doi]. [DOI] [PubMed]
- 41. Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, et al. (1987) Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science 237: 268–275. [DOI] [PubMed] [Google Scholar]
- 42. Rogerson FM, Dimopoulos N, Sluka P, Chu S, Curtis AJ, et al. (1999) Structural determinants of aldosterone binding selectivity in the mineralocorticoid receptor. J Biol Chem 274: 36305–36311. [DOI] [PubMed] [Google Scholar]
- 43. Monagas M, Garrido I, Lebron-Aguilar R, Bartolome B, Gomez-Cordoves C (2007) Almond (Prunus dulcis (Mill.) D.A. Webb) skins as a potential source of bioactive polyphenols. J Agric Food Chem 55: 8498–8507 10.1021/jf071780z [doi]. [DOI] [PubMed] [Google Scholar]
- 44. Coban D, Milenkovic D, Chanet A, Khallou-Laschet J, Sabbe L, et al. (2012) Dietary curcumin inhibits atherosclerosis by affecting the expression of genes involved in leukocyte adhesion and transendothelial migration. Mol Nutr Food Res 56: 1270–1281 10.1002/mnfr.201100818 [doi]. [DOI] [PubMed] [Google Scholar]
- 45. Goto T, Saito Y, Morikawa K, Kanamaru Y, Nagaoka S (2012) Epigallocatechin gallate changes mRNA expression level of genes involved in cholesterol metabolism in hepatocytes. Br J Nutr 107: 769–773 S0007114511003758 [pii];10.1017/S0007114511003758 [doi]. [DOI] [PubMed] [Google Scholar]
- 46. Chanet A, Milenkovic D, Claude S, Maier JA, Kamran KM, et al. (2013) Flavanone metabolites decrease monocyte adhesion to TNF-alpha-activated endothelial cells by modulating expression of atherosclerosis-related genes. Br J Nutr 110: 587–598 S0007114512005454 [pii];10.1017/S0007114512005454 [doi]. [DOI] [PubMed] [Google Scholar]
- 47. Garcia-Conesa MT, Tribolo S, Guyot S, Tomas-Barberan FA, et al. (2009) Oligomeric procyanidins inhibit cell migration and modulate the expression of migration and proliferation associated genes in human umbilical vascular endothelial cells. Mol Nutr Food Res 53: 266–276 10.1002/mnfr.200800134 [doi]. [DOI] [PubMed] [Google Scholar]
- 48. Spencer JP (2009) Flavonoids and brain health: multiple effects underpinned by common mechanisms. Genes Nutr 4: 243–250 10.1007/s12263-009-0136-3 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Milenkovic D, Jude B, Morand C (2013) miRNA as molecular target of polyphenols underlying their biological effects. Free Radic Biol Med 64: 40–51 S0891-5849(13)00274-8 [pii];10.1016/j.freeradbiomed.2013.05.046 [doi]. [DOI] [PubMed] [Google Scholar]
- 50. Jiang R, Jacobs DR Jr, Mayer-Davis E, Szklo M, Herrington D, et al. (2006) Nut and seed consumption and inflammatory markers in the multi-ethnic study of atherosclerosis. Am J Epidemiol 163: 222–231 kwj033 [pii];10.1093/aje/kwj033 [doi]. [DOI] [PubMed] [Google Scholar]
- 51. Spiller GA, Jenkins DA, Bosello O, Gates JE, Cragen LN, et al. (1998) Nuts and plasma lipids: an almond-based diet lowers LDL-C while preserving HDL-C. J Am Coll Nutr 17: 285–290. [DOI] [PubMed] [Google Scholar]
- 52. Spiller GA, Miller A, Olivera K, Reynolds J, Miller B, et al. (2003) Effects of plant-based diets high in raw or roasted almonds, or roasted almond butter on serum lipoproteins in humans. J Am Coll Nutr 22: 195–200. [DOI] [PubMed] [Google Scholar]
- 53. Hamalainen M, Nieminen R, Vuorela P, Heinonen M, Moilanen E (2007) Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators Inflamm 2007: 45673 10.1155/2007/45673 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Terra X, Montagut G, Bustos M, Llopiz N, Ardevol A, et al. (2009) Grape-seed procyanidins prevent low-grade inflammation by modulating cytokine expression in rats fed a high-fat diet. J Nutr Biochem 20: 210–218 S0955-2863(08)00053-3 [pii];10.1016/j.jnutbio.2008.02.005 [doi]. [DOI] [PubMed] [Google Scholar]
