Abstract
Background
Care bundles have been shown to improve outcomes, reduce hospital readmissions and reduce length of hospital stay; therefore increasing the speed of uptake and delivery of care bundles should be a priority in order to deliver more timely improvements and consistent high-quality care. Previous studies have detailed the difficulties of obtaining full compliance to bundle elements but few have described the underlying reasons for this. In order to improve future implementation this paper investigates the challenges encountered by clinical teams implementing a chronic obstructive pulmonary disease (COPD) care bundle and describes actions taken to overcome these challenges.
Methods
An initial retrospective documentary analysis of data from seven clinical implementation teams was undertaken to review the challenges faced by the clinical teams. Three focus groups with healthcare professionals and managers explored solutions to these challenges developed during the project.
Results
Documentary analysis identified 28 challenges which directly impacted implementation of the COPD care bundle within five themes; staffing, infrastructure, process, use of improvement methodology and patient and public involvement. Focus groups revealed that the five most significant challenges for all groups were: staff too busy, staff shortages, lack of staff engagement, added workload of the bundle and patient coding issues. The participants shared facilitating factors used to overcome issues including: shifting perceptions to improve engagement, further education sessions to increase staff participation and gaining buy-in from managers through payment frameworks.
Conclusions
Maximising the impact of a care bundle relies on its successful and timely implementation. Teams implementing the COPD care bundle encountered challenges that were common to all teams and sites. Understanding and learning from the challenges faced by previous endeavours and identifying the facilitators to overcoming these barriers provides an opportunity to mitigate issues that waste time and resources, and ensures that training can be tailored to the anticipated challenges.
Keywords: COPD Exacerbations, Pulmonary Rehabilitation
Key messages.
With popularity of the concept of care bundles growing, the need for future implementation teams to be informed of the key challenges involved in implementing a care bundle is essential.
Focus groups revealed that the five most significant challenges to successful implementation were: staff too busy, staff shortages, lack of staff engagement, added workload of the bundle and patient coding issues.
Maximising the impact of a care bundle relies on its successful and timely implementation. A prior understanding of the challenges that teams may encounter provides an opportunity to mitigate these issues, saving time and resource and ensuring training tailored to the anticipated challenges.
Introduction
Chronic obstructive pulmonary disease (COPD) remains a major health problem associated with a high mortality and morbidity.1 Beginning in 2009, a COPD discharge care bundle was implemented across seven acute hospital sites in northwest London with support from the National Institute of Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care (CLAHRC) for Northwest London (NWL). The care bundle was implemented to address the recognised variation in clinical care of patients with acute exacerbation of COPD.1–5 Care bundles are a selection of four to five evidence-based practices that should be delivered to all patients, and have been demonstrated to be an effective tool for improving outcomes in patients with COPD.6–8
The COPD discharge care bundle included five evidence-based elements to be provided at discharge from hospital following an acute exacerbation of COPD. The five elements were:
If the patient is a smoker, offer smoking cessation assistance;
Refer for assessment for pulmonary rehabilitation;
Give written information about COPD including British Lung Foundation (BLF) self-management booklet, oxygen alert card and information about patient support groups (BLF Breathe Easy Group).
Demonstrate satisfactory use of inhalers.
Follow-up appointment to be made with a specialist prior to discharge.
The initial pilot study suggested that the 30-day readmission rate was 5.6% lower after initiation of the COPD care bundle,8 although the results were not statistically significant they provided sufficient evidence that further implementation would be beneficial to patients. Implementation first spread to other hospitals across London as part of a Commissioning for Quality and Innovation (CQUIN) payment framework, which makes a proportion of providers’ income conditional on achieving targets.9 This study describes the implementation of the COPD care bundle across seven sites from September 2010 to April 2012. The retrospective analysis conducted within this study does not refer to data collected during the pilot study.
CLAHRCs were developed to accelerate the implementation of evidence into practice, addressing the disconnect between the development of new innovations and their implementation in routine clinical practice.10 NIHR CLAHRC for NWL employs a quality improvement collaborative approach, providing funding and support to front line healthcare teams to improve practice. As the teams implemented the COPD care bundles, CLAHRC NWL supported them by providing the tools for monitoring delivery of the bundle. The COPD care bundle implementation showed varied compliance patterns and delivery (table 1) with average compliance between sites ranging from 42.8% to 84.4% during the study period. Compliance was measured as the team's ability to deliver all elements of the care bundle (100% compliance = all elements delivered). Monthly compliance measures at individual site level also showed wide variability which indicated that although the COPD care bundle seemed simple in concept, it may not be implemented optimally in practice, a finding noted for care bundles in other settings.11
Table 1.
Acute care organisation; hospital site and improvement team composition
| Acute care organisation | A | B | C | D |
|---|---|---|---|---|
| Number of sites | 1 | 3 | 2 | 1 |
| Approximate number of beds | 429 | 1258 | 631 | 400 |
| Presence of emergency department | Yes | Yes | Yes | Yes |
| Number of bundle team members | 8 | 14 | 10 | 15 |
| Data used in documentary analysis | Yes | Yes | Yes | Yes |
| Team participated in focus groups (n) | Yes (6) | Yes (5) | No | Yes (6) |
| Total number of bundles delivered at time of study | 150 | 327 | 389 | 186 |
| Achieved CQUIN target | Yes | Yes | Yes | Yes |
CQUIN, Commissioning for Quality and Innovation.
Care bundles have been shown to improve outcomes, reduce hospital readmissions and reduce length of hospital stay,7 11–13 therefore increasing the speed of uptake and delivery of fully compliant care bundles should be a priority in order to deliver more timely improvements and more consistent high-quality care. While previous studies have detailed the difficulties of obtaining full compliance to bundle elements,12 14 15 few have described the underlying reasons for this. In order to improve compliance with bundle elements and hasten uptake there is a need for detailed information on the experience of implementing bundles to be shared. This will provide necessary information on the context in which implementation of evidence takes place.16 To understand the context and experience of implementing a COPD care bundle, teams were consulted for their experience and perceptions. This study describes the challenges identified by improvement teams in delivering a COPD care bundle in practice and explores the actions taken by teams to overcome these issues.
Methods
Documentary analysis
Implementation teams across all sites recorded a range of quantitative and qualitative data throughout the projects. The CLAHRC NWL approach supports the use of a number of quality improvement tools that allow the team to capture their data in a continuous and iterative fashion. The data were recorded using an online web-based reporting tool which is now called the Web Improvement Support in Healthcare (WISH) system.16 WISH allows teams to easily record their data and produce improvement measure reports using Statistical Process Control (SPC), enabling teams to identify when there are significant changes to their measures. Teams reviewed improvement measures that provided information on the progress of bundle implementation, including the number of patients who had received the bundle with compliance of bundle delivery reported as SPC run charts. The teams also recorded qualitative progress feedback using Plan-Do-Study-Act (PDSA) cycles17 which provided a structure for teams to document their implementation plans, the successes and failures of these plans, and any changes they had made as a result. The teams provided structured reports at defined time points (6, 12 and 18 months) during their projects including the teams’ reflections on the application of a range of quality improvement tools and techniques used for the implementation of the COPD care bundle. Documentary analysis of these data was performed by the CLAHRC NWL research team using Nvivo V.9, a qualitative data analysis software tool. The qualitative analysis was based on a thematic framework approach.18 Initial themes were identified from a small sample of source data and applied across the whole dataset to generate detailed examples of the themes.
As the skills of those delivering the interventions are important for successful implementation,19 an exercise was also conducted to identify how challenges found in the documentary analysis were related to the skills of the staff implementing the bundle. To do this, the range of knowledge and skills required for quality improvement identified by the Institute for Healthcare Improvement (IHI)20 were combined with the Donabedian model for assessing healthcare quality to form a competency framework21 (table 2).
Table 2.