- 55.Toufektsian MC, de LM, Nagy N, Salen P, Donati MB, et al.. (2008) Chronic dietary intake of plant-derived anthocyanins protects the rat heart against ischemia-reperfusion injury. J Nutr 138: 747–752. 138/4/747 [pii]. [DOI] [PubMed]
- 56. Sheng R, Gu ZL, Xie ML, Zhou WX, Guo CY (2007) EGCG inhibits cardiomyocyte apoptosis in pressure overload-induced cardiac hypertrophy and protects cardiomyocytes from oxidative stress in rats. Acta Pharmacol Sin 28: 191–201 10.1111/j.1745-7254.2007.00495.x [doi]. [DOI] [PubMed] [Google Scholar]
- 57. Bauersachs J, Widder JD (2008) Endothelial dysfunction in heart failure. Pharmacol Rep 60: 119–126. [PubMed] [Google Scholar]
- 58. Scherrer-Crosbie M, Ullrich R, Bloch KD, Nakajima H, Nasseri B, et al. (2001) Endothelial nitric oxide synthase limits left ventricular remodeling after myocardial infarction in mice. Circulation 104: 1286–1291. [DOI] [PubMed] [Google Scholar]
- 59. Merx MW, Liehn EA, Janssens U, Lutticken R, Schrader J, et al. (2004) HMG-CoA reductase inhibitor simvastatin profoundly improves survival in a murine model of sepsis. Circulation 109: 2560–2565 10.1161/01.CIR.0000129774.09737.5B [doi];01.CIR.0000129774.09737.5B [pii]. [DOI] [PubMed] [Google Scholar]
- 60. Ricchiuti V, Lapointe N, Pojoga L, Yao T, Tran L, et al. (2011) Dietary sodium intake regulates angiotensin II type 1, mineralocorticoid receptor, and associated signaling proteins in heart. J Endocrinol 211: 47–54 JOE-10-0458 [pii];10.1530/JOE-10-0458 [doi]. [DOI] [PubMed] [Google Scholar]
- 61. Schafer A, Fraccarollo D, Hildemann S, Christ M, Eigenthaler M, et al. (2003) Inhibition of platelet activation in congestive heart failure by aldosterone receptor antagonism and ACE inhibition. Thromb Haemost 89: 1024–1030 10.1267/THRO03061024 [doi];03061024 [pii]. [PubMed] [Google Scholar]
- 62. Nishiyama A, Yao L, Nagai Y, Miyata K, Yoshizumi M, et al. (2004) Possible contributions of reactive oxygen species and mitogen-activated protein kinase to renal injury in aldosterone/salt-induced hypertensive rats. Hypertension 43: 841–848 10.1161/01.HYP.0000118519.66430.22 [doi];01.HYP.0000118519.66430.22 [pii]. [DOI] [PubMed] [Google Scholar]
- 63. Mano A, Tatsumi T, Shiraishi J, Keira N, Nomura T, et al. (2004) H (2004) Aldosterone directly induces myocyte apoptosis through calcineurin-dependent pathways. Circulation 110: 317–323 10.1161/01.CIR.0000135599.33787.CA [doi];01.CIR.0000135599.33787.CA [pii]. [DOI] [PubMed] [Google Scholar]
- 64.Mill JG, Milanez MC, de Resende MM, Gomes MG, Leite CM (2003) Spironolactone prevents cardiac collagen proliferation after myocardial infarction in rats. Clin Exp Pharmacol Physiol 30: 739–744. 3906 [pii]. [DOI] [PubMed]
- 65. Rocha R, Stier CT Jr, Kifor I, Ochoa-Maya MR, Rennke HG, et al. (2000) Aldosterone: a mediator of myocardial necrosis and renal arteriopathy. Endocrinology 141: 3871–3878. [DOI] [PubMed] [Google Scholar]
- 66. Palfi A, Bartha E, Copf L, Mark L, Gallyas F, et al. (2009) Alcohol-free red wine inhibits isoproterenol-induced cardiac remodeling in rats by the regulation of Akt1 and protein kinase C alpha/beta II. J Nutr Biochem 20: 418–425 S0955-2863(08)00100-9 [pii];10.1016/j.jnutbio.2008.04.009 [doi]. [DOI] [PubMed] [Google Scholar]
- 67. Matsuyama T, Tanaka Y, Kamimaki I, Nagao T, Tokimitsu I (2008) Catechin safely improved higher levels of fatness, blood pressure, and cholesterol in children. Obesity (Silver Spring) 16: 1338–1348 oby200860 [pii];10.1038/oby.2008.60 [doi]. [DOI] [PubMed] [Google Scholar]
- 68. Brown AL, Lane J, Coverly J, Stocks J, Jackson S, et al. (2009) Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: randomized controlled trial. Br J Nutr 101: 886–894 S0007114508047727 [pii];10.1017/S0007114508047727 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.