Professional affiliation of focus group partcipants
| Professional affiliation | Focus group 1 | Focus group 2 | Focus group 3 |
|---|---|---|---|
| Consultant | 1 | 1 | 1 |
| Nurse/clinical nurse specialist | 2 | 3 | 3 |
| Physiotherapist | 1 | 1 | – |
| Pharmacist | 1 | – | – |
| Project manager | 1 | – | 1 |
| Senior executive/manager | – | – | 1 |
Focus groups
All COPD care bundle implementation teams from northwest London were invited to participate in the focus groups. Researchers aimed to recruit between five and eight participants for each group as it has been found that this is an ideal size for non-commercial focus groups.22 These smaller groups allow for more in-depth discussion and make participants more comfortable to share their views.22 Three focus groups took place involving implementation team members from five of the seven hospital sites across northwest London. Seventeen healthcare professionals and healthcare managers participated in the three groups with group size ranging from five to six participants. The professional affiliation of participants for each focus group is shown in table 2.
The focus groups provided an opportunity for the implementation teams to discuss and rank challenges identified by the initial documentary analysis as well as present further challenges not previously identified. A semistructured discussion followed where the teams discussed the solutions and facilitators that were developed during the project to overcome the challenges identified. The focus groups were facilitated by an independent researcher (LL or HM) who had not been involved in the implementation work. The sessions were audio recorded and an additional researcher made field notes during the focus groups.
The audio recording and field notes were imported into Nvivo 9 and analysed using thematic content analysis.23 The workshop data were categorised based on the thematic challenges and within these categories, solutions identified were deductively derived. Analysis was undertaken by two investigators to aid in the reliability of coding. The data from each focus group were analysed individually and then aggregated. Quotes from individuals were used to illustrate the themes arising collectively from the different sites.
Results
Table 1 displays the characteristics of the hospital sites and improvement teams that participated in the study. Additional metrics have been provided to allow comparison between organisations and sites.
Documentary analysis
The documentary analysis uncovered a number of challenges identified by the teams. Five high level themes with 28 associated challenges were identified during the analysis (table 3).
Table 3.
Themes and associated challenges identified in documentary analysis
| High level themes | Associated challenges |
|---|---|
| Staffing |
|
| Infrastructure |
|
| Process |
|
| Methodology |
|
| Patient and public involvement |
|
BLF, British Lung Foundation; COPD, chronic obstructive pulmonary disease; GP, general practitioner; PDSA, Plan-Do-Study-Act.
Focus groups
The results from the documentary analysis were summarised to produce a competency table (table 4). This triangulation verified that the range of challenges encountered by teams were linked to skills and knowledge domains and related to quality improvement within health systems. This information was used to structure the focus group discussion.
Table 4.
Challenges aligned to the IHI Quality Improvement Competency Framework
| Donabedian model of quality |
|||
|---|---|---|---|
| Practice-based learning and improvement competency | |||
| Domain | Structure (staffing/infrastructure) | Process (care processes/knowledge exchange) | Outcomes (clinical outcomes/sustainable change) |
| Customer knowledge—patients’ perspectives and needs | Effectively engaging patients in the implementation of the bundle | Ensuring the COPD care bundle meets the needs of the patients | Demonstrating the COPD care bundle improves outcomes aligned to the needs of the patients |
| Variation and measurement—collection and analysis of data | Connectedness of IT systems to collect data at the patient level | Accuracy and resource to collect data on care processes | Linkage of process data and outcome data at a patient level |
| Leading, following and making changes in healthcare—demonstration of change management skills | Engaging and training staff with the necessary skills for implementation and evaluation of complex interventions | Delivering required care processes to reduce variation | Sustaining change |
| Developing new, locally useful knowledge—demonstrating development and use of PDSA cycles/model for improvement | Staff capacity to develop skills required for iterative design and feedback | Understanding the connectivity between care processes | Linking data to behaviour change and demonstrating causal linkage |
| Systems-based practice competency | |||
| Healthcare as process/system—engaging all patients in the healthcare system | Representativeness of patient involved and engaged | Capturing all patients eligible for intervention | Demonstrating population level health benefits |
| Collaboration—networking, joint working and sharing knowledge and ideas within and across organisations and between similar services | Engagement between care providers and third sector organisations | Sharing information about patient care | Competition versus collaboration |
| Social context and accountability—understanding conditions from a social perspective linking with health and social care model | Range of issues affecting disease beyond the biomedical model of healthcare | Conflicting priorities between different health and social care organisations and third sector providers | Demonstrating and sustaining system wide changes |
IHI, Institute for Healthcare Improvement; PDSA, Plan-Do-Study-Act.
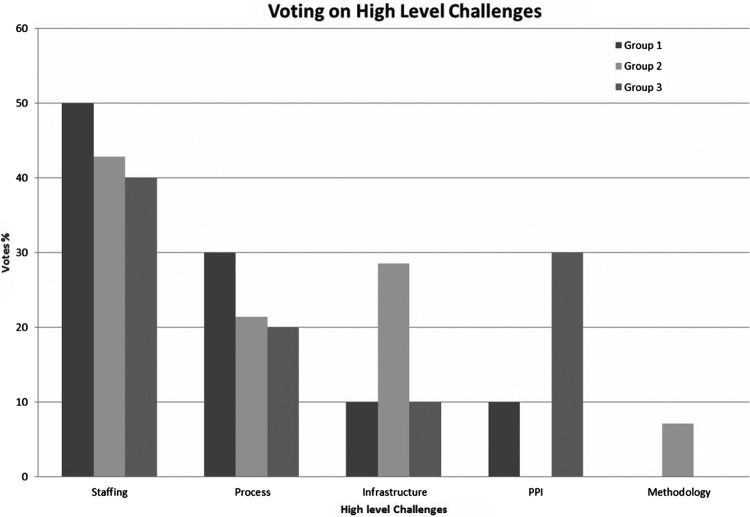
The focus groups were shown the challenges identified in the documentary analysis and participants were given the opportunity to rank the high-level themes according to impact of the challenge. Although the group discussed all challenges identified in the documentary analysis, this section focuses on the findings for the five most significant challenges, as ranked by the focus groups. All focus groups identified staffing as the greatest high-level challenge to implementation (figure 1).
Figure 1.
Focus group ranking of high-level themes Patient and Public Involvement (PPI).
The five most significant challenges to bundle implementation were: staff too busy, staff shortages, lack of staff engagement, added workload of the bundle and patient coding issues. These challenges and their suggested solutions are summarised in table 5.
Table 5.
Top 5 challenges and facilitators
| Challenge | Solution | Rationale | Focus group comments |
|---|---|---|---|
| Staff too busy | Use of a multidisciplinary team | Bundle initiated more consistently and less impact on one person's workload | “Having multidisciplinary people get involved helps with the initiation of the bundle. Because even if one person misses it a physio or nurse comes and starts it and even a pharmacist can say this patient isn't on a bundle and start one” (Physiotherapist, group 1) |
| Staff shortages | None identified | Viewed as out of staff control | “There are some things we will never have solutions for… because we can’t, we don’t have any power over that” (Nurse, group 2) |
| Staff engagement | Change perception of the bundle from research to best practice Finding project champions Having a CQUIN in place Educating the staff on the benefits of the bundle |
Demonstrated to staff that this should be done for every patient regardless of the bundle Aided in getting people on board and motivating staff to complete the bundle Allowed the staff to see the financial importance of bundle delivery and was key in gaining support from managers Allowed staff to recognise the improvement the bundle makes to patient's care |
“It was about educating and changing the perceptions…that really the two things we asked them to do, are what they should be doing during their everyday work anyway like inhaler technique and smoking cessation...So once the perception was changed and actually it was part of their everyday role, they didn't see it as an add-on to what they were already doing” (Nurse, group 1) “If you are going to do it, find a champion on the ward. Nurses do not respond to someone who comes on to the ward as much as they do to someone they already work with” (Consultant, group 3) “When the CQUIN was introduced there were financial penalties for non-completion which meant managers were more interested in encouraging staff to complete the bundle” (Physiotherapist, group 2) “If nurses don’t understand something they are not going to do it. The benefits of PR(Pulmonary rehab), inhaler technique, of course they want to do it because they want to provide the best care but if they don't understand it they aren’t going to do it. If you have someone there explaining than it is much better” (Clinical Nurse Specialist, group 2) |
| Added workload of the bundle | Changing the perception of the work involved in delivering the bundle | No longer considered extra work but as part of the standard of care required for all patients | “A large part was changing the perception of the bundle, they envisaged it as more time consuming than it actually was, because they are constantly being given more paperwork around various diseases and to them it was just another piece of paper that they thought would be a lot of work” (Nurse, group 1) |
| Patient coding issues | Engaging coders in the project Educating junior doctors on coding technique |
Coders aware of the bundle project and able to properly code patients Eligible patient accurately coded and started on the bundle |
“The team asked the coders to talk us through their process…by understanding their process we were able to help them understand ours” (Nurse, group 1) “Making sure that education is paramount for junior doctors… so that they are aware how to code the conditions” (Consultant, group 2) |
CQUIN, Commissioning for Quality and Innovation.
Focus group participants reported that staffing issues had a negative impact on the uptake of the bundle. Staff perception was initially a major barrier to uptake of the bundle as many staff believed the bundle may be “just another piece of paper” (Nurse, group 1) that would result in extra work. Participants also commented that the bundle had an impact on their workload and was often seen as too time consuming, with one participant commenting that initially the nurses had to “stop what they were doing to do the bundle” (Clinical Nurse Specialist (CNS), group 2). These issues led to a lack of staff engagement resulting in eligible patients missing out on receiving all elements of the bundle.
One site found that presenting the bundle as best practice resulted in staff being more likely to engage with the project and more willing to complete the bundles. The response to the bundle was much more positive when the staff saw it as “simply a way of recording the activities they were already doing” (Consultant, group 1). Once the bundle had been positioned as best practice it was no longer considered extra work but as part of the standard of care required for all patients. “We are doing this anyways, we are just trying to make sure that everybody gets it and it is going to be part of provding a better service to the patients” (Consultant, group 1).
Staff being too busy was consistently voted as a major challenge at all focus groups. Two teams identified the use of a multidisciplinary team as a facilitator. The teams found that having multiple professionals involved in the delivery of the bundle allowed the workload to be shared thus having less of an impact on one professional's responsibilities. This allowed the bundle to be completed more often and for fewer eligible patients to be missed. While staff shortages were identified as a significant challenge by all sites, teams did not identify or suggest any potential solutions for this area. It was often considered to be outside of the teams’ control and therefore solutions were not considered possible. It was evident from the discussions that the staff group most likely to be impacted by shortages was nurses.
Having a CNS, designated bundle nurse or project champion on the team was also identified as being a key facilitator to staff engagement issues. Participants commented that having a bundle champion or CNS aided in “getting people on board” (Clinical lead, group 2) and motivating staff members to complete the bundles. One team stated that having a champion also allows for the project to be rolled out in new setting more smoothly as it allowed staff to learn from someone they already knew. “If you are going to do it, find a champion on the ward. Nurses do not respond to someone who comes on to the ward as much as they do to someone they already work with” (Consultant, group 3).
Participants also identified that having a CQUIN was a key contributor to the uptake of the project and staff engagement. Having a CQUIN in place improved use of the bundle and allowed the staff to see the financial importance of delivering the bundle. The CQUIN also played a key role in having mangers and senior staff involved in encouraging and supporting the implementation of the bundle. “When the CQUIN was introduced there were financial penalties for non-completion which meant managers were more interested in encouraging staff to complete the bundle” (Physiotherapist, group 1).
The only challenge that was ranked in the top five outside the staffing theme was patient diagnostic coding; which was identified as a significant challenge for all teams. Participants revealed that they had encountered major problems with erroneous data with one consultant commenting that “50% of the data out of the warehouse are not COPD’ because ‘coding is reliant on many people in many systems” (Clinical lead, group 3). The issues related to patient coding resulted in eligible patients not receiving the bundle. Teams identified a range of solutions to address this challenge including performing baseline audits of patient coding to assess for coding accuracy, working with coders to understand the coding process, engaging coders in the project and educating junior doctors on coding processes.
Discussion
The COPD care bundle has been designed to support the uniform implementation of best practice, providing a mechanism for delivering of evidence based care, while improving documentation and reducing variation of care processes. While care bundles have been shown to be effective in improving care processes and outcomes in a range of conditions and settings, challenges still remain in their effective design and implementation.12 13 24 This is the first in-depth study to investigate staff perceptions of the challenges and facilitators of delivering a COPD care bundle. While this study provides a specific and focused overview of the challenges and potential facilitators associated with the implementation of a COPD care bundle in the acute hospital setting, the findings may be used by future teams to understand the potential challenges of implementing a bundle as well as ways to overcome them. The results of this study demonstrate a range of factors impacted the successful implementation of the COPD care bundle, which resonate with the findings from research in other studies of implementation.25–27 The solutions identified emphasise the need for ongoing support, education and engagement of staff as well as the importance of incentives to motivate staff and gain managerial buy-in.
Four of the five most significant challenges identified in this study relate to staffing, reflecting the importance of staff engagement and ensuring staff have the necessary skills and support to deliver improvements when implementing a care bundle. Highly engaged staff are associated with better outcomes and higher productivity and considered ‘essential for making change and improvement happen’19 emphasising the need for early and consistent staff engagement throughout bundle projects. The role of ‘adopter’ has been identified in previous studies as integral to achieving success in implementation of evidence and innovations into practice.19 25 26 This study further explored staff perceptions and their role as ‘adopters’ providing insight into how organisations may support staff to address potential issues and ensure maximum engagement during improvement initiatives. The challenges identified around patient coding highlight the need for robust coding measures to underpin the delivery of change and the need for clinicians to be adequately educated and confident that the patient diagnosis is correct and accurately recorded. Although coding issues continue to be long-standing concerns within hospitals, recent reports have demonstrated that overall the quality and accuracy of coding is improving.28 29
Importantly, this study also highlights facilitators and actions taken by staff during the implementation of the COPD care bundle. Implementation teams overcame many challenges by ensuring a multidisciplinary team structure in delivering elements of the COPD care bundle, for example, ward nurses offered smoking cessation referrals, physiotherapists assessed for pulmonary rehabilitation, and CNS advised on self-management. Fulbrook and Mooney27 found similar results in their study on the implementation of a care bundle in critical care and noted that “if ownership of the care bundle is widespread…its implementation is more likely to be successfully sustained.” Different staff members played key roles in overcoming challenges throughout the projects. ‘Bundle champions’ encouraged staff engagement and changed staff perceptions, respiratory clinicians engaged clinical coding staff, and respiratory nurse specialists supported ward nurses, an important aspect of sustaining change.
Implementing bundles which achieve 100% compliance often involves prolonged periods of learning and adaption.11 Planning for this time is imperative and will help teams to set realistic goals and anticipate training needs. While all teams reported variable monthly compliance throughout the duration of the projects it is important to note that all teams did achieve 100% compliance at specific points in time allowing each to meet their CQUIN targets. This variability demonstrates that while challenges are encountered, teams often require time and experience as well as ongoing support to address challenges and improve compliance over time.
With popularity of the concept of bundles growing, the need for future implementation teams to be informed of the key challenges involved in implementing a care bundle is essential. As bundles seem relatively simple in concept, there is a risk that future teams may begin new bundle projects or alter existing bundles without recognising the inherent challenges involved in delivering a care bundle and the potential impact it may have on staff and systems.30 This paper offers important lessons from frontline teams that have implemented the COPD care bundle and describes the challenges. The paper also provides information on facilitators used to mitigate these challenges which can be used to inform future implementation teams in overcoming similar barriers.
A potential limitation is the generalisability of the results as the thoughts and opinions collected from participants may not be representative of all staff involved in the implementation of the COPD care bundles however similar results are supported in the literature.11 12 27 These findings may not be applicable to care bundles in all other disease areas but reinforce that implementation of evidence into care is not without challenges which should be considered before implementation begins.
Maximising the impact of a care bundle relies on its successful and timely implementation. A priori understanding of the challenges that teams may encounter provides an opportunity to mitigate these issues, saving time and resource and ensuring training tailored to the anticipated challenges. Shared learning of the facilitators can also equip organisations with the knowledge of the importance of broader and effective stakeholder engagement early on in implementation efforts, ensuring the team have the knowledge and skills, and the perception of having sufficient time to ensure successful implementation. It has been recognised that there is a need to understand the mechanisms that make improvement projects work.16 29 This study provides invaluable information on what has made the COPD care bundle ‘work’ for teams looking to begin or improve their own bundle implementation. The findings from this study may allow future teams to establish early on the challenges associated with implementing care bundles and help teams to mitigate issues where possible, improving uptake and hastening implementation to deliver the best care possible faster.
Acknowledgments
The authors would like to acknowledge the work undertaken to deliver the quality improvement initiative by the implementation teams at Chelsea and Westminster Hospitals Trust, Imperial College NHS Trust, West Middlesex Hospitals Trust and Northwest London Hospitals Trust. The authors would also like to thank Dilys Lai, Bobby Mann, Trish Winn and Nick Hopkinson for their contribution.
Footnotes
Contributors: SE, LL and HM conceived the study. LL, HM and SG performed the documentary analysis. LL and HM undertook the focus groups. LL, SG, HM and CH wrote the first draft; all authors contributed to the revision of the manuscript.
Disclaimer: This article presents independent research commissioned by the National Institute for Health Research (NIHR) under the Collaborations for Leadership in Applied Health Research and Care (CLAHRC) programme for North West London. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Funding: National Institute for Health Research (NIHR) Collaborations for Leadership in Applied Health Research and Care (CLAHRC).
Competing interests: None.
Ethics approval: Ethics approval was not required for this work as it is part of a service evaluation and improvement project. All participants were NHS staff and provided verbal consent for the recording of the focus groups and were informed that all data would be anonymised for publication.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Buckingham R, Lowe D, Pursey N, et al. Report of the National Chronic Obstructive Pulmonary Disease Audit 2008: clinical audit of COPD exacerbations admitted to acute NHS units across the UK. Lodnon, 2008:1–81 [Google Scholar]
- 2.British Thoracic Society. Ready for Home? 2010
- 3.Institute of Medicine. Crossing the quality chasm a new health system for the 21st century. National Academies Press, 2001 [PubMed] [Google Scholar]
- 4.Department of Health. Report of the high level group on clinical effectiveness. London, 2007 [Google Scholar]
- 5.Right Care. The NHS Atlas of variation in healthcare reducing unwarranted variation to increase value and improve quality. London, 2011 [Google Scholar]
- 6.Resar R, Griffin FA, Haraden C, et al. Using care bundles to improve health care quality innovation series. Cambridge, 2012 [Google Scholar]
- 7.Winn T, Noone M, Buxton M. How a COPD care bundle is reducing readmissions. Health Serv J 2011;November [Google Scholar]
- 8.Hopkinson NS, Englebretsen C, Cooley N, et al. Designing and implementing a COPD discharge care bundle. Thorax 2012;67:90–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NHS Institute for Innovation and Improvement. Commisioning for Quality and Innovation (CQUIN) payment framework. 2012. [cited 2012 Oct 25]. http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_091443
- 10.Cooksey SD, Cooksey D. A review of UK health research funding: a review of UK health research funding. London, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hampton DC, Griffith D, Howard A. Evidence-based clinical improvement for mechanically ventilated patients. Rehabil Nurs 2003;30:160–5 [DOI] [PubMed] [Google Scholar]
- 12.Crunden E, Boyce C, Woodman H, et al. An evaluation of the impact of the ventilator care bundle. Nurs Crit Care 2005;10:242–6 [DOI] [PubMed] [Google Scholar]
- 13.Koehler BE, Richter KM, Youngblood L, et al. Reduction of 30-day postdischarge hospital readmission or emergency department (ED) visit rates in high-risk elderly medical patients through delivery of a targeted care bundle. J Hosp Med 2009;4:211–18 [DOI] [PubMed] [Google Scholar]
- 14.Bird D, Zambuto A, O'Donnell C, et al. Adherence to ventilator-associated pneumonia bundle and incidence of ventilator-associated pneumonia in the surgical intensive care unit. Arch Surg 2010;145:465–70 [DOI] [PubMed] [Google Scholar]
- 15.DuBose JJ, Inaba K, Shiflett A, et al. Measurable outcomes of quality improvement in the trauma intensive care unit: the impact of a daily quality rounding checklist. J Trauma 2008;64:22–7 [DOI] [PubMed] [Google Scholar]
- 16.Curcin V, Woodcock T, Poots AJ, et al. Model-driven approach to data collection and reporting for quality improvement. J Biomed Inform. 2014;in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langley GJ, Moen R, Nolan K. The improvement guide: a practical approach to enhancing organizational performance. San Francisco: Jossey-Bass Publishers, 1996 [Google Scholar]
- 18.Pope C, Ziebland S, Mays N. Analysing qualitative data. BMJ 2000;320:5–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Kings Fund. Leadership and engagement for improvement in the NHS-Together we can. London, 2012 [Google Scholar]
- 20.Batalden P, Berwick D, Bisognano M, et al. Knowledge domains for health professional students seeking competency in the continual improvement & innovation of health care. Boston, 1998:1–2 [Google Scholar]
- 21.Donabedian A. The quality of care. How can it be assessed? JAMA 1998;260:1743–8 [DOI] [PubMed] [Google Scholar]
- 22.Krueger RA, Casey MA. Focus groups: a practical guide for applied research fourth edition. Thousand Oaks: Sage Publications Ltd, 2009:63–81 [Google Scholar]
- 23.Carol Grbich. Qualitative research in health: an introduction. St. Leonards: Allen and Unwin, 1999:233–4 [Google Scholar]
- 24.Barochia AV, Cui X, Vitberg D, et al. Bundled care for septic shock: an analysis of clinical trials. Crit Care Med 2012;38:668–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenhalgh T, Robert G, Macfarlane F, et al. Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Q 2004;82:581–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grol R, Grimshaw J. Research into practice I from best evidence to best practice: effective implementation of change in patients’ care. Lancet 2003;362:1225–31 [DOI] [PubMed] [Google Scholar]
- 27.Fulbrook P, Mooney S. Care bundles in critical care: a practical approach to evidence-based practice. Nurs Crit Care 2003;8:249–55 [DOI] [PubMed] [Google Scholar]
- 28.Chan KS, Fowles JB, Weiner JP. Review: electronic health records and the reliability and validity of quality measures: a review of the literature. Med Care Res Rev 2010;67:503–27 [DOI] [PubMed] [Google Scholar]
- 29.Burns EM, Rigby E, Mamidanna R, et al. Systematic review of discharge coding accuracy. J Public Health (Bangkok) 2012;34:138–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mackay AJ, Donaldson GC, Patel ARC, et al. Usefulness of the chronic obstructive pulmonary disease assessment test to evaluate severity of COPD exacerbations. Am J Respir Crit Care Med 2012;185:1218–24. http://www.atsjournals.org/doi/abs/10.1164/rccm.201110-1843OC [DOI] [PubMed] [Google Scholar]